A dramatic enhancing effect of InBr3 towards the oxidative Sonogashira cross-coupling reaction of 2-ethynylanilines†
Abstract
The addition of InBr3 to the oxidative Sonogashira cross-coupling reaction of 2-ethynylaniline with (E)-trimethyl(3,3,3-trifluoroprop-1-enyl)silane led to a dramatic increase in the reactivity to afford the corresponding 1,3-enynes bearing a trifluoromethyl group on their terminal sp2 carbon. The subsequent cyclization of these 1,3-enynes under palladium catalysis provides access to the corresponding indoles bearing a 3,3,3-trifluoroprop-1-enyl group at their 2-position.
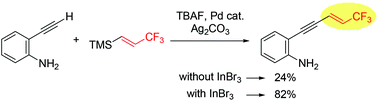

 Please wait while we load your content...
Please wait while we load your content...