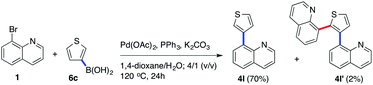Synthesis of 8-heteroaryl nitroxoline analogues via one-pot sequential Pd-catalyzed coupling reactions†
Received
17th November 2015
, Accepted 4th December 2015
First published on 5th January 2016
Abstract
A series of 8-heteroaryl substituted quinolines were prepared, either by direct C–H arylation of five-membered heteroarenes, or Pd-catalyzed coupling of organoboron reagents with bromoquinolines. The use of (benzo)thiophenyl or (benzo)furanyl boron coupling partners allowed further C–H functionalization on the five-membered heteroaryl ring with aryl bromides in one flask to access a variety of polyconjugated molecular architectures. The developed methodology represents a simple approach towards 8-arylated analogues of the biologically interesting nitroxoline core.
Introduction
8-Arylquinoline is one of the pharmaceutically important scaffolds present in many molecules which have shown a variety of biological activity,1 and has also been designed as a key structural element in materials science.2 Recently, we have described the synthesis of new potential cathepsin B inhibitors based on 1,3,5-triazin-2(1H)-one3 and nitroxoline (8-hydroxy-5-nitroquinoline)4 scaffolds. To further explore the structure–activity relationship an efficient synthetic approach to obtain a variety of 8-(hetero)arylquinoline derivatives was required. Therefore, we focused on metal-catalyzed arylations, which have found a wide application in the synthesis of bi(hetero)aryl systems. Indeed, the quinoline moiety has often been derivatized via Pd-,5 Ni-,6 Rh-,7 Cu-,8 and Ag-catalysed9 reactions producing mostly 2- and 4-arylated products. More recently, two methods for catalytic arylation of quinolines at the 8-position have been described,10,11 of which direct functionalization with a Rh(NHC)-based catalyst11 appeared as the most attractive. In the course of development of cross-coupling synthetic methodologies some individual examples of quinoline arylation at the 8-position have also appeared in the literature. For example, the Suzuki reaction of 5-bromoindanone and (quinoline-8-yl)boronic acid gave the corresponding cross-coupling product in good yield.12 Quinoline-8-yl triflate was used as a coupling partner in the decarboxylative arylation with potassium arylcarboxylates13 and arylation with potassium (hetero)aryltrifluoroborates14 catalyzed by Pd catalysts, while 5-methoxy-8-chloroquinoline coupled with phenylboronic acid in the presence of Ni(C) catalyst under relatively harsh reaction conditions.15 An example of Negishi cross-coupling of 8-chloroquinoline catalysed by a Ni(II)/diethyl phosphite system16 and regioselective functionalization of a quinoline moiety via magnesiation17 was described by Knochel and co-workers. Macdonald et al. described an elegant synthetic approach to phosphodiesterase type 4 photoaffinity probe by applying a cross-coupling step between 8-bromo-6-quinolinic acid and 3-aminobenzeneboronic acid.18
Results and discussion
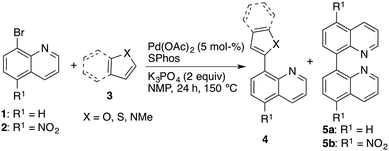
From the retrosynthetic point of view, 8-heteroarylquinoline derivatives could simply be prepared by metal-catalyzed heteroarylation via C–H activation of furans, thiophenes, pyrroles, oxazoles, thiazoles, and their benzo derivatives with 8-haloquinolines. In most cases, intramolecular arylations or intermolecular C5-arylations of C2-substituted five membered heterocycles have been extensively studied.19 However, the direct arylation of some fundamental heterocycles, such as furan, thiophene, and pyrrole to prepare their 2-arylated derivatives was found to be problematic.20 Guerchais, Doucet, and co-workers documented palladium-catalyzed direct heteroarylation of 8-bromoquinoline in moderate to good yields.21 Being aware of their successful examples we set out our synthetic approach towards 8-heteroarylquinoline derivatives via direct Pd-catalysed arylation of furan, thiophene, 1-methyl-1H-pyrrole, and their benzo derivatives. The starting material, 8-bromo-5-nitroquinoline (2), was prepared from commercially available 8-bromoquinoline (1). The most appropriate nitration reagent for 8-bromoquinoline was found to be an excess of KNO3 in concentrated sulphuric acid, wherein the reaction time played a crucial role in obtaining a good yield (86%) of the product 2.
Having the starting materials in hand, we initiated the coupling chemistry with the model reaction of 8-bromoquinoline (1) with furan. After thorough screening of different catalytic systems and reaction conditions22 we found Pd(OAc)2 (5 mol%) in combination with SPhos (10 mol%) and K3PO4 as the base in NMP at 150 °C as optimal reaction conditions. When 5 equivalents of furan were used, we achieved a complete conversion of 8-bromoquinoline yielding 4a (Table 1) together with a significant amount of side product 5a (ratio 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a = 1
5a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) produced by dimerization of 8-bromoquinoline. However, we were able to reduce the dimerization process of 8-bromoquinoline under the given reaction conditions only by applying a large excess of furan. When furan was used as a co-solvent (NMP-furan in a ratio of 1
2.4) produced by dimerization of 8-bromoquinoline. However, we were able to reduce the dimerization process of 8-bromoquinoline under the given reaction conditions only by applying a large excess of furan. When furan was used as a co-solvent (NMP-furan in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)), the complete conversion of 8-bromoquinoline was achieved in 24 h at 150 °C and the ratio of products 4a
1 (v/v)), the complete conversion of 8-bromoquinoline was achieved in 24 h at 150 °C and the ratio of products 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (10
5a (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) changed in favour of the desired product 4a. Under the optimized reaction conditions both bromoquinolines 1 and 2 can be successfully coupled with furan, thiophene, and 1-methyl-1H-pyrrole only if they are used in a large excess (Table 1). On the other hand, the method, using a large excess of heteroarene, was found to be impractical for benzo[b]furan (3e) and benzo[b]thiophene (3f); for this reason only 5 equivalents of 3e and 3f were used. Even though, arylation of benzo[b]furan with 8-bromoquinoline (1) progressed up to 73% conversion in 24 h, it was accompanied by the pronounced dimerization of 8-bromoquinoline. Consequently, we were able to isolate only 8% of the pure product 4e. Slightly better but still unsatisfactory results were obtained with benzo[b]thiophene (Table 1, entry 6). It should be mentioned that the optimized reaction conditions failed for the coupling of 8-bromo-5-nitroquinoline (2) either with benzo[b]furan or benzo[b]thiophene resulting in a rather complex reaction mixture. For comparison, we also found that 8-bromoquinoline is much less reactive than the 2- or 3-bromo analogues, since they can be successfully coupled with furan at lower temperature (130 °C) using Pd(OAc)2 (1 mol%) and PPh3 as a ligand.
1) changed in favour of the desired product 4a. Under the optimized reaction conditions both bromoquinolines 1 and 2 can be successfully coupled with furan, thiophene, and 1-methyl-1H-pyrrole only if they are used in a large excess (Table 1). On the other hand, the method, using a large excess of heteroarene, was found to be impractical for benzo[b]furan (3e) and benzo[b]thiophene (3f); for this reason only 5 equivalents of 3e and 3f were used. Even though, arylation of benzo[b]furan with 8-bromoquinoline (1) progressed up to 73% conversion in 24 h, it was accompanied by the pronounced dimerization of 8-bromoquinoline. Consequently, we were able to isolate only 8% of the pure product 4e. Slightly better but still unsatisfactory results were obtained with benzo[b]thiophene (Table 1, entry 6). It should be mentioned that the optimized reaction conditions failed for the coupling of 8-bromo-5-nitroquinoline (2) either with benzo[b]furan or benzo[b]thiophene resulting in a rather complex reaction mixture. For comparison, we also found that 8-bromoquinoline is much less reactive than the 2- or 3-bromo analogues, since they can be successfully coupled with furan at lower temperature (130 °C) using Pd(OAc)2 (1 mol%) and PPh3 as a ligand.
Table 1 Synthesis of the 8-heteroarylquinolines via Pd-catalyzed direct arylation
Since we found some limitations in the direct arylation approach with unsubstituted five-membered heteroarenes and being aware that it would be difficult to access targeted C3-quinolinyl heteroarenes we turned to the coupling chemistry with the model reaction of 8-bromoquinoline (1) with (furan-3-yl)boronic acid testing different catalytic systems and reaction conditions.22 The reaction was found to be rather sluggish at temperatures below 80 °C in toluene in the presence of Pd(PPh3)4 or Pd(OAc)2 complexes. Thus, a set of different Pd-catalysts, most of which were generated in situ from a Pd-source and triphenylphosphine ligand, were tested in several solvents at 100 °C. Most of the commonly used Pd-precatalysts more or less successfully coupled 8-bromoquinoline with (furan-3-yl)boronic acid. However, the catalyst system Pd(OAc)2/PPh3, K2CO3 in the mixture of 1,4-dioxane/water 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was chosen to be the most practical. Moreover, the addition of water to the reaction mixture could also contribute to sufficiently solubilize the base.
1 (v/v) was chosen to be the most practical. Moreover, the addition of water to the reaction mixture could also contribute to sufficiently solubilize the base.
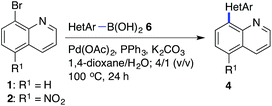
In continuation, the scope of Suzuki–Miyaura cross-coupling of bromoquinolines 1 and 2 with different heteroarylboronic acids 6 was investigated by applying optimized reaction conditions. The reactions with both substrates 1 and 2 were complete in 3–24 h providing the corresponding 8-heteroarylquinoline coupling products 4 in good yields (Table 2). Since microwave (MW) conditions have been shown to be beneficial in some examples of Suzuki–Miyaura reactions,23 we ran selected reactions under microwave irradiation at 100 °C in a 1,4-dioxane/water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system for 3 h. However, our results show that there is no beneficial effect of using MW conditions, and in some cases even lower yields of the isolated products 4 were obtained (Table 2, yields in brackets).
1) solvent system for 3 h. However, our results show that there is no beneficial effect of using MW conditions, and in some cases even lower yields of the isolated products 4 were obtained (Table 2, yields in brackets).
Table 2 Suzuki–Miyaura cross-coupling of 1 and 2 with heteroarylboronic acids 6
Suzuki–Miyaura cross-coupling of 8-bromoquinolines 1 and 2 with heteroarylboronic acids 6a–g allowed the introduction of a variety of pyrrolyl, furanyl, thiophenyl, and their benzo analogues at the 8-position of the quinoline nucleus in good to excellent yields. In the case of using N-Boc-pyrrole-2-boronic acid (Table 2, entries 4 and 11) as the coupling partner the Boc group was removed in the course of the reaction, as also noticed by other researchers.24
When 1 was coupled with (thiophene-3-yl)boronic acid (6c) in the presence of Pd(OAc)2 and PPh3 at temperatures higher than 100 °C, beside 4l we also isolated a minor quantity (2%) of the side product 4l′, 8,8′-(thiophene-2,3-diyl)diquinoline, arising from the arylation of the thiophene ring of the already formed Suzuki coupling product 4l with 8-bromoquinoline (Scheme 1).
 |
| | Scheme 1 Coupling reaction between 1 and 6c at elevated temperature. | |
This result suggested that a one-pot Suzuki–Miyaura coupling reaction followed by C–H arylation of an already installed heteroarene moiety could be feasible. The development of multistep synthetic sequences in a single flask, such as tandem, cascade, or sequential reactions is highly desirable in the chemistry community. There are a number of advantages of such processes, such as reduction of cost, time, and waste. Additionally, such a methodology enables the production of large molecular diversity and the introduction of a wide degree of complexity in a single transformation.25 There are several examples where the Suzuki–Miyaura coupling reaction was combined in a one-pot procedure, for example with C–H cross-coupling reaction,26 Heck coupling,27 isoxazole fragmentation,28 Michael addition,29 aza-Michael reaction,30 and condensation reaction.31
Investigation towards one-pot sequential procedure was initiated with the Suzuki–Miyaura cross-coupling between 8-bromoquinoline (1) and (furan-3-yl)boronic acid (6b) employing the catalyst Pd(OAc)2–PPh3, and a 1,4-dioxane/H2O solvent system. The subsequent C–H arylation was then carried out by simply adding 4-bromoacetophenone after the completion of the Suzuki coupling. In order to obtain high conversions, additional 5 mol% of the catalyst (Pd(OAc)2) without the additional loading of the ligand (PPh3) was required. The resulting reaction mixture was further stirred for 24 h while raising the temperature to 120 °C. Pleasingly, the expected arylated product 7a was obtained in a high 80% isolated yield (Scheme 2). A 1,4-dioxane–H2O, 4/1 (v/v) solvent system was found to be superior among the tested solvents (toluene, 1,4-dioxane, NMP, 2-propanol, and water). Even though 2-propanol32 showed promising results in the Suzuki–Miyaura cross-coupling reaction, the subsequent C–H activation step was almost completely inhibited in this solvent. The Suzuki–Miyaura cross-coupling products of 1 and 2 with commercially available heteroarylboronic acids 6b, 6c, 6e, 6f, and 6g allowed efficient subsequent C–H arylation of (benzo)furan, (benzo)thiophene, and pyrrole ring using different arylbromides as electrophiles, mostly affording monoarylated final products 7 (Scheme 2).
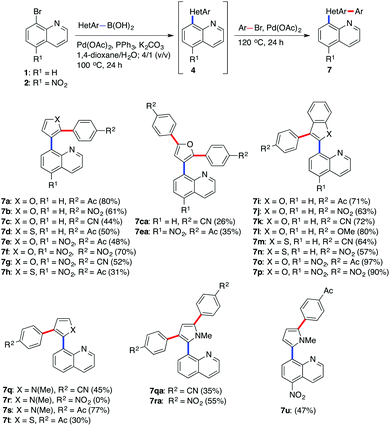 |
| | Scheme 2 One-pot synthesis of quinoline derivatives 7 by sequential Suzuki–Miyaura cross-coupling/C–H arylation reaction. | |
Detailed structural analysis of the monoarylated products 7a–h using X-ray analysis and 2D NMR techniques confirmed the formation of C2 arylated products that is in consistency with the literature data published to a large extent by Doucet and co-workers on C3 substituted furans,33 pyrroles,34 and thiophenes.35 The C–H arylation of 8-(thiophene-2-yl)- and 8-(N-methylpyrrol-2-yl)quinolines 4b and 4d (Scheme 2, products 7q–t) predominantly occurred at the C3 position rather than at C5. In case of arylation of nitro analogue 4j with 4-bromoacetophenone the C5 site selectivity was prevalent (Scheme 2, example 7u). To clarify the synthesis of 7t, the Suzuki–Miyaura reaction between 8-bromoquinoline (1) and potassium thiophene-2-yl trifluoroborate was achieved in 1,4-dioxane at 80 °C in the presence of a catalyst, which was synthesized form 2-aminobiphenyl, Pd(OAc)2 and SPhos (catalyst A).32 However, our results indicate that the catalyst A is not successful in performing the C–H arylation synthetic step, consequently, the arylation step was then performed with loading of Pd(OAc)2 (5 mol%), PPh3 (10 mol%), and K2CO3 and heating the reaction mixture at 120 °C for 24 h. The final product 7t was isolated in a modest 30% yield.
According to the 1H NMR spectrum of the crude reaction mixtures only traces of diarylated products were detected. However, in four particular cases we were able to isolate the diarylated products 7ca, 7ea, 7qa and 7ra in pure form (Scheme 2). To minimize the formation of the diarylated products 0.80 equivalents of the aryl bromides were used in the arylation step assuming the complete conversion of the Suzuki–Miyaura cross-coupling reaction.
According to the results of regioselectivity in the above-mentioned examples one could suggest the directing influence of the quinoline nitrogen in C–H activations,36 which is rather small due to a six-membered palladacyclic transition state and can be, in particular examples (Scheme 2, examples of monoarylated product 7u and diarylated products 7ca and 7ea), overwritten by the grater reactivity of the C-5 position. Additionally, we speculate that the nitro group in the case of the C-5 arylated product 7u may also have influence on the reactivity of the C-5 position, since in the analogue product 7s (lacking nitro functionality) C-3 arylation occurred. The examination of the set of aryl bromides as the coupling partners revealed that the aryl halides bearing an electron-accepting group reacted relatively well with the 8-heteroarylquinoline derivatives under the given reaction conditions. 4-Bromoanisole, bearing an electron-donating group, was found to be less reactive. However, by simply elevating the reaction temperature from 120 °C to 150 °C we were able to isolate the desired product 7l in an 80% yield (Scheme 2).
Furthermore, we were able to accomplish a three-step one-pot transformation, which included the Suzuki–Miyaura cross-coupling reaction of 2 and 6b followed by two C–H arylations of the furan-3-yl moiety of the formed intermediate 4n using two different aryl bromides. The corresponding product 8 was isolated in a reasonable 45% yield (Scheme 3).
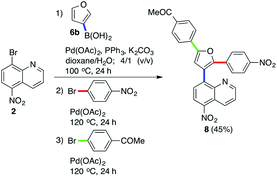 |
| | Scheme 3 One-pot synthesis of polyarylated furan. | |
Finally, we examined the possibility of combining the Suzuki–Miyaura cross-coupling reaction, direct C–H arylation reaction, and hydrogenation reaction in a one-pot synthetic procedure. The Suzuki–Miyaura cross-coupling reaction of 8-bromo-5-nitroquinoline (2) and benzo[b]furan-2-yl boronic acid (6f) delivered product 4q which was arylated with 4-bromonitrobenzene and the crude reaction mixture was then exposed to the atmosphere (1 atm) of hydrogen at room temperature for 24 h. The final product 9 (Scheme 4) was isolated in good (70%) overall yield.
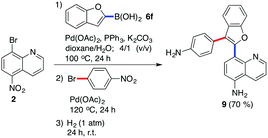 |
| | Scheme 4 One-pot Suzuki–Miyaura cross-coupling/arylation/hydrogenation reaction sequence. | |
Conclusions
In summary, an original two-step single-flask palladium-catalyzed Suzuki–Miyaura cross-coupling/direct C–H arylation sequence was developed starting from readily available 8-bromo- and 8-bromo-5-nitroquinoline. The method enables a straightforward derivatization of primarily formed heteroaryl Suzuki–Miyaura cross-coupling products with aryl bromides as proelectrophiles. In addition, we have demonstrated that our single-flask multicoupling approach successfully allowed the preparation of a 2,5-non-symmetrically diarylated furanyl quinoline product 8 by implementing two consecutive arylation steps. This sequential synthetic approach supplies a variety of hetero(aryl) functionalized quinolines being potential inhibitors of the cathepsin family of enzymes. Furthermore, the photophysical and electrochemical properties of some synthesized products are under investigation.
Experimental
General information
Unless specified, commercial-grade reagents were used without further purification. Reactions were monitored by analytical thin-layer chromatography (TLC) or reverse-phase HPLC. Visualization of the developed TLC chromatogram was performed by UV absorbance or aqueous potassium permanganate. Flash chromatography was performed on 230–400-mesh silica gel with the indicated solvent system. Yields refer to chromatographically and spectroscopically (1H and 13C NMR) homogeneous materials. Melting points are uncorrected. Infra-red spectra were recorded on a FT-IR spectrometer and are reported in reciprocal centimeters (cm−1). Routine nuclear magnetic resonance spectra were recorded either on a Bruker Avance DPX 300 or Avance III 500 MHz spectrometer. Chemical shifts for 1H NMR spectra are recorded in parts per million (ppm) from tetramethylsilane as an internal standard. Data are reported as follows: chemical shift, multiplicity, (s = singlet, d = doublet, t = triplet, q = quartet, qn = quintet, sext = sextet, sept = septet, m = multiplet, and br = broad), number of equivalent nuclei (by integration), coupling constants (J) quoted in Hertz to the nearest 0.25 Hz. Chemical shifts for the 13C NMR spectra are recorded in parts per million from tetramethylsilane using the central peak of the solvent resonance as an internal standard. All spectra were obtained with complete proton decoupling. High-resolution mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS instrument by electrospray ionization operating at a resolution of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 full widths at half height.
000 full widths at half height.
8-Bromo-5-nitroquinoline (2).
8-Bromoquinoline (1) (2.3 g, 11.2 mmol) was slowly added to a mixture of H2SO4 (7 mL, 98%) and KNO3 (4.5 g, 44.6 mmol) at 0 °C. The reaction mixture was allowed to warm to room temperature at which it was stirred for 2 h. The mixture was basified with a saturated solution of Na2CO3 to pH = 9 and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure to obtain 2.42 g (86%) of pure product 2.
Light yellow solid; Rf (light petroleum/EtOAc = 5/3) 0.48. FT-IR (ATR, neat): 3087, 1594, 1511, 1487, 1393, 1323, 1286, 921, 841, 806, 776 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.17 (dd, J = 4.0, 1.5 Hz, 1H), 9.07 (dd, J = 9.0, 1.5 Hz, 1H), 8.26 (d, J = 8.5 Hz, 1H), 8.20 (d, J = 8.5 Hz, 1H), 7.74 (dd, J = 9.0, 4.5 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 152.2, 145.0, 144.9, 133.3, 132.6, 131.4, 124.7, 122.1. HRMS (ESI−): m/z calcd for C9H6BrN2O2 (M + H)+: 252.9607; found: 252.9609. Elemental analysis calcd for C9H5BrN2O2: C, 42.72; H, 1.99; N, 11.07; found: C, 42.76; H, 1.83; N, 10.90.
General procedure for the synthesis of 8-heteroarylquinolines via Pd-catalyzed direct arylation (4a–j)
The corresponding quinoline (1, 2) (0.50 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol), SPhos (20.5 mg, 0.050 mmol) and K3PO4 (212 mg, 1.00 mmol) were dissolved in NMP. In the case of direct arylation of furan (3a), thiophene (3b), 2-methylthiophene (3c) and 1-methyl-1H-pyrrole (3d) the latter were added as a co-solvent (1 mL and 1 mL of NMP). In the case of direct arylation of benzo[b]furan (3e) and benzo[b]thiophene (3f) 5 equiv. (2.50 mmol) and 2 mL of NMP were added. The reaction mixture was stirred in a sealed glass tube at 150 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which water was added (3 mL) and the product was extracted into dichloromethane (2 × 3 mL). The combined organic layers were dried (Na2SO4) and filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 4.
8-(Furan-2-yl)quinoline (4a).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Colorless oil (47 mg, 48%); Rf (light petroleum/EtOAc = 10/1) 0.51. FT-IR (ATR, neat): 3060, 1582, 1501, 1015, 818, 790, 736 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.00 (dd, J = 4.0, 2.0 Hz, 1H), 8.24 (dd, J = 7.5, 1.5 Hz, 1H), 8.16 (dd, J = 8.5, 2.0 Hz, 1H), 7.81 (d, J = 3.5 Hz, 1H), 7.71 (dd, J = 8.0, 1.5 Hz, 1H), 7.60–7.75 (m, 2H), 7.43 (dd, J = 8.0, 4.0 Hz, 1H), 6.61 (dd, J = 3.5, 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 151.3, 149.9, 144.3, 141.9, 136.4, 128.9, 128.6, 127.0, 126.4, 126.0, 121.0, 113.0, 112.1. HRMS (ESI+): m/z calcd for C13H10NO (M + H)+: 196.0757; found: 196.0756.
8-(Thiophen-2-yl)quinoline (4b).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 50/1). Light yellow oil (62 mg, 59%); Rf (light petroleum/EtOAc = 10/1) 0.44. FT-IR (ATR, neat): 3065, 1494, 1311, 937, 820, 787, 694 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.01 (dd, J = 4.0, 2.0 Hz, 1H), 8.16 (dd, J = 8.0, 2.0 Hz, 1H), 8.06 (dd, J = 7.5, 1.5 Hz, 1H), 7.79 (dd, J = 3.5, 1.0 Hz, 1H), 7.73 (dd, J = 8.0, 1.5 Hz, 1H), 7.55 (dd, J = 8.0, 7.5 Hz, 1H), 7.48 (dd, J = 5.0, 1.0 Hz, 1H), 7.43 (dd, J = 8.0, 4.0 Hz, 1H), 7.17 (dd, J = 5.0, 3.5 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 149.5, 144.6, 139.7, 136.3, 133.0, 128.7, 128.0, 127.9, 127.1, 126.7, 126.6, 126.4, 121.2. HRMS (ESI+): m/z calcd for C13H10NS (M + H)+: 212.0528; found: 212.0528.
8-(5-Methylthiophen-2-yl)quinoline (4c).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Light green oil (44 mg, 39%); Rf (light petroleum/EtOAc = 10/1) 0.49. FT-IR (ATR, neat): 2914, 1496, 1474, 1046, 827, 794, 753, 650 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.00 (dd, J = 4.0, 2.0 Hz, 1H), 8.15 (dd, J = 8.5, 2.0 Hz, 1H), 8.01 (dd, J = 7.5, 1.5 Hz, 1H), 7.70 (dd, J = 8.0, 1.5 Hz, 1H), 7.57 (d, J = 3.5 Hz, 1H), 7.55–7.52 (m, 1H), 7.42 (dd, J = 8.0, 4.0, 1H), 6.82 (dd, J = 3.5, 1.0 Hz, 1H), 2.57 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 149.3, 144.6, 142.6, 137.3, 136.3, 133.4, 128.7, 127.4, 126.7, 126.6, 126.4, 124.9, 121.1, 15.3. HRMS (ESI+): m/z calcd for C14H12NS (M + H)+: 226.0685; found: 226.0684.
8-(1-Methyl-1H-pyrrol-2-yl)quinoline (4d).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 50/1). Colorless oil (63 mg, 61%); Rf (light petroleum/EtOAc = 5/3) 0.46. FT-IR (ATR, neat): 3046, 2940, 1498, 1314, 958, 831, 706 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.95 (dd, J = 4.0, 2.0 Hz, 1H), 8.18 (dd, J = 8.0, 2.0 Hz, 1H), 7.83 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (dd, J = 7.0, 1.5 Hz, 1H), 7.58–7.55 (m, 1H), 7.39 (dd, J = 8.0, 4.0 Hz, 1H), 6.84–6.83 (m, 1H), 6.32–6.29 (m, 2H), 3.47 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 150.4, 147.0, 136.1, 133.0, 132.2, 131.9, 128.5, 127.9, 126.1, 123.2, 120.9, 110.0, 107.7, 35.3. HRMS (ESI+): m/z calcd for C14H13N2 (M + H)+: 209.1073; found: 209.1074.
8-(Benzofuran-2-yl)quinoline (4e).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (9 mg, 8%); mp = 102–104 °C; Rf (light petroleum/EtOAc = 3/1) 0.46. FT-IR (ATR, neat): 3030, 1499, 1450, 1259, 1109, 821, 792, 741 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.07 (dd, J = 4.1, 1.8 Hz, 1H), 8.50 (dd, J = 7.4, 1.4 Hz, 1H), 8.30 (d, J = 0.9 Hz, 1H), 8.22 (dd, J = 8.2, 1.8 Hz, 1H), 7.81 (dd, J = 8.1, 1.4 Hz, 1H), 7.70–7.64 (m, 2H) 7.57 (dd, J = 8.2, 0.9 Hz, 1H), 7.49 (dd, J = 8.2, 4.1 Hz, 1H), 7.34–7.30 (m, 1H), 7.27–7.24 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 154.2, 153.0, 150.1, 144.9, 136.5, 129.9, 128.6, 128.4, 128.2, 127.4, 126.3, 124.5, 122.7, 121.6, 121.2, 110.9, 109.6. HRMS (ESI+): m/z calcd for C17H12NO (M + H)+: 246.0913; found: 246.0914. Elemental analysis calcd for C17H11NO: C, 83.25; H, 4.52; N, 5.71; found: C, 82.79; H, 4.33; N, 5.62.
8-(Benzo[b]thiophen-2-yl)quinoline (4f).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (37 mg, 28%); mp = 79–80 °C; Rf (light petroleum/EtOAc = 3/1) 0.41. FT-IR (ATR, neat): 3049, 1508, 1379, 1306, 932, 822, 740 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.07 (dd, J = 4.2, 1.8 Hz, 1H), 8.21 (dd, J = 8.2, 1.9 Hz, 1H), 8.17 (dd, J = 7.3, 1.4 Hz, 1H), 8.10 (s, 1H), 7.90–7.85 (m, 2H), 7.82 (dd, J = 8.1, 1.4 Hz, 1H), 7.62–7.59 (m, 1H), 7.48 (dd, J = 8.2, 4.1 Hz, 1H), 7.38–7.31 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 149.7, 145.1, 141.6, 140.4, 139.7, 136.5, 133.0, 129.2, 128.8, 128.1, 126.4, 124.2, 124.1, 123.9, 123.7, 121.9, 121.4. HRMS (ESI+): m/z calcd for C17H12NS (M + H)+: 262.0685; found: 262.0683. Elemental analysis calcd for C17H11NS: C, 78.13; H, 4.28; N, 5.36; found: C, 77.64; H, 3.87; N, 5.21.
8-(Furan-2-yl)-5-nitroquinoline (4g).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (53 mg, 44%); mp = 153–156 °C; Rf (light petroleum/EtOAc = 10/1) 0.47. FT-IR (ATR, neat): 3117, 2920, 1499, 1322, 1183, 1025, 948, 811, 787, 738 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.11 (dd, J = 9.0, 2.0 Hz, 1H), 9.07 (dd, J = 4.0, 2.0 Hz, 1H), 8.43 (d, J = 8.5 Hz, 1H), 8.28 (d, J = 8.5 Hz, 1H), 8.08 (d, J = 3.5 Hz, 1H), 7.67–7.65 (m, 2H), 6.66 (dd, J = 3.5, 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 150.5, 149.9, 143.9, 143.5, 143.1, 134.0, 132.3, 125.1, 123.7, 123.2, 121.8, 117.4, 113.0. HRMS (ESI+): m/z calcd for C13H9N2O3 (M + H)+: 241.0608; found: 241.0608.
5-Nitro-8-(thiophen-2-yl)quinoline (4h).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 25/1). Yellow solid (68 mg, 53%); mp = 144–147 °C; Rf (light petroleum/EtOAc = 10/1) 0.35. FT-IR (ATR, neat): 3088, 1558, 1498, 1418, 1397, 1327, 1305, 902, 851, 812 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.15 (dd, J = 9.0, 1.5 Hz, 1H), 9.11 (dd, J = 4.0, 1.5 Hz, 1H), 8.44 (d, J = 8.5 Hz, 1H), 8.17, (d, J = 8.5 Hz, 1H), 7.94 (d, J = 4.0 Hz, 1H), 7.69 (dd, J = 9.0, 4.0 Hz, 1H), 7.65 (d, J = 5.0, 1H), 7.22 (d, J = 5.0, 4.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 149.9, 143.8, 143.1, 139.9, 137.8, 132.4, 131.9, 128.9, 126.9, 125.1, 124.8, 123.9, 122.0. HRMS (ESI+): m/z calcd for C13H8N2O2S (M + H)+: 257.0385; found: 257.0387.
8-(5-Methylthiophen-2-yl)-5-nitroquinoline (4i).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (47 mg, 35%); mp = 162–164 °C; Rf (light petroleum/EtOAc = 3/1) 0.46. FT-IR (ATR, neat): 2916, 1497, 1469, 1442, 1393, 1356, 844, 803, 783 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.15 (dd, J = 8.9, 1.7 Hz, 1H), 9.08 (dd, J = 4.1, 1.7 Hz, 1H), 8.42 (d, J = 8.4 Hz, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.75 (d, J = 3.8, 1H), 7.67 (dd, J = 8.9, 4.1 Hz, 1H), 6.88 (d, J = 3.8 Hz, 1H), 2.60 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 149.6, 147.2, 143.6, 142.4, 140.2, 135.3, 132.4, 129.2, 125.4, 125.3, 123.9, 123.8, 122.1, 15.4. HRMS (ESI+): m/z calcd for C14H11N2O2S (M + H)+: 271.0536; found: 271.0536. Elemental analysis calcd for C14H10N2O2S: C, 62.21; H, 3.73; N, 10.36; found: C, 62.15; H, 3.87; N, 10.37.
8-(1-Methyl-1H-pyrrol-2-yl)-5-nitroquinoline (4j).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Orange solid (72 mg, 57%); mp = 119–120 °C; Rf (light petroleum/EtOAc = 5/3) 0.51. FT-IR (ATR, neat): 3119, 1509, 1497, 1328, 1311, 846, 814, 793, 735 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.08–9.05 (m, 2H), 8.40 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.64 (dd, J = 9.0, 4.0 Hz, 1H), 6.91–6.90 (m, 1H), 6.44 (dd, J = 3.5, 2.0 Hz, 1H), 6.34–6.33 (m, 1H), 3.52 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 151.1, 146.3, 144.3, 140.0, 132.0, 130.8, 129.2, 125.2, 124.4, 123.6, 121.7, 112.4, 108.5, 35.8. HRMS (ESI+): m/z calcd for C14H12N3O2 (M + H)+: 254.0924; found: 254.0925. Elemental analysis calcd for C14H11N3O2: C, 66.40; H, 4.38; N, 16.59; found: C, 66.17; H, 4.11; N, 16.37.
General procedure for Suzuki–Miyaura coupling (4k–r).
The corresponding quinoline (1, 2) (0.25 mmol), boronic acid (6a–g) (0.375 mmol), Pd(OAc)2 (2.8 mg, 0.0125 mmol), PPh3 (6.6 mg, 0.025 mmol) and K2CO3 (69 mg, 0.5 mmol) were dissolved in a mixture of 1,4-dioxane (0.8 mL) and water (0.2 mL). The reaction mixture was stirred in a sealed glass tube at 100 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which water was added (3 mL) and the product was extracted into dichloromethane (2 × 3 mL). The combined organic layers were dried (Na2SO4) and filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 4.
8-(Furan-3-yl)quinoline (4k).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 50/1). Colourless oil (42 mg, 85%); Rf (light petroleum/EtOAc = 5/3) 0.44. FT-IR (ATR, neat): 3045, 1511, 1497, 1158, 1028, 871, 794, 756, 645 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.98 (dd, J = 4.1, 1.8 Hz, 1H), 8.56 (s, 1H), 8.18 (dd, J = 8.3, 1.8 Hz, 1H), 7.87 (dd, J = 7.2, 1.4 Hz, 1H), 7.73 (dd, J = 8.2, 1.4 Hz, 1H) 7.57–7.54 (m, 2H), 7.43 (dd, J = 8.2, 4.1 Hz, 1H), 7.00 (d, J = 1.9 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 149.7, 145.7, 143.1, 142.3, 136.4, 131.5, 128.8, 127.8, 126.8, 126.4, 122.8, 121.1, 110.6. HRMS (ESI+): m/z calcd for C13H10NO (M + H)+: 196.0757; found: 196.0757.
8-(Thiophen-3-yl)quinoline (4l).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow oil (37 mg, 70%); Rf (light petroleum/EtOAc = 5/3) 0.48. FT-IR (ATR, neat): 3034, 1607, 1529, 1133, 866, 830, 793, 754 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.97 (dd, J = 4.1, 1.8 Hz, 1H), 8.16 (dd, J = 8.2, 1.8 Hz, 1H), 7.89 (dd, J = 3.0, 1.3 Hz, 1H), 7.85 (dd, J = 7.2, 1.5 Hz, 1H), 7.75 (dd, J = 8.1, 1.4 Hz, 1H), 7.66 (dd, J = 5.0, 1.4 Hz, 1H), 7.56–7.53 (m, 1H), 7.42 (dd, J = 5.0, 3.0 Hz, 1H), 7.39 (dd, J = 8.2, 4.1 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 150.0, 145.8, 139.5, 136.3, 135.1, 129.7, 129.4, 128.8, 127.3, 126.3, 124.7, 124.5, 121.0. HRMS (ESI+): m/z calcd for C13H10NS (M + H)+: 212.0528; found: 212.0530. Elemental analysis calcd for C13H9NS: C, 73.90; H, 4.29; N, 6.63; found: C, 73.75; H, 4.16; N, 6.57.
8-(1H-Pyrrol-2-yl)quinoline (4m).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (28 mg, 57%); mp = 86–90 °C; Rf (light petroleum/EtOAc = 10/1) 0.46. FT-IR (ATR, neat): 3305, 1775, 1498, 1312, 1111, 1081, 802, 721 cm−1. 1H NMR (500 MHz, CDCl3): δ 12.62 (s, 1H), 8.90 (dd, J = 4.2, 1.9 Hz, 1H), 8.18–8.11 (m, 2H), 7.61–7.58 (m, 1H), 7.55–7.50 (m, 1H), 7.41 (dd, J = 8.3, 4.2 Hz, 1H), 7.03–7.01 (m, 1H), 6.91–6.89 (m, 1H), 6.35–6.32 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 148.5, 144.6, 137.1, 131.6, 129.1, 129.0, 126.8, 125.0, 124.9, 120.8, 119.1, 108.9, 107.0. HRMS (ESI+): m/z calcd for C13H11N2 (M + H)+: 195.0917; found: 195.0916.
8-(Furan-3-yl)-5-nitroquinoline (4n).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (51 mg, 85%); mp = 117–119 °C; Rf (light petroleum/EtOAc = 3/1) 0.49. FT-IR (ATR, neat): 3121, 1510, 1500, 1315, 1158, 1143, 868, 808 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.09 (dd, J = 8.9, 1.7 Hz, 1H), 9.07–9.05 (m, 1H), 8.74 (s, 1H), 8.39 (d, J = 8.2 Hz, 1H), 7.90 (d, J = 8.2, 1H), 7.66 (dd, J = 8.9, 4.1 Hz, 1H), 7.59–7.58 (m, 1H), 7.01–7.00 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 150.4, 145.5, 145.1, 143.4, 142.9, 138.9, 132.3, 125.2, 124.9, 123.7, 121.8 (2C), 110.2. HRMS (ESI+): m/z calcd for C13H9N2O3 (M + H)+: 241.0608; found: 241.0611. Elemental analysis calcd for C13H8N2O3: C, 65.00; H, 3.36; N, 11.66; found: C, 64.67; H, 3.50; N, 12.04.
5-Nitro-8-(thiophen-3-yl)quinoline (4o).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (58 mg, 90%); mp = 143–145 °C; Rf (light petroleum/EtOAc = 3/1) 0.44. FT-IR (ATR, neat): 3089, 1492, 1326, 1291, 1208, 1140, 851, 793, 774 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.11–9.07 (m, 2H), 8.40 (d, J = 8.2 Hz, 1H), 8.06 (dd, J = 3.0, 1.3 Hz, 1H), 7.93 (d, J = 8.2 Hz, 1H), 7.68–7.63 (m, 2H), 7.49–7.46 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 150.9, 145.5, 144.1, 142.0, 138.0, 132.1, 129.6, 127.4, 127.2, 125.2, 124.6, 123.6, 121.9. HRMS (ESI+): m/z calcd for C13H9N2O2S (M + H)+: 257.0379; found: 257.0376. Elemental analysis calcd for C13H8N2O2S: C, 60.93; H, 3.15; N, 10.93; found: C, 60.66; H, 2.94; N, 10.84.
5-Nitro-8-(1H-pyrrol-2-yl)quinoline (4p).
Radial chromatography on silica gel (eluting with petroleum ether/dichloromethane; 2/1). Orange solid (37 mg, 62%); mp = 147–148 °C; Rf (light petroleum/EtOAc = 3/1) 0.48. FT-IR (ATR, neat): 3307, 1562, 1494, 1311, 1044, 857, 743 cm−1. 1H NMR (500 MHz, CDCl3): δ 12.68 (s, 1H), 9.25 (dd, J = 9.0, 1.5 Hz, 1H), 8.99 (dd, J = 4.0, 1.5 Hz, 1H), 8.42 (d, J = 8.5 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 7.66 (dd, J = 9.0, 4.0 Hz, 1H), 7.14–7.13 (m, 1H), 7.09–7.07 (m, 1H), 6.41–6.39 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 149.1, 143.7, 140.9, 135.8, 133.2, 130.1, 126.1, 123.6, 122.9, 122.3, 122.2, 111.8, 110.4. HRMS (ESI+): m/z calcd for C13H10N3O2 (M + H)+: 240.0768; found: 240.0766. Elemental analysis calcd for C13H9N3O2: C, 65.27; H, 3.79; N, 17.56; found: C, 65.11; H, 3.69; N, 17.13.
8-(Benzofuran-2-yl)-5-nitroquinoline (4q).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (57 mg, 79%); mp = 183–185 °C; Rf (light petroleum/EtOAc = 3/1) 0.49. FT-IR (ATR, neat): 2919, 1562, 1499, 1449, 1312, 834, 812, 740 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.15–9.14 (m, 1H), 9.12–9.10 (m, 1H), 8.55–8.53 (m, 2H), 8.47–8.45 (m, 1H), 7.74–7.70 (m, 2H), 7.59–7.58 (m, 1H), 7.41–7.38 (m, 1H), 7.31–7.28 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 154.7, 151.1, 150.7, 144.4, 144.1, 134.6, 132.4, 129.5, 126.0, 124.8 (2C), 123.8, 123.2, 122.3, 121.7, 113.6, 111.2. HRMS (ESI+): m/z calcd for C17H11N2O3 (M + H)+: 291.0764; found: 291.0766.
8-(Benzo[b]thiophen-2-yl)-5-nitroquinoline (4r).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (53 mg, 69%); mp = 149–152 °C; Rf (light petroleum/EtOAc = 5/3) 0.49. FT-IR (ATR, neat): 3051, 1509, 1492, 1329, 1303, 815, 791, 739 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.17–9.14 (m, 2H), 8.46 (d, J = 8.5 Hz, 1H), 8.28 (d, J = 8.5 Hz, 1H), 8.24 (s, 1H), 7.93–7.90 (m, 2H), 7.73 (dd, J = 9.0, 4.0 Hz, 1H), 7.41–7.39 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 150.1, 144.4, 143.8, 143.0, 139.8, 138.9, 138.3, 132.3, 126.2, 126.1, 125.4, 124.6, 124.5, 124.3, 124.0, 122.0, 121.9. HRMS (ESI+): m/z calcd for C17H11N2O2S (M + H)+: 307.0536; found: 307.0538.
Procedure for Suzuki–Miyaura coupling with potassium thiophene-2-trifluoroborate
Quinoline 1 (0.50 mmol), potassium thiophene-2-trifluoroborate (1.50 mmol) and the Pd catalyst A (36 mg, 0.05 mmol) were dissolved in 1,4-dioxane. The reaction mixture was stirred in a sealed glass tube at 80 °C for 48 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which water was added (5 mL) and the product was further extracted into dichloromethane (2 × 5 mL). The combined organic layers were dried (Na2SO4),and filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 4b.
General procedure for one-pot sequential Suzuki–Miyaura coupling and direct C–H functionalization
The corresponding quinoline (1, 2) (0.50 mmol), boronic acid 6 (0.75 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol), PPh3 (13.1 mg, 0.05 mmol) and K2CO3 (207 mg, 1.5 mmol) were dissolved in a mixture of 1,4-dioxane (1.6 mL) and water (0.4 mL). The reaction mixture was stirred in a sealed glass tube at 100 °C for 24 h under an inert atmosphere. After allowing the mixture to cool to r.t. the glass tube was opened to which the aryl bromide (0.40 mmol) and Pd(OAc)2 (5.6 mg, 0.025 mmol) were added. The reaction mixture was again sealed and further stirred at 120 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which water was added (5 mL) and the product was further extracted with dichloromethane (2 × 5 mL). The combined organic layers were dried (Na2SO4), filtered and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 7.
8,8′-(Thiophene-2,3-diyl)diquinoline (4l′).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 5/1). Yellow solid (3 mg, 2%); mp = 242–247 °C; Rf (light petroleum/EtOAc = 1/1) 0.48. FT-IR (ATR, neat): 3048, 1572, 1492, 905, 900, 825, 785, 750, 729, 667 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.90 (dd, J = 4.2, 1.8 Hz, 1H), 8.85 (dd, J = 4.2, 1.8 Hz, 1H), 8.13–8.09 (m, 2H), 7.65–7.60 (m, 3H), 7.47 (d, J = 5.2 Hz, 1H), 7.41 (dd, J = 7.2, 1.4 Hz, 1H), 7.38–7.33 (m, 3H), 7.21–7.18 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 149.9, 149.8, 146.8, 146.6, 138.3, 136.9, 136.4, 136.0 (2C), 133.8, 132.3, 131.5, 131.2, 128.5, 128.4, 127.5, 127.0, 126.0, 125.9, 125.3, 120.9, 120.8. HRMS (ESI+): m/z calcd for C22H15N2S (M + H)+: 339.0950; found: 339.0951.
1-{4-[3-(Quinolin-8-yl)furan-2-yl]phenyl}ethan-1-one (7a).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (100 mg, 80%); mp = 180–185 °C; Rf (light petroleum/EtOAc = 5/3) 0.48. FT-IR (ATR, neat): 2923, 1671, 1602, 1357, 1261, 831, 795, 730 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.91 (dd, J = 4.0, 2.0 Hz, 1H), 8.24 (dd, J = 8.5, 2.0 Hz, 1H), 7.89 (dd, J = 8.0, 1.5 Hz, 1H), 7.75–7.74 (AA′BB′, J = 8.5 Hz, 2H), 7.71 (dd, J = 7.0, 1.5 Hz, 1H), 7.67 (d, J = 2.0 Hz, 1H), 7.57–7.54 (m, 1H), 7.46–7.43 (m, 3H), 6.76 (d, J = 1.7 Hz, 1H), 2.52 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.5, 150.5, 148.5, 146.5, 142.2, 136.4, 135.6, 135.1, 133.3, 131.1, 128.9, 128.3, 128.2, 126.4, 125.3, 122.2, 121.4, 116.4, 26.5. HRMS (ESI+): m/z calcd for C21H16NO2 (M + H)+: 314.1176; found: 314.1171.
8-[2-(4-Nitrophenyl)furan-3-yl]quinoline (7b).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (77 mg, 61%); mp = 217–220 °C; Rf (light petroleum/EtOAc = 5/3) 0.31. FT-IR (ATR, neat): 3124, 1771, 1753, 1240, 1056, 831, 693 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.88 (dd, J = 4.0, 2.0 Hz, 1H), 8.26 (dd, J = 8.5, 2.0 Hz, 1H), 8.01–7.99 (AA′BB′, J = 9.0 Hz, 2H), 7.92 (dd, J = 8.0, 1.5 Hz, 1H), 7.73 (dd, J = 7.0, 1.5 Hz, 1H), 7.70 (d, J = 2.0 Hz, 1H), 7.61–7.58 (m, 1H), 7.50–7.44 (m, 3H), 6.77 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 150.7, 147.4, 146.3, 146.0, 143.0, 137.2, 136.5, 132.9, 131.0, 128.9, 128.6, 126.4, 125.6 (2C), 123.7, 123.6 (2C), 121.5, 116.7. HRMS (ESI+): m/z calcd for C19H13N2O3 (M + H)+: 317.0921; found: 317.0920.
4-[3-(Quinolin-8-yl)furan-2-yl]benzonitrile (7c).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Orange solid (52 mg, 44%); mp = 106–110 °C; Rf (light petroleum/EtOAc = 5/3) 0.40. FT-IR (ATR, neat): 3108, 2219, 1605, 1499, 1063, 828, 797, 753 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.89 (dd, J = 4.0, 2.0 Hz, 1H), 8.24 (dd, J = 8.5, 2.0 Hz, 1H), 7.90 (dd, J = 8.0, 1.5 Hz, 1H), 7.71 (dd, J = 7.0, 1.5 Hz, 1H), 7.67 (d, J = 2.0 Hz, 1H), 7.59–7.56 (m, 1H), 7.46–7.40 (m, 5H), 6.75 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 150.6, 147.6, 146.3, 142.6, 136.4, 135.4, 133.0, 132.0 (2C), 131.0, 128.9, 128.5, 126.4, 125.6 (2C), 122.9, 121.5, 119.0, 116.5, 109.8. HRMS (ESI+): m/z calcd for C20H13N2O (M + H)+: 297.1022; found: 297.1022.
1-{4-[3-(Quinolin-8-yl)thiophen-2-yl]phenyl}ethan-1-one (7d).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (66 mg, 50%); mp = 207–210 °C; Rf (light petroleum/EtOAc = 5/3) 0.38. FT-IR (ATR, neat): 3043, 1675, 1599, 1357, 1265, 828, 794, 723 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.90 (dd, J = 4.0, 2.0 Hz, 1H), 8.20 (dd, J = 8.5, 2.0 Hz, 1H), 7.81 (dd, J = 8.0, 2.0 Hz, 1H), 7.71–7.70 (AA′BB′, J = 8.5 Hz, 2H), 7.49–7.48 (m, 1H), 7.46–7.40 (m, 3H), 7.35 (d, J = 5.0 Hz, 1H), 7.28–7.26 (m, 2H), 2.51 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.6, 150.4, 146.7, 139.6, 139.1, 137.1, 136.2, 135.6, 135.2, 132.7, 131.7, 130.9, 128.8 (2C), 128.3 (2C), 127.8, 126.2, 124.6, 121.2, 26.5. HRMS (ESI+): m/z calcd for C21H16NOS (M + H)+: 330.0947; found: 330.0945.
1-{4-[3-(5-Nitroquinolin-8-yl)furan-2-yl]phenyl}ethan-1-one (7e).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (69 mg, 48%); mp = 218–220 °C; Rf (light petroleum/EtOAc = 5/3) 0.39. FT-IR (ATR, neat): 3137, 1673, 1601, 1515, 1500, 1335, 1250, 812 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.10 (dd, J = 8.9, 1.7 Hz, 1H), 8.99 (dd, J = 4.1, 1.7 Hz, 1H), 8.35 (d, J = 8.0 Hz, 1H), 7.81–7.78 (m, 3H), 7.70 (d, J = 1.8, 1H), 7.86 (dd, J = 8.9, 4.1 Hz, 1H), 7.43–7.42 (m, 2H), 6.79 (d, J = 1.8 Hz, 1H), 2.54 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.3, 151.3, 149.5, 146.2, 145.0, 142.6, 141.0, 135.7, 134.8, 132.2, 129.2, 128.5 (2C), 125.9 (2C), 124.3, 124.1, 121.9, 120.7, 116.0, 26.5. HRMS (ESI+): m/z calcd for C21H15N2O4 (M + H)+: 359.1026; found: 359.1023. Elemental analysis calcd for C21H14N2O4: C, 70.39; H, 3.94; N, 7.82; found: C, 70.40; H, 3.70; N, 7.52.
5-Nitro-8-[2-(4-nitrophenyl)furan-3-yl]quinoline (7f).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (101 mg, 70%); mp = 254–256 °C; Rf (light petroleum/EtOAc = 5/3) 0.51. FT-IR (ATR, neat): 3091, 1595, 1500, 1324, 1103, 1072, 851, 795, 705 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.10 (dd, J = 8.9, 1.7 Hz, 1H), 8.96 (dd, J = 4.1, 1.7 Hz, 1H), 8.39 (d, J = 8.0 Hz, 1H), 8.06–8.04 (m, 2H), 7.82 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 1.9 Hz, 1H), 7.69 (dd, J = 8.9, 4.1 Hz, 1H), 7.48–7.46 (m, 2H), 6.79 (d, J = 1.9 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 151.5, 148.3, 146.5, 146.0, 145.3, 143.3, 140.4, 136.6, 132.3, 129.1, 126.1 (2C), 124.3, 124.2, 123.9 (2C), 122.0, 121.9, 116.3. HRMS (ESI+): m/z calcd for C19H12N3O5 (M + H)+: 362.0771; found: 362.0770.
4-[3-(5-Nitroquinolin-8-yl)furan-2-yl]benzonitrile (7g).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (71 mg, 52%); mp = 157–161 °C; Rf (light petroleum/EtOAc = 5/3) 0.46. FT-IR (ATR, neat): 3084, 2221, 1604, 1504, 1328, 1068, 896, 840, 786, 728 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.09 (dd, J = 8.9, 1.6 Hz, 1H), 8.96 (dd, J = 4.2, 1.6 Hz, 1H), 8.38 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 1.7 Hz, 1H), 7.69 (dd, J = 8.9, 4.2 Hz, 1H), 7.48–7.46 (m, 2H), 7.42–7.40 (m, 2H), 6.78 (d, J = 1.7 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 151.4, 148.5, 146.0, 145.2, 143.0, 140.5, 134.7, 132.2, 132.1 (2C), 129.0, 126.0 (2C), 124.3, 124.2, 121.9, 121.3, 118.7, 116.2, 110.7. HRMS (ESI+): m/z calcd for C20H12N3O3 (M + H)+: 342.0873; found: 342.0870.
1-{4-[3-(5-Nitroquinolin-8-yl)thiophen-2-yl]phenyl}ethan-1-one (7h).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Orange solid (46 mg, 31%); mp = 205–209 °C; Rf (light petroleum/EtOAc = 5/3) 0.42. FT-IR (ATR, neat): 3100, 1675, 1597, 1513, 1333, 1263, 864, 797, 736, 725 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.06 (dd, J = 9.0, 2.0 Hz, 1H), 8.98 (dd, J = 4.0, 2.0 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 7.76–7.74 (AA′BB′, J = 8.5 Hz, 2H), 7.64 (dd, J = 9.0, 4.0 Hz, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.52 (d, J = 5.0 Hz, 1H), 7.34 (d, J = 5.0 Hz, 1H), 7.26–7.24 (AA′BB′, J = 8.5 Hz, 2H), 2.53 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.2, 151.2, 146.3, 144.7, 143.0, 140.8, 138.8, 135.7, 135.3, 132.3, 132.1, 129.8, 129.0 (2C), 128.6 (2C), 125.1, 124.2, 123.9, 121.8, 26.5. HRMS (ESI+): m/z calcd for C21H15N2O3S (M + H)+: 375.0798; found: 375.0789.
4,4′-[3-(Quinolin-8-yl)furan-2,5-diyl]dibenzonitrile (7ca).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 5/1). Orange solid (41 mg, 26%); mp > 250 °C; Rf (light petroleum/EtOAc = 1/1) 0.68. FT-IR (ATR, neat): 3117, 2221, 1599, 1497, 938, 827, 790, 758 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.90 (dd, J = 4.0, 2.0 Hz, 1H), 8.28 (dd, J = 8.5, 2.0 Hz, 1H), 7.95 (dd, J = 8.0, 1.5 Hz, 1H), 7.89–7.87 (AA′BB′, J = 8.5 Hz, 2H), 7.75 (dd, J = 7.0, 1.5 Hz, 1H), 7.73–7.71 (AA′BB′, J = 8.5 Hz, 2H), 7.63–7.60 (m, 1H), 7.52–7.45 (m, 5H), 7.17 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 151.5, 150.8, 148.6, 146.2, 136.6, 134.6, 134.1, 132.7 (2C), 132.2, 132.1 (2C), 131.0, 129.0, 128.9126.5, 125.8 (2C), 125.4, 124.3 (2C), 121.7, 118.9, 118.8, 114.9, 110.9, 110.5. HRMS (ESI+): m/z calcd for C27H16N3O (M + H)+: 398.1288; found: 398.1282.
1,1′-{[3-(5-Nitroquinolin-8-yl)furan-2,5-diyl]bis(4,1-phenylene)}bis(ethan-1-one) (7ea).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 5/1). Yellow solid (67 mg, 35%); mp = 220–224 °C; Rf (light petroleum/EtOAc = 5/3) 0.27. FT-IR (ATR, neat): 3131, 1669, 1598, 1505, 1328, 1261, 1181, 838, 787 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.12 (dd, J = 8.9, 1.7 Hz, 1H), 9.01 (dd, J = 4.1, 1.7 Hz, 1H), 8.39 (d, J = 8.0 Hz, 1H), 8.06–8.04 (m, 2H), 7.91–7.89 (m, 2H), 7.86 (d, J = 8.0, 1H), 7.84–7.82 (m, 2H), 7.71 (dd, J = 8.9, 4.1 Hz, 1H), 7.52–7.50 (m, 2H), 7.19 (s, 1H), 2.65 (s, 3H), 2.56 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.3, 197.2, 152.5, 151.5, 149.9, 146.1, 145.3, 140.4, 136.1, 136.0, 134.3, 134.0, 132.3, 129.2, 129.0 (2C), 128.6 (2C), 125.9 (2C), 124.3, 124.2, 124.0 (2C), 123.0, 121.9, 113.6, 26.6, 26.5. HRMS (ESI+): m/z calcd for C29H21N2O5 (M + H)+: 477.1445; found: 477.1443. Elemental analysis calcd for C29H20N2O5: C, 73.10; H, 4.23; N, 5.88; found: C, 73.18; H, 3.89; N, 5.59.
1-{4-[2-(Quinolin-8-yl)benzofuran-3-yl]phenyl}ethan-1-one (7i).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Light yellow solid (103 mg, 71%); mp = 153–155 °C; Rf (light petroleum/EtOAc = 5/3) 0.63. FT-IR (ATR, neat): 2917, 1678, 1604, 1266, 1182, 957, 831, 795, 750, 727 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.79 (dd, J = 4.0, 2.0 Hz, 1H), 8.20 (dd, J = 8.5, 2.0 Hz, 1H), 7.92 (dd, J = 8.0, 1.5 Hz, 1H), 7.84–7.77 (m, 4H), 7.64 (d, J = 8.0 Hz, 1H), 7.57–7.54 (m, 1H), 7.46–7.44 (AA′BB′, J = 8.5 Hz, 2H), 7.41–7.38 (m, 2H), 7.35–7.32 (m, 1H), 2.56 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.7, 155.1, 151.4, 150.8, 146.5, 138.6, 136.2, 135.3, 132.5, 129.9, 129.0 (2C), 128.6, 128.5 (2C), 128.3, 127.4, 126.1, 124.8, 123.2, 121.5, 120.0, 119.5, 111.7, 26.5. HRMS (ESI+): m/z calcd for C25H18NO2 (M + H)+: 364.1332; found: 364.1328. Elemental analysis calcd for C25H17NO2: C, 82.63; H, 4.72; N, 3.85; found: C, 82.42; H, 4.51; N, 3.76.
8-[3-(4-Nitrophenyl)benzofuran-2-yl]quinoline (7j).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 20/1). Yellow solid (92 mg, 63%); mp = 71–76 °C; Rf (light petroleum/EtOAc = 5/3) 0.60. FT-IR (ATR, neat): 3065, 1596, 1510, 1341, 1104, 851, 792, 746 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.71 (dd, J = 4.0, 2.0 Hz, 1H), 8.21 (dd, J = 8.5, 2.0 Hz, 1H), 8.10–8.08 (AA′BB′, J = 9.0 Hz, 2H), 7.96 (dd, J = 8.0, 1.5 Hz, 1H), 7.89 (dd, J = 7.0, 1.5 Hz, 1H), 7.76 (d, J = 7.5 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.62–7.59 (m, 1H), 7.51–7.49 (AA′BB′, J = 9.0 Hz, 2H), 7.44–7-35 (m, 3H). 13C NMR (125 MHz, CDCl3): δ 155.1, 151.7, 150.7, 146.3, 146.0, 140.9, 136.3, 132.3, 130.2, 129.4, 129.3 (2C), 128.7, 128.0, 126.2, 125.1, 123.7 (2C), 123.4, 121.7, 119.7, 118.7, 111.8. HRMS (ESI+): m/z calcd for C23H15N2O3 (M + H)+: 367.1077; found: 367.1073.
4-[2-(Quinolin-8-yl)benzofuran-3-yl]benzonitrile (7k).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (100 mg, 72%); mp = 89–93 °C; Rf (light petroleum/EtOAc = 5/3) 0.58. FT-IR (ATR, neat): 3047, 2224, 1607, 1494, 1451, 960, 829, 793, 745, 727 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.72 (dd, J = 4.0, 2.0 Hz, 1H), 8.20 (dd, J = 8.5, 2.0 Hz, 1H), 7.94 (dd, J = 8.0, 1.5 Hz, 1H), 7.87 (dd, J = 7.0, 1.5 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 8.0, 1H), 7.61–7.58 (m, 1H), 7.52–7.50 (AA′BB′, J = 8.5 Hz, 2H), 7.45–7.43 (AA′BB′, J = 8.5 Hz, 2H), 7.42–7.39 (m, 2H), 7.36–7-33 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 155.1, 151.4, 150.6, 146.0, 138.8, 136.2, 132.3, 132.1 (2C), 130.0, 129.5, 129.4 (2C), 128.7, 128.0, 126.1, 125.0, 123.3, 121.6, 119.7, 119.0, 118.9, 111.8, 110.0. HRMS (ESI+): m/z calcd for C24H15N2O (M + H)+: 347.1179; found: 347.1174.
8-[3-(4-Methoxyphenyl)benzofuran-2-yl]quinoline (7l).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (112 mg, 80%); mp = 51–57 °C; Rf (light petroleum/EtOAc = 5/1) 0.54. FT-IR (ATR, neat): 2834, 1512, 1450, 1286, 1244, 1171, 1017, 828, 792, 744 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.88 (dd, J = 4.0, 2.0 Hz, 1H), 8.18 (dd, J = 8.5, 2.0 Hz, 1H), 7.87 (dd, J = 8.0, 1.5 Hz, 1H), 7.76–7.75 (m, 2H), 7.62 (d, J = 8.0 Hz, 1H), 7.52–7.49 (m, 1H), 7.40 (dd, J = 8.5, 4.0 Hz, 1H), 7.37–7.34 (m, 1H), 7.31–7.28 (m, 3H), 6.80–6.79 (AA′BB′, J = 8.5 Hz, 2H), 3.76 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 158.4, 155.0, 150.8, 150.2, 146.9, 136.1, 132.7, 130.4, 130.2 (2C), 129.4, 129.0, 128.6, 126.0, 125.3, 124.4, 122.7, 121.4, 120.2, 119.8, 113.9 (2C), 111.6, 55.1. HRMS (ESI+): m/z calcd for C24H18NO2 (M + H)+: 352.1332; found: 352.1329.
4-[2-(Quinolin-8-yl)benzo[b]thiophen-3-yl]benzonitrile (7m).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (93 mg, 64%); mp = 181–184 °C; Rf (light petroleum/EtOAc = 5/3) 0.58. FT-IR (ATR, neat): 3055, 2227, 1603, 1488, 1362, 969, 823, 788, 738 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.86 (dd, J = 4.0, 2.0 Hz, 1H), 8.17 (dd, J = 8.5, 2.0 Hz, 1H), 7.93–7.92 (m, 1H), 7.82 (dd, J = 8.0, 1.5 Hz, 1H), 7.70–7.68 (m, 1H), 7.57 (dd, J = 7.0, 1.5 Hz, 1H), 7.49–7.48 (AA′BB′, J = 8.5 Hz, 2H), 7.46–7.43 (m, 1H), 7.41–7.38 (m, 5H). 13C NMR (125 MHz, CDCl3): δ 150.4, 146.5, 141.0, 140.6, 138.8, 138.6, 136.2, 133.9, 132.9, 132.8, 131.9, 130.7, 129.0, 128.5, 125.9, 124.8, 124.6, 122.6, 122.3, 121.4, 118.9, 110.4. HRMS (ESI+): m/z calcd for C24H15N2S (M + H)+: 363.0950; found: 363.0949.
8-[3-(4-Nitrophenyl)benzo[b]thiophen-2-yl]quinoline (7n).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (87 mg, 57%); mp = 153–156 °C; Rf (light petroleum/EtOAc = 5/3) 0.63. FT-IR (ATR, neat): 2916, 1594, 1511, 1342, 1307, 1101, 824, 788, 735 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.86 (dd, J = 4.0, 2.0 Hz, 1H), 8.18 (dd, J = 8.5, 2.0 Hz, 1H), 8.07–8.05 (AA′BB′, J = 9.0 Hz, 2H), 7.95–7.93 (m, 1H), 7.84 (dd, J = 8.0, 1.5 Hz, 1H), 7.72–7.70 (m, 1H), 7.60 (dd, J = 7.0, 1.5 Hz, 1H), 7.47–7.44 (m, 3H), 7.43–7.39 (m, 3H). 13C NMR (125 MHz, CDCl3): δ 150.5, 146.5, 143.1, 140.6, 139.0, 138.8, 136.3, 133.6, 132.9, 132.7, 130.8 (3C), 129.1, 128.5, 126.0, 124.9, 124.7, 123.5 (2C), 122.6, 122.3, 121.5. HRMS (ESI+): m/z calcd for C23H15N2O2S (M + H)+: 383.0849; found: 383.0851.
1-{4-[2-(5-Nitroquinolin-8-yl)benzofuran-3-yl]phenyl}ethan-1-one (7o).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (158 mg, 97%); mp = 148–152 °C; Rf (light petroleum/EtOAc = 5/3) 0.63. FT-IR (ATR, neat): 1676, 1605, 1531, 1515, 1329, 1265, 953, 828, 737 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.99 (dd, J = 9.0, 2.0 Hz, 1H), 8.72 (dd, J = 4.0, 2.0 Hz, 1H), 8.37 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.87–7.85 (AA′BB′, J = 8.5 Hz, 2H), 7.77 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.57 (dd, J = 9.0, 4.0 Hz, 1H), 7.46–7.35 (m, 4H), 2.57 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.5, 155.3, 151.1, 148.6, 145.8, 145.6, 138.2, 136.6, 135.6, 131.9, 130.0, 129.0 (2C), 128.6 (2C), 128.2, 125.8, 124.1, 124.0, 123.6, 121.9, 121.6, 120.4, 111.8, 26.6. HRMS (ESI+): m/z calcd for C25H17N2O4 (M + H)+: 409.1183; found: 409.1186. Elemental analysis calcd for C25H16N2O4: C, 73.52; H, 3.95; N, 6.86; found: C, 73.22; H, 3.68; N, 6.46.
5-Nitro-8-[3-(4-nitrophenyl)benzofuran-2-yl]quinoline (7p).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (148 mg, 90%); mp = 189–194 °C; Rf (light petroleum/EtOAc = 7/1) 0.44. FT-IR (ATR, neat): 3080, 1597, 1508, 1492, 1340, 854, 746, 703 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.00 (dd, J = 9.0, 2.0 Hz, 1H), 8.64 (dd, J = 4.0, 1.5 Hz, 1H), 8.42 (d, J = 8.0 Hz, 1H), 8.13 (AA′BB′, J = 9.0 Hz, 2H), 8.10 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.57 (dd, J = 9.0, 4.0 Hz, 1H), 7.49–7.45 (m, 3H), 7.38 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 155.2, 151.0, 148.9, 146.6, 145.9, 145.2, 140.6, 136.1, 132.0, 129.9, 129.5 (2C), 128.0, 126.0, 124.2, 124.0, 123.8 (3C), 121.6, 121.0, 120.1, 111.9. HRMS (ESI+): m/z calcd for C23H14N3O5 (M + H)+: 409.1183; found: 409.1186.
4-[1-Methyl-5-(quinolin-8-yl)-1H-pyrrol-3-yl]benzonitrile (7q).
Radial chromatography on silica gel (eluting with petroleum ether/diethyl ether; 2/1). White solid (56 mg, 45%); mp = 136–141 °C; Rf (light petroleum/EtOAc = 5/3) 0.63. FT-IR (ATR, neat): 2918, 2212, 1601, 1345, 1178, 930, 844, 829, 797, 714 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.96 (dd, J = 4.0, 2.0 Hz, 1H), 8.26 (dd, J = 8.5, 2.0 Hz, 1H), 7.93 (dd, J = 8.0, 2.0 Hz, 1H), 7.56–7.51 (m, 2H), 7.46 (dd, J = 8.5, 4.0 Hz, 1H), 7.30–7.28 (AA′BB′, J = 8.5 Hz, 2H), 7.09–7.08 (AA′BB′, J = 8.5 Hz, 2H), 6.88 (d, J = 3.0 Hz, 1H), 6.55 (d, J = 3 Hz, 1H), 3.35 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 151.0, 147.6, 141.8, 136.4, 133.4, 131.8 (2C), 131.7, 129.8, 129.1, 128.7, 127.3 (2C), 126.4, 123.1, 122.2, 121.4, 119.6, 107.7, 107.4, 35.0. HRMS (ESI+): m/z calcd for C21H16N3 (M + H)+: 310.1339; found: 310.1337.
1-{4-[1-Methyl-2-(quinolin-8-yl)-1H-pyrrol-3-yl]phenyl}ethan-1-one (7s).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (100 mg, 77%); mp = 75–78 °C; Rf (light petroleum/EtOAc = 5/3) 0.46. FT-IR (ATR, neat): 2923, 1670, 1597, 1354, 1265, 1223, 1182, 832, 796, 726 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.98 (dd, J = 4.0, 2.0 Hz, 1H), 8.26 (dd, J = 8.5, 2.0 Hz, 1H), 7.91 (dd, J = 8.0, 1.5 Hz, 1H), 7.65–7.63 (AA′BB′, J = 8.5 Hz, 2H), 7.56 (dd, J = 7.0, 1.5 Hz, 1H), 7.53–7.50 (m, 1H), 7.46 (dd, J = 8.5, 4.0 Hz, 1H), 7.11–7.09 (AA′BB′, J = 8.5 Hz, 2H), 6.88 (d, J = 3.0 Hz, 1H), 6.59 (d, J = 3.0 Hz, 1H), 3.37 (s, 3H), 2.47 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.7, 151.0, 147.8, 142.1, 136.4, 133.5, 133.4, 132.1, 129.6, 128.9, 128.7, 128.3 (2C), 126.9 (2C), 126.5, 122.9, 122.8, 121.4, 107.8, 35.0, 26.4. HRMS (ESI+): m/z calcd for C22H19N2O (M + H)+: 327.1492; found: 327.1488.
1-{4-[2-(Quinolin-8-yl)thiophen-3-yl]phenyl}ethan-1-one (7t).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 10/1). Yellow solid (39 mg, 30%); mp = 122–123 °C; Rf (light petroleum/EtOAc = 5/1) 0.49. FT-IR (ATR, neat): 2918, 2850, 1671, 1594, 1493, 1358, 1264, 1109, 955, 829, 759 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.07 (dd, J = 4.0, 2.0 Hz, 1H), 8.22 (dd, J = 8.0, 2.0 Hz, 1H), 8.14 (dd, J = 7.5, 1.5 Hz, 1H), 8.01–7.99 (AA′BB′, J = 8.5 Hz, 2H), 7.83–7.78 (m, 4H), 7.662–7.59 (m, 1H), 7.52–7.48 (m, 2H), 2.64 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.4, 149.5, 145.0, 144.5, 140.9, 139.3, 136.5, 135.5, 132.6, 129.1 (2C), 128.8, 127.6 (2C), 127.5, 126.5, 125.4 (2C), 124.3, 121.4, 26.6. HRMS (ESI+): m/z calcd for C21H16NOS (M + H)+: 330.0947; found: 330.0944.
4,4′-[1-Methyl-5-(quinolin-8-yl)-1H-pyrrole-2,4-diyl]dibenzonitrile (7qa).
Radial chromatography on silica gel (eluting with petroleum ether/diethyl ether; 1/1). Light yellow solid (57 mg, 35%); mp = 147–151 °C; Rf (light petroleum/EtOAc = 1/1) 0.44. FT-IR (ATR, neat): 2921, 2224, 1602, 1491, 1171, 913, 836, 791, 729 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.01 (dd, J = 4.0, 2.0 Hz, 1H), 8.30 (dd, J = 8.5, 2.0 Hz, 1H), 7.97 (dd, J = 8.0, 2.0 Hz, 1H), 7.75–7.73 (AA′BB′, J = 8.5 Hz, 2H), 7.69–7.67 (AA′BB′, J = 8.5 Hz, 2H), 7.61–7.54 (m, 2H), 7.51 (dd, J = 8.5, 4.0 Hz, 1H), 7.34–7.33 (AA′BB′, J = 8.5 Hz, 2H), 7.13–7.12 (AA′BB′, J = 8.5 Hz, 2H), 6.74 (s, 1H), 3.37 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 151.2, 147.4, 141.0, 137.4, 136.6, 134.1, 133.6, 133.3, 132.3 (2C), 131.9 (2C), 131.3, 129.5, 128.8, 128.7 (2C), 127.5 (2C), 126.6, 122.9, 121.7, 119.4, 118.9, 110.2, 110.1, 108.2, 33.8. HRMS (ESI+): m/z calcd for C28H19N4 (M + H)+: 411.1604; found: 411.1597.
8-[1-Methyl-3,5-bis(4-nitrophenyl)-1H-pyrrol-2-yl]quinoline (7ra).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 3/1). Orange solid (99 mg, 55%); mp = 123–127 °C; Rf (light petroleum/EtOAc = 1/1) 0.43. FT-IR (ATR, neat): 2925, 1589, 1505, 1330, 1105, 852, 725 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.02 (dd, J = 4.0, 2.0 Hz, 1H), 8.33–8.31 (m, 3H), 8.00 (dd, J = 8.0, 2.0 Hz, 1H), 7.94–7.92 (AA′BB′, J = 9.0 Hz, 2H), 7.75–7.73 (AA′BB′, J = 9.0 Hz, 2H), 7.62 (dd, J = 7.0, 1.5 Hz, 1H), 7.59–7.56 (m, 1H), 7.53 (dd, J = 8.0, 4.0 Hz, 1H), 7.18–7.17 (AA′BB′, J = 9.0 Hz, 2H), 6.84 (s, 1H), 3.41 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 151.3, 147.3, 146.3, 145.1, 143.1, 139.2, 136.7, 134.3, 134.0, 133.6, 131.1, 129.7, 128.9, 128.6 (2C), 127.4 (2C), 126.6, 124.0 (2C), 123.6 (2C), 122.7, 121.8, 110.9, 34.0. HRMS (ESI+): m/z calcd for C26H19N4O4 (M + H)+: 451.1401; found: 451.1394.
1-{4-[1-Methyl-5-(5-nitroquinolin-8-yl)-1H-pyrrol-2-yl]phenyl}ethan-1-one (7u).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 5/1). Orange solid (68 mg, 47%); mp = 191–195 °C; Rf (light petroleum/EtOAc = 2/1) 0.35. FT-IR (ATR, neat): 2921, 1673, 1599, 1318, 1268, 789, 754, 732 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.13–9.10 (m, 2H), 8.45 (d, J = 8.0 Hz, 1H), 8.05–8.04 (AA′BB′, J = 8.5 Hz, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.70–7.66 (m, 3H), 6.60 (d, J = 4 Hz, 1H), 6.57 (d, J = 4 Hz, 1H), 3.51 (s, 3H), 2.65 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.6, 151.3, 146.2, 144.5, 139.5, 137.7, 137.2, 135.3, 134.4, 132.2, 129.4, 128.6 (2C), 128.5 (2C), 124.5, 123.8, 121.9, 113.1, 110.8, 35.1, 26.6. HRMS (ESI+): m/z calcd for C22H18N3O3 (M + H)+: 372.1343; found: 372.1341.
Procedure for the three-step one-pot transformation (Suzuki–Miyaura coupling and two C–H functionalizations)
8-Bromo-5-nitroquinoline (2) (127 mg, 0.50 mmol), (furan-3-yl)boronic acid (6b) (84 mg, 0.75 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol), PPh3 (13.1 mg, 0.05 mmol) and K2CO3 (207 mg, 1.5 mmol) were dissolved in a mixture of 1,4-dioxane (1.6 mL) and water (0.4 mL). The reaction mixture was stirred in a sealed glass tube at 100 °C for 24 h under an inert atmosphere. After allowing the mixture to cool to r.t. the glass tube was opened to which 1-bromo-4-nitrobenzene (81 mg, 0.40 mmol) and Pd(OAc)2 (5.6 mg, 0.025 mmol) were added. The reaction mixture was again sealed and further stirred at 120 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which 4-bromoacetophenone (80 mg, 0.4 mmol) and Pd(OAc)2 (5.6 mg, 0.025 mmol) were added. The reaction mixture was sealed for the second time and further stirred at 120 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which water was added (5 mL) and the product was further extracted with dichloromethane (2 × 5 mL). The combined organic layers were dried (Na2SO4) and filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 8.
1-{4-[5-(4-Nitrophenyl)-3-(5-nitroquinolin-8-yl)furan-2-yl]phenyl}ethan-1-one (8).
Radial chromatography on silica gel (eluting with petroleum ether/dichloromethane; 1/1). Orange solid (87 mg, 45%); mp > 250 °C; Rf (light petroleum/EtOAc = 1/1) 0.68. FT-IR (ATR, neat): 2852, 1671, 1596, 1509, 1501, 1329, 1262, 1229, 850, 815, 704 cm−1. 1H NMR (500 MHz, CDCl3): δ 9.12 (dd, J = 9.0, 1.5 Hz, 1H), 8.98 (dd, J = 4.0, 1.5 Hz, 1H), 8.43 (d, J = 8.0 Hz, 1H), 8.10–8.05 (m, 4H), 7.92–7.88 (m, 3H), 7.72 (dd, J = 7.0, 4.0 Hz, 1H), 7.56–7.54 (AA′BB′, J = 9.0 Hz, 2H), 7.18 (s, 1H), 2.66 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 197.2, 153.3, 151.6, 148.6, 146.6, 146.0, 145.6, 139.9, 136.4, 136.1, 133.7, 132.4, 129.1 (3C), 126.1 (2C), 124.4 (2C), 124.3, 124.2 (2C), 124.0 (2C), 121.9, 113.7, 26.6. HRMS (ESI+): m/z calcd for C27H18N3O6 (M + H)+: 480.1190; found: 480.1187.
Procedure for the three-step one-pot transformation (Suzuki–Miyaura coupling followed by C–H functionalization and hydrogenation)
8-Bromo-5-nitroquinoline (2) (127 mg, 0.50 mmol), benzo[b]furan-2-ylboronic acid (6f) (121 mg, 0.75 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol), PPh3 (13.1 mg, 0.05 mmol) and K2CO3 (207 mg, 1.5 mmol) were dissolved in a mixture of 1,4-dioxane (1.6 mL) and water (0.4 mL). The reaction mixture was stirred in a sealed glass tube at 100 °C for 24 h under an inert atmosphere. After allowing the mixture to cool to r.t. the glass tube was opened to which 1-bromo-4-nitrobenzene (81 mg, 0.40 mmol) and Pd(OAc)2 (5.6 mg, 0.025 mmol) were added. The reaction mixture was again sealed and further stirred at 120 °C for 24 h under an inert atmosphere. The mixture was allowed to cool to r.t. to which a flow of H2 (1 atm) was passed. The reaction mixture was further stirred at r.t. for 24 h. After the reaction was complete, water was added (5 mL) and the product was further extracted with dichloromethane (2 × 5 mL). The combined organic layers were dried (Na2SO4), filtered and the solvent was evaporated under reduced pressure. The crude product was purified by radial chromatography to yield pure product 9.
8-[3-(4-Aminophenyl)benzofuran-2-yl]quinolin-5-amine (9).
Radial chromatography on silica gel (eluting with petroleum ether/EtOAc; 1/1). Orange solid (98 mg, 70%); mp = 202–204 °C; Rf (dichloromethane/methanol = 10/1) 0.38. FT-IR (ATR, neat): 3369, 3340, 1585, 1448, 1361, 1273, 1173, 1024, 823, 745 cm−1. 1H NMR (500 MHz, CDCl3): δ 8.88 (dd, J = 4.0, 1.5 Hz, 1H), 8.15 (dd, J = 8.5, 1.5 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.34 (dd, J = 8.5, 4.0 Hz, 1H), 7.31–7.28 (m, 1H), 7.27–7.24 (m, 1H), 7.19–7.17 (AA′BB′, J = 8.5 Hz, 2H), 6.72 (d, J = 8.0 Hz, 1H), 6.58–6.57 (AA′BB′, J = 8.5 Hz, 2H), 4.35 (s, 2H), 3.61 (s, 2H). 13C NMR (125 MHz, CDCl3): δ 154.8, 151.0, 150.6, 147.8, 144.9, 143.6, 133.7, 130.0 (2C), 129.4, 129.2, 123.8, 123.5, 122.4, 120.7, 120.1, 119.7, 119.0, 118.4, 115.1 (2C), 111.4, 109.3. HRMS (ESI+): m/z calcd for C23H17N3O (M + H)+: 352.1444; found: 352.1443.
Acknowledgements
We thank the Slovenian Research Agency for financial support through grant P1-0179 and H. B. thanks Slovene Human Resources Development and Scholarship Fund for the fellowship. We also acknowledge Centre of Excellence EN-FIST.
Notes and references
- Selected examples:
(a) D. Macdonald, H. Perrier, S. Liu, F. Laliberté, R. Rasori, A. Robichaud, P. Masson and Z. Huang, J. Med. Chem., 2000, 43, 382 CrossRef;
(b) C. Q. Huang, K. Wilcoxen, J. R. McCarty, M. Haddach, T. R. Webb, J. Gu, Y.-F. Xie, D. E. Grigoriadis and C. Chen, Bioorg. Med. Chem. Lett., 2003, 13, 3375 CrossRef CAS PubMed;
(c)
T. Eicher, S. Hauptmann and A. Speicher, The Chemistry of Heterocycles, WILEY-VCH, 2nd edn, 2003 CrossRef;
(d) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC.
-
(a) K. L. Garner, L. F. Parkes, J. D. Piper and J. A. G. Williams, Inorg. Chem., 2010, 49, 476 CrossRef CAS PubMed;
(b) S. Tao, L. Li, J. Yu, Y. Jiang, Y. Zhou, C.-S. Lee, S.-T. Lee, X. Zhang and O. Kwon, Chem. Mater., 2009, 21, 1284 CrossRef CAS.
- I. Sosič, B. Mirković, S. Turk, B. Štefane, J. Kos and S. Gobec, Eur. J. Med. Chem., 2011, 46, 4684 Search PubMed.
-
(a) I. Sosič, B. Mirković, K. Arenz, B. Štefane, J. Kos and S. Gobec, J. Med. Chem., 2013, 56, 521 CrossRef PubMed;
(b) B. Štefane, F. Požgan, I. Sosič and S. Gobec, Tetrahedron Lett., 2012, 53, 1964 CrossRef;
(c) B. Mirković, M. Renko, S. Turk, I. Sosič, Z. Jevnikar, N. Obermajer, D. Turk, S. Gobec and J. Kos, ChemMedChem, 2011, 6, 1351 CrossRef PubMed.
-
(a) A. Larivée, J. J. Mousseau and A. B. Charette, J. Am. Chem. Soc., 2008, 130, 52 CrossRef PubMed;
(b) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed;
(c) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291 CrossRef CAS PubMed;
(d) M. Wasa, B. T. Worrell and J.-Q. Yu, Angew. Chem., Int. Ed., 2010, 49, 1275 CrossRef CAS PubMed.
- M. Tobisu, I. Hyodo and N. Chatani, J. Am. Chem. Soc., 2009, 131, 12070 CrossRef CAS PubMed.
-
(a) A. M. Berman, J. C. Lewis, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 14926 CrossRef CAS PubMed;
(b) A. M. Berman, R. G. Bergman and J. A. Ellman, J. Org. Chem., 2010, 75, 7863 CrossRef CAS PubMed.
- D. Zhao, W. Wang, F. Yang, J. Lan, L. Yang, G. Gao and J. You, Angew. Chem., Int. Ed., 2009, 48, 3296 CrossRef CAS PubMed.
- I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194 CrossRef CAS PubMed.
- Y. Zhang, J. Gao, W. Li, H. Lee, B. Z. Lu and C. H. Senanayake, J. Org. Chem., 2011, 76, 6394 CrossRef CAS PubMed.
- J. Kwak, M. Kim and S. Chang, J. Am. Chem. Soc., 2011, 133, 3780 CrossRef CAS PubMed.
- E. Ballesteros, D. Moreno, T. Gómez, T. Rodríguez, J. Rojo, M. García-Valverde and T. Torroba, Org. Lett., 2009, 11, 1269 CrossRef CAS PubMed.
- L. Goossen, N. Rodríguez and C. Linder, J. Am. Chem. Soc., 2008, 130, 15248 CrossRef CAS PubMed.
- G. A. Molander, D. E. Petrillo, N. R. Landzberg, J. C. Rohanna and B. Biolatto, Synlett, 2005, 1763 CrossRef CAS.
- B. Lipshutz, J. A. Sclafani and P. A. Blomgren, Tetrahedron, 2000, 56, 2139 CrossRef CAS.
- A. Gavryushin, C. Kofink, G. Manolikakes and P. Knochel, Tetrahedron, 2006, 62, 7521 CrossRef CAS.
- N. Boudet, J. R. Lachs and P. Knochel, Org. Lett., 2007, 9, 5525 CrossRef CAS PubMed.
- D. Macdonald, H. Perrier, S. Liu, F. Laliberté, P. M. Robichaud and Z. Huang, J. Med. Chem., 2000, 43, 3820 CrossRef CAS PubMed.
- For review see: R. Rossi, F. Bellina, M. Lessi and C. Manzini, Adv. Synth. Catal., 2014, 356, 17 CrossRef CAS.
-
(a) T. E. Enrico, A. Lazareva and O. Daugulis, J. Org. Chem., 2011, 76, 471 CrossRef PubMed;
(b) H. Y. Fu and H. Doucet, Eur. J. Org. Chem., 2011, 7163 CrossRef CAS;
(c) A. Ohta, Y. Akita, T. Ohkuwa, M. Chiba, R. Fukunaga, A. Miyafuji, T. Nakata, N. Tani and Y. Aoyagi, Heterocycles, 1990, 31, 1951 CrossRef CAS.
- J. Laroche, K. Beydoun, V. Guerchais and H. Doucet, Catal. Sci. Technol., 2013, 3, 2072 CAS.
- For complete list see the ESI.†.
- For examples see:
(a) S. W. McDaniel, C. M. Keyari, K. C. Rider, N. R. Natale and P. Diaz, Tetrahedron Lett., 2011, 52, 5656 CrossRef CAS PubMed;
(b) J. Koubachi, S. El Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, J. Org. Chem., 2007, 72, 7650 CrossRef CAS PubMed;
(c) M. Larhed and A. Hallberg, J. Org. Chem., 1996, 61, 9582 CrossRef CAS;
(d) M. Larhed, G. Lindeberg and A. Hallberg, Tetrahedron Lett., 1996, 37, 8219 CrossRef CAS.
- N. Kudo, M. Perseghini and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1282 CrossRef CAS PubMed.
- For general references see:
(a)
L. F. Tietze, G. Brasche and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed;
(b) K. C. Nicolaou, T. Montagnon and S. A. Snyder, Chem. Commun., 2003, 551 RSC;
(c) A. Padwa, Pure Appl. Chem., 2004, 76, 1933 CrossRef CAS;
(d) G. Wu, W. Yin, H. C. Shen and Y. Huang, Green Chem., 2012, 14, 580 RSC.
-
(a) J. Koubachi, S. El Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, J. Org. Chem., 2007, 72, 7650 CrossRef CAS PubMed;
(b) V. Gembus, J.-F. Bonfanti, O. Querolle, P. Jubault, V. Levacher and C. Hoarau, Org. Lett., 2012, 14, 6012 CrossRef CAS PubMed.
- S. Paul, T. Gorai, A. Koley and J. K. Ray, Tetrahedron Lett., 2011, 52, 4051 CrossRef CAS.
- J. Velcicky, A. Soicke, R. Steiner and H.-G. Schmalz, J. Am. Chem. Soc., 2011, 133, 6948 CrossRef CAS PubMed.
-
(a) M. Ghosh, A. Ahmed, S. Dhara and J. K. Ray, Tetrahedron Lett., 2013, 54, 4837 CrossRef CAS;
(b) A. Ahmed, Y. Nuree and J. K. Ray, Tetrahedron Lett., 2013, 54, 665 CrossRef CAS.
- X. Bao, W. Yao, Q. Zhu and Y. Xu, Eur. J. Org. Chem., 2014, 7443 CrossRef CAS.
- M. Ghosh, A. Ahmed, R. Singha and J. K. Ray, Tetrahedron Lett., 2015, 56, 353 CrossRef CAS.
- For details see ESI.†.
- For examples see:
(a) D. Roy, S. Mom, M. Beaupérin and H. Doucet, Angew. Chem., Int. Ed., 2010, 49, 6650 CrossRef CAS PubMed;
(b) S. Bensaid and H. Doucet, Tetrahedron, 2012, 68, 7655 CrossRef CAS;
(c) J. Roger and H. Doucet, Eur. J. Org. Chem., 2010, 4412 CAS;
(d) I. Ozdemir, Y. Gök, O. Ozeroglu, H. Doucet and C. Bruneau, Eur. J. Inorg. Chem., 2010, 1798 CrossRef CAS.
- L. Zhao, C. Bruneau and H. Doucet, ChemCatChem, 2013, 5, 255 CrossRef CAS.
- Selected examples:
(a) J. J. Dong and H. Doucet, Eur. J. Org. Chem., 2010, 611 CrossRef CAS;
(b) J. J. Dong, D. Roy, R. J. Roy, M. Ionita and H. Doucet, Synthesis, 2011, 3530 CAS;
(c) J. Roger, F. Požgan and H. Doucet, Adv. Synth. Catal., 2010, 352, 696 CrossRef CAS;
(d) A. Beladhria, K. Beydoun, H. B. Ammar, R. B. Salem and H. Doucet, Synthesis, 2012, 2264 CAS.
- For review see:
(a) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed;
(b) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob02364e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a = 1
5a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4) produced by dimerization of 8-bromoquinoline. However, we were able to reduce the dimerization process of 8-bromoquinoline under the given reaction conditions only by applying a large excess of furan. When furan was used as a co-solvent (NMP-furan in a ratio of 1
2.4) produced by dimerization of 8-bromoquinoline. However, we were able to reduce the dimerization process of 8-bromoquinoline under the given reaction conditions only by applying a large excess of furan. When furan was used as a co-solvent (NMP-furan in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)), the complete conversion of 8-bromoquinoline was achieved in 24 h at 150 °C and the ratio of products 4a
1 (v/v)), the complete conversion of 8-bromoquinoline was achieved in 24 h at 150 °C and the ratio of products 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (10
5a (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) changed in favour of the desired product 4a. Under the optimized reaction conditions both bromoquinolines 1 and 2 can be successfully coupled with furan, thiophene, and 1-methyl-1H-pyrrole only if they are used in a large excess (Table 1). On the other hand, the method, using a large excess of heteroarene, was found to be impractical for benzo[b]furan (3e) and benzo[b]thiophene (3f); for this reason only 5 equivalents of 3e and 3f were used. Even though, arylation of benzo[b]furan with 8-bromoquinoline (1) progressed up to 73% conversion in 24 h, it was accompanied by the pronounced dimerization of 8-bromoquinoline. Consequently, we were able to isolate only 8% of the pure product 4e. Slightly better but still unsatisfactory results were obtained with benzo[b]thiophene (Table 1, entry 6). It should be mentioned that the optimized reaction conditions failed for the coupling of 8-bromo-5-nitroquinoline (2) either with benzo[b]furan or benzo[b]thiophene resulting in a rather complex reaction mixture. For comparison, we also found that 8-bromoquinoline is much less reactive than the 2- or 3-bromo analogues, since they can be successfully coupled with furan at lower temperature (130 °C) using Pd(OAc)2 (1 mol%) and PPh3 as a ligand.
1) changed in favour of the desired product 4a. Under the optimized reaction conditions both bromoquinolines 1 and 2 can be successfully coupled with furan, thiophene, and 1-methyl-1H-pyrrole only if they are used in a large excess (Table 1). On the other hand, the method, using a large excess of heteroarene, was found to be impractical for benzo[b]furan (3e) and benzo[b]thiophene (3f); for this reason only 5 equivalents of 3e and 3f were used. Even though, arylation of benzo[b]furan with 8-bromoquinoline (1) progressed up to 73% conversion in 24 h, it was accompanied by the pronounced dimerization of 8-bromoquinoline. Consequently, we were able to isolate only 8% of the pure product 4e. Slightly better but still unsatisfactory results were obtained with benzo[b]thiophene (Table 1, entry 6). It should be mentioned that the optimized reaction conditions failed for the coupling of 8-bromo-5-nitroquinoline (2) either with benzo[b]furan or benzo[b]thiophene resulting in a rather complex reaction mixture. For comparison, we also found that 8-bromoquinoline is much less reactive than the 2- or 3-bromo analogues, since they can be successfully coupled with furan at lower temperature (130 °C) using Pd(OAc)2 (1 mol%) and PPh3 as a ligand.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)c
5)c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7)b
2.7)b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b
1)b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a
1)a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was chosen to be the most practical. Moreover, the addition of water to the reaction mixture could also contribute to sufficiently solubilize the base.
1 (v/v) was chosen to be the most practical. Moreover, the addition of water to the reaction mixture could also contribute to sufficiently solubilize the base.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system for 3 h. However, our results show that there is no beneficial effect of using MW conditions, and in some cases even lower yields of the isolated products 4 were obtained (Table 2, yields in brackets).
1) solvent system for 3 h. However, our results show that there is no beneficial effect of using MW conditions, and in some cases even lower yields of the isolated products 4 were obtained (Table 2, yields in brackets).
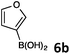
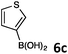
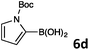
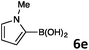
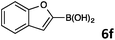
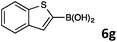
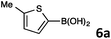
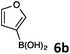
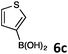
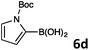
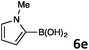
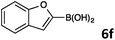

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 full widths at half height.
000 full widths at half height.