The synthesis of heterosaccharides related to the fucoidan from Chordaria flagelliformis bearing an α-l-fucofuranosyl unit†
Abstract
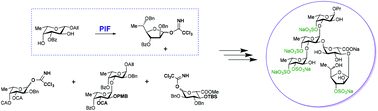
Sulfated polysaccharides, fucoidans, from brown algae are built up mainly of α-L-fucopyranosyl units and form a group of natural biopolymers with a wide spectrum of biological activities. Systematic synthesis of oligosaccharides representing fucoidans’ fragments gives molecular probes for detecting pharmacophores within fucoidan polysaccharide chains. Recently, it was discovered that the fucoidan from brown seaweed Chordaria flagelliformis contains not only α-L-fucopyranosyl units but also α-L-fucofuranosyl ones. To establish the influence of the unusual α-L-fucofuranose residue on the biological activity and conformational properties of fucoidans, the synthesis of selectively O-sulfated pentasaccharides, which represent the main repeating unit of the fucoidan from C. flagelliformis, was performed. The features of the synthesis were the use of the pyranoside-into-furanoside rearrangement to prepare the fucofuranoside precursor and remote stereocontrolling participation of O-acyl groups to manage stereoselective α-bond formation in glycosylation reactions.

 Please wait while we load your content...
Please wait while we load your content...