Yi Sing Gee, Neils J. M. Goertz, Michael G. Gardiner and Christopher J. T. Hyland
Org. Biomol. Chem., 2016,14, 2498-2503
DOI:
10.1039/C5OB02577J,
Paper
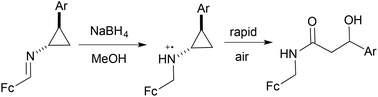
The in situ reduction of ferrocenyl cyclopropylimines to the corresponding amines triggers a facile oxidative ring-opening to yield the formal four-electron oxidation products: N-ferrocenylmethyl β-hydroxyamides. This process is believed to proceed via generation of a ferrocinium ion in the presence of air, leading to facile formation of a distonic radical cation that is ultimately trapped by oxygen.