Cintia Anton-Torrecillas, María Isabel Loza, José Brea and Jose C. Gonzalez-Gomez
Org. Biomol. Chem., 2016,14, 2264-2271
DOI:
10.1039/C5OB02624E,
Paper
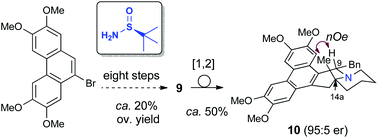
The first preparation of enantioenriched phenanthroquinolizidines with a quaternary center at C14a was accomplished in seven steps from readily available starting materials. Key steps were an efficient dynamic kinetic allylation of a diastereomeric mixture of chiral tert-butylsulfinyl ketimines and the construction of a piperidine E ring by rhodium catalyzed hydroformylation. The Stevens rearrangement of the corresponding N-benzyl derivatives took place smoothly, allowing the installation of a benzyl moiety at C9 in a trans relationship with the methyl group. The cytoxicity of the prepared phenanthroquinolizidines was evaluated against different human cancer cell lines.