Synthesis of glycoaminooxy acid and N-oxyamide-linked glycolipids†
Abstract
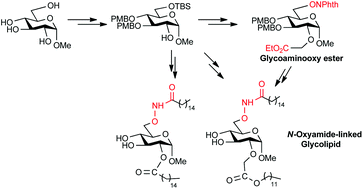
Aminooxyl sugar derivatives are versatile building blocks for the generation of various glycoconjugates with interesting bioactivities. We report herein a synthetic method for the preparation of orthogonally protected glycoaminooxy acid from methyl α-D-glycopyranoside in 7 steps. The key steps involve the selective protection, O-alkylation and Mitsunobu reaction. Fully deprotected N-oxyamide-linked novel glycolipids can be easily generated from the glycoaminooxy ester or from the 2-hydroxy free sugar in 5 or 6 steps.
 Please wait while we load your content...
Please wait while we load your content...