Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Perenosins: a new class of anion transporter with anti-cancer activity†
Wim
Van Rossom
,
Daniel J.
Asby
,
Ali
Tavassoli
and
Philip A.
Gale
 *
*
Department of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: philip.gale@soton.ac.uk
First published on 1st February 2016
Abstract
A new class of anion transporter named ‘perenosins’ consisting of a pyrrole linked through an imine to either an indole, benzimidazole or indazole is reported. The indole containing members of the perenosin family function as effective transmembrane Cl−/NO3− antiporters and HCl cotransporters in a manner similar to the prodigiosenes. The compounds reduce the viability of MDA-MB-231 and MCF-7.
Introduction
Prodigiosin is naturally occurring tripyrrolic compound,1 produced by a group of microorganisms including Serratia marcescens (Fig. 1).2 Although the compound was first isolated in pure form in 1929,3 it was not until 1977 that Fullan and co-workers demonstrated that prodigiosin has anti-tumour activity. Since that time the anti-cancer properties of many different natural and synthetic prodigiosenes have been explored.4,5 Prodigiosin has been shown to passively transport HCl across lipid bilayer membranes and it is proposed that the anti-cancer properties of this class of compounds may be linked to this process.6,7 Many prodigiosenes have also shown potent antimicrobial,8 antimalarial9 and immunosuppressive activity.10 Unfortunately, the high toxicity of prodigiosin and its analogues prevents their use in the clinic.11 Closely related compounds including the tambjamines12,13 and obatoclax,14 having similar structures, have been shown to exhibit similar biological properties (Fig. 1). Obatoclax mesylate GX15-070, an indole-based prodigiosin analogue, is currently in clinical trials, being evaluated in solid tumors and hematological neoplasms.Pyrrole and indole groups are found in many synthetic anion receptor systems. Examples that have been employed in lipid bilayer anion transport include amidopyrrole functionalized with a basic methylimidazole group that was shown to co-transport HCl,15 calixpyrrole-based transporters16 including strapped systems that trigger apoptosis in cells due to influx of NaCl,17 and indole functionalized thioureas.18 This latter class of compound have also been used as carboxylate transporters.19
In this paper we report the synthesis of a new class of anion transporter with structures inspired by prodigiosin. Known as ‘perenosins’20 these compounds contain a pyrrole hydrogen bond donor linked through an imine to an indole, benzimidazole or indazole. Compounds with a range of lipophilicities have been prepared and their anion complexation and transport properties studied.
Results and discussion
Synthesis and characterization
Perenosins 1a–e, 2 and 3 (Fig. 2) were prepared using a condensation reaction (EtOH, MgSO4, room temperature, 24 h) of 3,5-dimethylpyrrole-2-carboxaldehyde with a reduced 7-nitroindole, 7-nitrobenzimidazole or 7-nitroindazole (H2, Pd/C 10%, EtOH, room temperature, 5 h). The non-commercial 7-nitroindoles (except for R = MeO) were readily obtained from the respective 2-nitroaniline starting material through an iodination – Sonagashira reaction – base assisted cyclization pathway. For 5-methoxy-7-nitroindole an alternative Fisher indole synthesis – decarboxylation route was followed. Further details are provided in the ESI.†Compounds 1a–e, 2 and 3 all obey ‘Lipinski's rule of 5’ (except the non-protonated form of compound 1d which has a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P slightly over 5).23 The clog
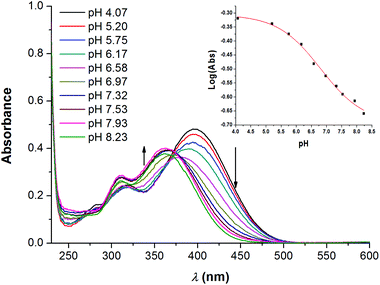
P slightly over 5).23 The clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of 1a–e, 2, 3 were calculated with VCCLabs (Table 1).22 Although initially hypothesised by J. T. Davis et al., no direct correlation between the basicity of prodigiosenes and their anti-cancer properties was found.24 pKa is, however, an indication at which pH the compounds are protonated and therefore at what point an increased affinity for anions is to be expected. The apparent pKa values for 1a–e and 2 were determined via a spectrophotometric method, previously described by Manderville (Fig. 3 and Table 1).25 In solution, protonated perenosins are dark yellow to orange with absorbance maxima above 380 nm. As free-base the compounds are mostly lightly yellow and absorb at a lower wavelength. Gradual variation of pH allows monitoring of the change in ionization state and determination of the pKa values from plots of log(Abs) versus pH (see ESI†).
P of 1a–e, 2, 3 were calculated with VCCLabs (Table 1).22 Although initially hypothesised by J. T. Davis et al., no direct correlation between the basicity of prodigiosenes and their anti-cancer properties was found.24 pKa is, however, an indication at which pH the compounds are protonated and therefore at what point an increased affinity for anions is to be expected. The apparent pKa values for 1a–e and 2 were determined via a spectrophotometric method, previously described by Manderville (Fig. 3 and Table 1).25 In solution, protonated perenosins are dark yellow to orange with absorbance maxima above 380 nm. As free-base the compounds are mostly lightly yellow and absorb at a lower wavelength. Gradual variation of pH allows monitoring of the change in ionization state and determination of the pKa values from plots of log(Abs) versus pH (see ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s, Hill coefficient (n), and pKa and calculated log
270 s, Hill coefficient (n), and pKa and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (clog
P (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) (free-base and protonated form) for perenosins 1a–e, 2 and 3
P) (free-base and protonated form) for perenosins 1a–e, 2 and 3
| Transporter | pKa | EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s 270 s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (mol%) b (mol%) |
n | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (error)c P (error)c |
clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P protonated (error)c P protonated (error)c |
|---|---|---|---|---|---|
a Literature value.21
b Cl−/NO3− assay using POPC/chol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at pH 7.2.
c Values calculated with VCCLabs.22
d not determined. 3) at pH 7.2.
c Values calculated with VCCLabs.22
d not determined.
|
|||||
| 1a | 6.84 | 0.0773 | 1.11 | 2.98 (±0.60) | 2.05 (±1.20) |
| 1b | 5.38 | 1.1922 | 1.52 | 3.84 (±0.61) | 3.37 (±0.59) |
| 1c | 6.81 | 0.0301 | 1.00 | 3.36 (±0.66) | 2.41 (±1.17) |
| 1d | 6.65 | 0.0299 | 1.00 | 5.16 (±0.98) | 4.02 (±1.46) |
| 1e | 7.11 | 0.1859 | 1.33 | 2.85 (±0.74) | 2.00 (±1.16) |
| 2 | 7.18 | 6.4084 | 1.93 | 2.32 (±0.44) | 1.58 (±1.38) |
| 3 | n.d.d | 5.4305 | 2.21 | 2.44 (±0.50) | 1.48 (±1.26) |
| Prodigiosin | 7.16a | 0.0002 | 1.00 | 4.12 (±0.78) | 3.28 (±1.15) |
X-ray crystallographic analysis
A single crystal of 1a with HCl suitable for X-ray analysis was obtained via slow evaporation of a CDCl3 solution of 1a in the presence of a small excess of HCl. The structure (Fig. 4) reveals that the protonated perenosin 1a forms three hydrogen bonds to chloride to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (N–Cl distances 3.169(4)–3.223(3) Å, N–H⋯Cl angles 164.3–175.5°). The compound adopts a planar conformation allowing for conjugation throughout the molecule and a more rigid structure (Fig. 4b).
1 complex (N–Cl distances 3.169(4)–3.223(3) Å, N–H⋯Cl angles 164.3–175.5°). The compound adopts a planar conformation allowing for conjugation throughout the molecule and a more rigid structure (Fig. 4b).
 | ||
| Fig. 4 X-ray crystal structure of [1a + HCl]; (a) indicating the distance (given in Å) between donor and acceptor; (b) side view (Cl− omitted for clarity). | ||
1H NMR titration studies
To assess the affinity for the biologically important chloride and bicarbonate anions, 1H NMR titration studies were performed to obtain association constants (1 × 10−5 M DMSO-d6/0.5% H2O, 298 K). The apparent association constants were calculated using WinEqNMR 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 by fitting the titration data to a 1
26 by fitting the titration data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, as was found from Job plot analysis27 supported by the single-crystal X-ray analysis (Table 2). Upon stepwise addition of chloride to the protonated host [1a + HPF6] the pyrrole N–H (11.91 to 13.28 ppm), iminium N–H (11.82 to 13.00 ppm) and indole N–H (11.37 to 11.57 ppm) proton resonances shifted downfield. In addition, a substantial downfield shift for the indole proton in the 6-position (7.33 to 7.59 ppm) and a modest downfield shift for the imine C–H proton (8.64 to 8.71 ppm) was observed. For the remaining protons no significant changes in chemical shift were observed. Addition of TEAHCO3 or TBAH2PO4 to the protonated [1a + HPF6] resulted in deprotonation of the receptor, whereas upon addition of TBANO3 no notable changes in chemical shift were observed. Similar results have previously been observed with prodigiosin.28 Receptors 1b–e responded in a similar manner to the addition of chloride and were shown to possess a similar affinity for chloride. For compounds 2 and 3, having benzimidazole and indazole functionalities instead of the indole group, respectively, the association constant with chloride was 10 times lower presumably due to the different resonance forms present (see ESI†).
1 binding model, as was found from Job plot analysis27 supported by the single-crystal X-ray analysis (Table 2). Upon stepwise addition of chloride to the protonated host [1a + HPF6] the pyrrole N–H (11.91 to 13.28 ppm), iminium N–H (11.82 to 13.00 ppm) and indole N–H (11.37 to 11.57 ppm) proton resonances shifted downfield. In addition, a substantial downfield shift for the indole proton in the 6-position (7.33 to 7.59 ppm) and a modest downfield shift for the imine C–H proton (8.64 to 8.71 ppm) was observed. For the remaining protons no significant changes in chemical shift were observed. Addition of TEAHCO3 or TBAH2PO4 to the protonated [1a + HPF6] resulted in deprotonation of the receptor, whereas upon addition of TBANO3 no notable changes in chemical shift were observed. Similar results have previously been observed with prodigiosin.28 Receptors 1b–e responded in a similar manner to the addition of chloride and were shown to possess a similar affinity for chloride. For compounds 2 and 3, having benzimidazole and indazole functionalities instead of the indole group, respectively, the association constant with chloride was 10 times lower presumably due to the different resonance forms present (see ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between 1a–e, 2, 3 (1 × 10−5 M, 298 K) and anionic guests in DMSO-d6/0.5% H2Oa
1 complexation between 1a–e, 2, 3 (1 × 10−5 M, 298 K) and anionic guests in DMSO-d6/0.5% H2Oa
| Receptor | Cl– (M–1) (1 equiv. HPF6)b | Cl– (M–1) | HCO3– (M–1) |
|---|---|---|---|
| a Calculated using WinEqNMR2.26 Maximum error estimated to be ±15%. b The exchange between PF6− and Cl− is observed. c Only minor spectral changes were observed under these conditions. | |||
| 1a | 1340 | <5c | 8.98 |
| 1b | 4030 | 6.98 | Deprot. |
| 1c | 1670 | <5c | 9.50 |
| 1d | 1650 | <5c | 11.5 |
| 1e | 2320 | <5c | 14.4 |
| 2 | 318 | Deprot. | |
| 3 | 433 | <5c | Deprot. |
Addition of TBACl to 1a–e, 2, 3 (1 × 10−5 M DMSO-d6/0.5% H2O, 298 K) only perturbed the proton resonances minimally not allowing for an association constant to be calculated in this competitive solvent mixture (Table 2). Addition of TEAHCO3 to the free-base form of the receptors revealed the presence of a very weak interaction, presumably due to the more basic nature of the anion and the potential binding of the anion's proton to the imine functionality.
Transmembrane chloride transport studies
The anti-cancer properties of the prodigiosenes have been linked to their ability to transport passively chloride or H+/Cl− across vesicle and cell membranes.6,7 Consequently, the ability of the perenosins to facilitate chloride and proton transport across lipid bilayers was assessed using a combination of ion selective electrode (ISE) and fluorescence assays. To quantify the chloride efflux rate Hill plots were determined for 200 nm POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol liposomes (Table 1; see ESI†). Typically, unilamellar vesicles were prepared from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol (7
cholesterol liposomes (Table 1; see ESI†). Typically, unilamellar vesicles were prepared from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio), containing an intravesicular sodium chloride solution (489 mM with 5 mM phosphate buffer at pH 7.2), were suspended in an isotonic sodium nitrate solution (489 mM with 5 mM phosphate buffer at pH 7.2). Perenosins 1a–e, 2, 3 were added as a DMSO solutions and the resulting chloride efflux was monitored by a chloride selective electrode. At the end of the experiment detergent (octaethylene glycol monododecyl ether) was added to lyse the liposomes and calibrate the electrode to 100% chloride release.
3 ratio), containing an intravesicular sodium chloride solution (489 mM with 5 mM phosphate buffer at pH 7.2), were suspended in an isotonic sodium nitrate solution (489 mM with 5 mM phosphate buffer at pH 7.2). Perenosins 1a–e, 2, 3 were added as a DMSO solutions and the resulting chloride efflux was monitored by a chloride selective electrode. At the end of the experiment detergent (octaethylene glycol monododecyl ether) was added to lyse the liposomes and calibrate the electrode to 100% chloride release.
This assay and others described below are evidence that the perenosins are mediating chloride/nitrate antiport in this case. Through the addition of transporter 1a in various concentrations, a Hill plot29 was derived giving EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s of 0.0773 mol% (carrier to lipid). The more electron-deficient analogue 1b (R = CF3), having a higher affinity for chloride but a lower pKa, proved to be a less efficient chloride transporter with EC50
270 s of 0.0773 mol% (carrier to lipid). The more electron-deficient analogue 1b (R = CF3), having a higher affinity for chloride but a lower pKa, proved to be a less efficient chloride transporter with EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s of 1.1922 mol% (Fig. 5). The introduction of a methyl or pentyl substituent in the 5-position of the indole moiety provided a decrease of the EC50
270 s of 1.1922 mol% (Fig. 5). The introduction of a methyl or pentyl substituent in the 5-position of the indole moiety provided a decrease of the EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s value (EC50
270 s value (EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s 0.0301 and 0.0299 mol%, respectively). Presumably the increase in clog
270 s 0.0301 and 0.0299 mol%, respectively). Presumably the increase in clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P with respect to 1a resulted in improved chloride efflux. A higher pKavia the use of methoxy-derivative 1e which should be protonated more easily and is therefore expected to transport more efficiently, resulted in a less effective transporter than 1a–d (EC50
P with respect to 1a resulted in improved chloride efflux. A higher pKavia the use of methoxy-derivative 1e which should be protonated more easily and is therefore expected to transport more efficiently, resulted in a less effective transporter than 1a–d (EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s 0.1859 mol%) most probably attributed to the receptor's lower clog
270 s 0.1859 mol%) most probably attributed to the receptor's lower clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value (Table 1). The benzimidazole and indazole derivatives were shown to be poor lipid bilayer chloride transporters. Perenosin 1d with the lowest EC50
P value (Table 1). The benzimidazole and indazole derivatives were shown to be poor lipid bilayer chloride transporters. Perenosin 1d with the lowest EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s was found to be two orders of magnitude slower than prodigiosin (EC50
270 s was found to be two orders of magnitude slower than prodigiosin (EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 s 0.0299 and 0.0002, respectively).
270 s 0.0299 and 0.0002, respectively).
To investigate the effect of the pH on the transport activity of perenosins 1a–e and 2, chloride/nitrate antiport was followed at different pH values (pH 4.0, 6.2, 7.2 and 8.2; Fig. 6). Upon decreasing the pH from 7.2 to 6.2, an increase in transport is observed corresponding to the increased amount of receptor molecules being protonated. At pH 8.2 there is a significant drop in transport activity as presumably a significant proportion of the transporters are not protonated. This is evidence that only the protonated form of this perenosin is capable of transporting anions.
To further explore the transport mechanism operating in this system, a variety of ISE and fluorescence vesicle assays were performed altering the bilayer and the intra- or extravesicular solution composition. To probe whether metal ion–anion symport occurs POPC vesicles were loaded with different group 1 metal (Na+, K+, Cs+) chloride salts (see ESI†). The metal was found to have no effect on the rate of chloride efflux from the vesicles upon addition of compound 1a, evidence in support of a transport mechanism not involving metal cations.
The sulfate ion is highly hydrophilic and is more challenging to transport across the lipid bilayer than nitrate.30 Upon addition of perenosin to vesicles loaded with sodium chloride suspended in a sodium sulfate solution, no chloride efflux was observed (see ESI†). Upon addition of bicarbonate to the extravesicular solution, a chloride/bicarbonate antiport mechanism may be initiated. After the bicarbonate pulse at t = 120 s, a modest increase in extravesicular chloride concentration was noted (Fig. 7). The compounds proved to be quite poor bicarbonate transporters presumably due to deprotonation of the protonated perenosin. This is evidence in support of the protonated form of the receptor being the species that is capable of transporting anions across the bilayer.
In the sulfate assays no anion transport was observed, however due to the very small intravesicular volume, HCl co-transport along a pH gradient using ion-selective electrode assays is very hard to quantify. The possible presence of HCl co-transport was studied by fluorescence using a pH gradient assay. Vesicles containing sodium chloride (489 mM) and 1 mM 8-hydroxy-1,3,6-pyrenetrisulfonate (HPTS), a pH sensitive fluorescent dye were prepared.31 The vesicles were suspended in a solution of sodium sulfate (167 mM) and the HPTS fluorescence measured upon addition of a DMSO solution of compounds 1a–e, 2 (Fig. 8). An increase in pH was observed, corresponding to the deacidification of the vesicles via a H+/Cl− co-transport mechanism (with Cl−/OH− antiport being ruled out due to the decrease in transport observed at higher pH and with basic anions such as bicarbonate).
Prodigiosenes have been shown to transport HCl across the lipid bilayer via a mobile carrier mechanism.1 The Hill coefficients found for all indole-based perenosins and prodigiosin have a value of approximately 1 evidence in support of the hypothesis that the transport of a chloride ion can be performed by a single carrier molecule.32 The non-indole perenosins 2 and 3 have a Hill coefficient of 1.93 and 2.21, respectively, evidence in support of cooperative mechanism involving two carrier molecules transporting one chloride ion.
Evidence for a carrier mechanism was derived from U-tube experiments.33 Transporters 1a, 1c–e, 2 as a solution in chloroform (1 mM) were kept between two aqueous phases as a membrane model mimicking a vesicle assay (see ESI†). The source aqueous phase was loaded with sodium chloride (489 mM buffered to pH 7.2 with 5 mM sodium phosphate salts) and the receiving aqueous phase was loaded with sodium nitrate (489 mM buffered to pH 7.2 with 5 mM sodium phosphate salts). The large separation between the two aqueous phases rules out the possibility of transport via channel formation. Chloride transport was monitored using an ISE and showed that all the tested perenosins yielded an increase in chloride concentration in the receiving phase over time (5 days). These results support the hypothesis of a mobile carrier mechanism being the most likely mode of transport in this case.
Transport studies with the prodigiosenes show that the pKa of the transporter correlates well with the EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270s,24 however no clear correlation could be found between the pKa of perenosins and their EC50
270s,24 however no clear correlation could be found between the pKa of perenosins and their EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270s. However, compounds with a pKa value higher than 6.65 and a clog
270s. However, compounds with a pKa value higher than 6.65 and a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P between than 2.85 and 5.16, (supported by the log
P between than 2.85 and 5.16, (supported by the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P range stipulated by Quesada et al.,13b) appear to exhibit the best chloride transport properties.
P range stipulated by Quesada et al.,13b) appear to exhibit the best chloride transport properties.
Taking all the transport studies together the results show that the indole perenosins behave similarly to prodigiosin namely functioning as both a HCl cotransporter and a Cl−/NO3− antiporter6 and forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with the anion.
1 complex with the anion.
Hydrolysis studies
The rate of hydrolysis is an important factor within the set of pharmokinetic properties, the collective of bioavailability and processes of absorption, distribution, metabolism and elimination. As a model for all perenosins, the hydrolysis rate of 1a in phosphate buffer (0.1 M NaCl) was determined following the perturbation of the UV/Vis spectroscopic data over time (see ESI†). At pH 7.2 the half-life time of 1a was calculated, following first-order kinetics, to be 10.8 h.34 Lowering the pH to 4.0 shortened the half-life time of 1a to 6.6 h, consistent with the presence of the imine linker. Entrapment of 1a inside a vesicular lipid bilayer at pH 7.2 extended the life span of perenosin 1a six fold (half-life 60.2 h). Vesicles that fuse with the cancer cell membrane, potentially decorated with cancer cell selective receptors and fluorophores, therefore offer a potential route to administer future anti-cancer agents based upon the perenosin scaffold. An additional benefit would be the reduced toxicity for highly active compounds when administered whilst embedded inside liposomes.35Cell-based analysis
We performed preliminary studies to assess the effect of perenosins on the viability of cancerous and non-cancerous model cell lines. Compounds 1a–e, 2 were assessed for their effect on the viability of breast carcinoma MDA-MB-231 (invasive) and MCF-7 (non-invasive) model cell lines, as well as MCF-10A normal mammary model cells (Table 3). The cell lines were treated with increasing doses of perenosins for 24 h and the effect on the degree of cell viability was determined by MTT assays giving a dose–response curve (see ESI Fig. S85–S90†) that was used to determine the IC50 values for each compound in each cell line.| Compound | MDA-MB-231 (μM) | MCF-7 (μM) | MCF-10A (μM) |
|---|---|---|---|
| 1a | 9.07 ± 1.30 | 6.02 ± 1.27 | 15.43 ± 5.75 |
| 1b | 3.67 ± 0.05 | 4.13 ± 0.22 | 12.93 ± 3.23 |
| 1c | 5.10 ± 1.08 | 4.38 ± 0.30 | 11.92 ± 3.37 |
| 1d | 4.39 ± 0.80 | 3.84 ± 0.12 | 22.37 ± 7.74 |
| 1e | 9.92 ± 0.80 | 5.61 ± 1.48 | 18.28 ± 6.69 |
| 2 | 28.78 ± 2.33 | 20.22 ± 7.35 | 51.20 ± 15.31 |
All indole-based perenosins 1a–e were cytotoxic to the two malignant cell lines at low μM, with 1b, 1c and 1d being most potent. All the molecules tested here were less potent in the normal MCF-10A cells. Interestingly, 1d showed the largest selectivity (∼5.5 fold) for the cancerous cell lines tested here. The benzimidazole derivative 2 was found to be substantially less active (IC50 24.32 μM) than the other molecules. The most active compounds in cells (1b and 1d) were also the most lipophilic in the series (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.84 and 5.06, respectively). The reduced cytotoxicity observed in the normal MCF-10A cells suggests a potential mechanism for selective targeting of cancer cells with more potent derivatives of these molecules.
P 3.84 and 5.06, respectively). The reduced cytotoxicity observed in the normal MCF-10A cells suggests a potential mechanism for selective targeting of cancer cells with more potent derivatives of these molecules.
Conclusions
The perenosins represent a new class of highly effective anion transporters based upon the structure of prodigiosin. It has been demonstrated that indole-based perenosins are highly efficient chloride transporters and function as a mobile-carrier by an antiport and H+/Cl− symport mechanism of anion transport. The most lipophilic derivatives affect the viability of two breast cancer cell lines with ∼5.5-fold selectivity over normal breast cells. These compounds therefore represent excellent lead structures for further exploration of the potentially selective anti-cancer activities of this new class of molecules.Acknowledgements
W. V. R. and P. A. G. thank the European Union for a Marie Curie Career Integration Grant. P. A. G. thanks the Royal Society and the Wolfson Foundation for a Royal Society Wolfson Research Merit Award. D. J. A. and A. T. thank the Engineering and Physical Sciences Research Council (for EP/H04986X/1). The authors thank Dr Ethan Howe for assistance with the preparation of this manuscript.Notes and references
- J. T. Davis, Top. Heterocycl. Chem., 2010, 24, 145–176 CrossRef CAS.
- B. Bizio, Biblioteca Italiana o sia Giornale di Letteratura, Scienze e Arti Tomo, 1823, 30, 275–295 Search PubMed.
- F. Wrede and O. Hettche, Ber. Dtsch. Chem. Ges. B, 1929, 62, 2678–2687 CrossRef.
- N. P. Fullan, D. L. Lynch and D. H. Ostrow, Microbiol. Lett., 1977, 5, 157–161 Search PubMed.
- (a) R. A. Manderville, Curr. Med. Chem.: Anti-Cancer Agents, 2001, 1, 195–218 CrossRef CAS PubMed; (b) R. Perez-Tomas, Curr. Med. Chem., 2006, 13, 1859–1876 CrossRef CAS PubMed; (c) R. Perez-Tomas, B. Montaner, E. Llagostera and V. Soto-Cerrato, Biochem. Pharmacol., 2003, 66, 1447–1452 CrossRef CAS PubMed; (d) J. Regourd, A. A.-S. Ali and A. Thompson, J. Med. Chem., 2007, 50, 1528–1536 CrossRef CAS PubMed; (e) R. I. Sáez Díaz, S. M. Bennett and A. Thompson, ChemMedChem, 2009, 4, 742–745 CrossRef PubMed.
- J. L. Seganish and J. T. Davis, Chem. Commun., 2005, 5781–5783 RSC.
- (a) T. Sato, H. Konno, Y. Tanaka, T. Kataoka, K. Nagai, H. H. Wasserman and S. Ohkuma, J. Biol. Chem., 1998, 273, 21455–21462 CrossRef CAS PubMed; (b) S. Ohkuma, T. Sato, M. Okamoto, H. Matsuya, K. Arai, T. Kataoka, K. Nagai and H. H. Wasserman, Biochem. J., 1998, 334, 731–741 CrossRef CAS PubMed; (c) D. Yamamoto, Y. Kiyozuka, Y. Uemura, C. Yamamoto, H. Takemoto, H. Hirata, K. Tanaka, K. Hioki and A. Tsubura, J. Cancer Res. Clin. Oncol., 2000, 126, 191–197 CrossRef CAS PubMed.
- (a) R. F. Tsuji, J. Magae, M. Yamashita, K. Nagai and M. Yamasaki, J. Antibiot., 1992, 45, 1295–1302 CrossRef CAS PubMed; (b) E. Marchal, Md. I. Uddin, D. A. Smithen, C. L. A. Hawco, M. Lanteigne, D. P. Overy, R. G. Kerr and A. Thompson, RSC Adv., 2013, 3, 22967–22971 RSC.
- (a) A. J. Castro, Nature, 1967, 213, 903–904 CrossRef CAS PubMed; (b) E. Marchal, D. A. Smitchen, Md. I. Uddin, A. W. Robertson, D. L. Jakeman, V. Mollard, C. D. Goodman, K. S. MacDougall, S. A. McFarland, G. I. McFadden and A. Thompson, Org. Biomol. Chem., 2014, 12, 4132–4142 RSC.
- (a) N. R. Williamson, P. C. Fineran, T. Gristwood, S. R. Chawrai, F. J. Leeper and D. P. C. Salmond, Future Microbiol., 2007, 2, 605–618 CrossRef CAS PubMed; (b) P. S. Kim, C. Jochems, I. Grenga, R. N. Donahue, K. Y. Tsang, J. L. Gulley, J. Scholm and D. Fursaci, J. Immunol., 2014, 192, 2622–2633 CrossRef CAS PubMed.
- R. H. Wier, R. O. Egeberg, A. R. Lack and G. Leiby, Am. J. Med. Sci., 1952, 224, 70–76 CrossRef CAS PubMed.
- P. I. Hernandez, D. Moreno, A. A. Javier, T. Torroba, R. Perez-Tomas and R. Quesada, Chem. Commun., 2012, 48, 1556–1558 RSC.
- (a) N. J. Knight, E. Hernando, C. J. E. Haynes, N. Busschaert, H. J. Clarke, K. Takimoto, M. García-Valverde, J. G. Frey, R. Quesada and P. A. Gale, Chem. Sci., 2016, 7, 1600–1608 RSC; (b) V. Saggiomo, S. Otto, I. Marques, V. Félix, T. Torroba and R. Quesada, Chem. Commun., 2012, 48, 5274–5276 RSC.
- B. D. de Grenu, P. I. Hernandez, M. Espona, D. Quinonero, M. E. Light, T. Torroba, R. Perez-Tomas and R. Quesada, Chem. – Eur. J., 2011, 17, 14074–14083 CrossRef PubMed.
- P. A. Gale, M. E. Light, B. McNally, K. Navakhun, K. E. Sliwinski and B. D. Smith, Chem. Commun., 2005, 3773–3775 RSC.
- (a) C. C. Tong, R. Quesada, J. L. Sessler and P. A. Gale, Chem. Commun., 2008, 6321–6323 RSC; (b) M. G. Fisher, P. A. Gale, J. R. Hiscock, M. B. Hursthouse, M. E. Light, F. P. Schmidtchen and C. C. Tong, Chem. Commun., 2009, 3017–3019 RSC; (c) P. A. Gale, C. C. Tong, C. J. E. Haynes, O. Adeosun, D. E. Gross, E. Karnas, E. Sedenberg, R. Quesada and J. L. Sessler, J. Am. Chem. Soc., 2010, 132, 3240–3241 CrossRef CAS PubMed; (d) M. Yano, C. C. Tong, M. E. Light, F. P. Schmidtchen and P. A. Gale, Org. Biomol. Chem., 2010, 8, 4356–4363 RSC.
- S. K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed.
- (a) N. J. Andrews, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis, W. A. Harrell Jr. and P. A. Gale, Chem. Sci., 2011, 2, 256–260 RSC; (b) S. J. Moore, M. Wenzel, M. E. Light, R. Morley, S. J. Bradberry, P. Gómez-Iglesias, V. Soto-Cerrato, R. Pérez-Tomás and P. A. Gale, Chem. Sci., 2012, 3, 2501–2508 RSC.
- C. J. E. Haynes, S. N. Berry, J. Garric, J. Herniman, J. R. Hiscock, I. L. Kirby, M. E. Light, G. Perkes and P. A. Gale, Chem. Commun., 2013, 49, 246–248 RSC.
- The name ‘perenosin’ was derived from a fusion of the name prodigiosin and the Russian word Переносчик [perenoschik], which translates as ‘carrier’.
- V. Rizzo, A. Morelli, V. Pinciroli, D. Sciangula and R. D'Alessio, J. Pharm. Sci., 1999, 88, 73–78 CrossRef CAS PubMed.
- VCCLAB Virtual Computational Chemistry Laboratory. http://www.vcclab.org.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 2001, 46, 3–26 CrossRef CAS PubMed.
- E. Marchal, S. Rastogi, A. Thompson and J. T. Davis, Org. Biomol. Chem., 2014, 12, 7515–7522 CAS.
- M. S. Melvin, J. T. Tomlinson, G. Park, C. S. Day, G. R. Saluta, G. L. Kucera and R. A. Manderville, Chem. Res. Toxicol., 2002, 15, 734–741 CrossRef CAS PubMed.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- P. Job, Ann. Chim., 1928, 9, 113–203 CAS.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sanchez, T. Torriba and R. Quesada, Nat. Chem., 2009, 1, 138–144 CrossRef CAS PubMed.
- A. V. Hill, Biochem. J., 1913, 7, 471–480 CrossRef CAS PubMed.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC.
- N. R. Clement and J. M. Gould, Biochemistry, 1981, 20, 1534–1539 CrossRef CAS PubMed.
- A. V. Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 905 CrossRef PubMed.
- (a) N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernandez, R. Perez-Tomas and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed; (b) S. N. Berry, N. Busschaert, C. L. Frankling, D. Salter and P. A. Gale, Org. Biomol. Chem., 2015, 13, 3136–3143 RSC.
- P. L. Toutain and A. Bousquet-Mélou, J. Vet. Pharmacol. Ther., 2004, 27, 427–439 CrossRef CAS PubMed.
- C. R. Dass, Methods Mol. Biol., 2008, 437, 177–182 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses details, 1H NMR and 13C NMR spectra, X-ray crystallographic data, vesicle transport assay details, Hill plots, hydrolysis data, in vitro assays details, and other supporting figures. Data underlying this publication are available see: DOI: 10.5258/SOTON/386610. CCDC 1441636. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/C6OB00002A |
| This journal is © The Royal Society of Chemistry 2016 |