Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel protocol for the one-pot borylation/Suzuki reaction provides easy access to hinge-binding groups for kinase inhibitors†
A.
Hooper
,
A.
Zambon‡
and
C. J.
Springer‡
*
The Institute of Cancer Research, Cancer Research UK Cancer Therapeutics Unit, Division of Cancer Therapeutics, 15 Cotswold Road, Sutton, Surrey SM2 5NG, UK. E-mail: Caroline.Springer@icr.ac.uk
First published on 24th November 2015
Abstract
The one-pot borylation/Suzuki reaction is a very efficient means of accessing cross-coupling products of two aryl-halide partners that generally requires the use of specific catalysts or ligands and/or relatively long reaction times. This new microwave-assisted method provides a quick one-pot borylation/Suzuki reaction protocol that we applied to the synthesis of various bi- or poly-aryl scaffolds, including a variety of aryl and heteroaryl ring systems and the core frameworks of kinase inhibitors vemurafenib and GDC-0879.
Introduction
Kinases are key functional proteins that regulate signal transduction pathways in cells by catalyzing phosphorylation of serine, threonine, or tyrosine residues. Deregulation of protein kinases is implicated in many diseases including cancer, diabetes, and inflammation: as a consequence kinases have been major targets for recent small molecule drug development.1–3A common feature of most kinase inhibitors is the presence of a hinge-binding moiety,4 a group that is able to form hydrogen bonds to the cleft between N- and C-lobes of the kinase known as the hinge region.4 Typically, a hinge-binding structure consists of a hetero-aromatic group containing hydrogen bond donors and/or acceptors in either a mono- or bi-dentate fashion. This mode of binding mimics that of the adenosine ring of the natural kinase ligand, ATP.
The palladium-catalysed Suzuki–Miyaura reaction, which couples aryl halide and aryl boronic species for the formation of new C–C bonds,5–8 is particularly suited to access hinge binding fragments thanks to its tolerance of functional groups and mild reaction conditions. Furthermore, extensive literature describes a wide range of experimental procedures.9,10
Despite its wide scope, the Suzuki–Miyaura cross-coupling reaction has a number of limitations such as lack of availability, high expense and instability of certain boronic species. In order to circumvent these issues, Miyaura explored the use of bis(pinocolato)diboron as the boronic acid equivalent in a one-pot borylation/Suzuki reaction, which eliminates the need to isolate the boronic intermediate. Despite subsequent improvements to the methodology,11–15 current one-pot borylation/Suzuki protocols require double loading of specific catalysts, use of additional ligands or relatively long reaction times, and their scope is generally limited to one specific scaffold.16–18
Our aim was to develop a robust one-pot borylation/Suzuki protocol that employs one single loading of catalyst with no need for additional ligands and to use it to access a small panel of putative hinge binding fragments, which were then profiled for kinase selectivity.
Results and discussion
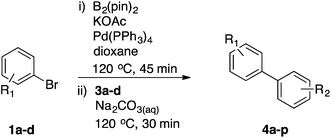
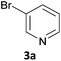
As a model reaction for the optimisation of the one-pot protocol, we selected the coupling of 5-bromoindanone 1a and 3-bromopyridine 3a to give 3-pyridinylindenone 4a, mediated by the formation of pinacolate boronic ester 2a. We reasoned that the structure of 4a could act as a basic scaffold for a kinase inhibitor, with the pyridine group acting as hinge binder and the indanone elaborating into the ATP binding pocket. Variation of this basic structure would then allow us to access a panel of compounds with potential kinase activity.The original borylation conditions, developed by Miyaura11 were assessed utilising Pd(dppf)Cl2 as the catalytic species and KOAc as the base. Although this yields the boronic ester 2a with 100% conversion, addition of 3-bromopyridine 3a, along with catalyst and base did not yield 4a (Table 1, entry 1). Pd(PPh3)4 as catalyst was assessed for the Suzuki step but again 4a was not formed (entry 2). However, the borylation reaction time could be shortened to 1 hour at 120 °C under microwave irradiation resulting in the formation of intermediate 2a. This is a significant time reduction from 18 h needed at 80 °C using both catalytic species (entries 3 and 4). From this point, only tetrakis(triphenylphosphine)palladium(0) as catalyst was utilized to avoid a mixture of catalytic species as this was deemed most suitable due to its wide use and cheaper cost.
| Entry | Cat1/base1 | t2 (h) | T (°C) | 2a (%) | Cat2/Base2 | t2 (h) | 4a (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (1 equiv.), B2(pin)2 (1.2 equiv.), catalyst (10 mol%) and base (3 equiv.) in dioxane (0.5 M) followed by 3a (1 equiv.), catalyst (10 mol%) and base (2 equiv.). b Conversion by LCMS. c Reactions performed in a microwave. d Initially heated to 80 °C over 18 h but after no product 2a formation was observed, it was heated to 120 °C in a microwave for 1 h. e Only indanone dimer observed. | |||||||
| 1 | Pd(dppf)Cl2/KOAc | 18 | 80 | 100 | Pd(dppf)Cl2/KOAc | 1 | 0 |
| 2 | Pd(dppf)Cl2/KOAc | 18 | 80 | 100 | Pd(PPh3)4/KOAc | 1 | 0 |
| 3 | Pd(dppf)Cl2/KOAc | 1 | 120c | 100 | Pd(PPh3)4/KOAc | 1 | 0 |
| 4 | Pd(PPh3)4/KOAc | 1 | 120c,d | 100 | Pd(PPh3)4/KOAc | 1 | 0 |
| 5 | Pd(PPh3)4/KOAc | 1 | 120c | 100 | Pd(PPh3)4/Na2CO3(aq) | 1 | 100 |
| 6 | Pd(PPh3)4/Na2CO3(aq) | 1 | 120c | 0e | — | — | — |
| 7 | Pd(PPh3)4/KOAc | 45 min | 120c | 100 | —/Na2CO3(aq) | 30 min | 100 |
To identify the best reaction conditions to access 4a for a one pot reaction, we postulated a bicyclic mechanism merging the catalytic cycles 1 and 2 elucidated by Miyaura and Suzuki for the borylation and coupling steps respectively,11,19 as outlined in Fig. 1. Cycle 1 is a typical borylation cycle with oxidative–addition of the first halide to the catalytic species, oxidising the palladium species from the neutral state of the Pd(PPh3)4 to the Pd(II) species. Base1 then displaces the halide within the catalytic complex, activating it for the subsequent transmetallation step with bis(pinocolato)diboron.11,19 A final reductive–elimination step recovers the Pd(0) catalytic species and completes cycle 1, releasing the aryl-boronic species; at this stage the reactions carried out in entries 1–4 stalled.
Similarly, catalytic cycle 2 is initiated by oxidative addition of the second halide to the catalyst and the displacement of the halide by Base2 followed by addition of the aryl boronate to form the biaryl-substituted palladium species. Complexation of a base to the aryl boronate is essential at this stage in order to accelerate the transmetallation step20 by forming a more reactive boronate to interact with the palladium species21 and facilitate an intramolecular transmetallation.22 We postulated that a stronger base is required at this step in cycle 2 than the one needed in cycle 1, which only has the role to displace the halide from the first palladium species.
We then applied a change of base to our model system, introducing sodium carbonate as second base after the formation of intermediate 2a, and pleasingly the pyridinylindenone product 4a is formed with 100% conversion (entry 5). As a further confirmation of the reaction mechanism, the introduction of sodium carbonate in the borylation step affords only the homocoupling product (entry 6).
Once the optimal base/catalyst system was identified, we worked to eliminate the second catalyst loading and decrease the reaction times. In these optimised conditions, 100% conversion to product 4a was obtained (entry 7) using a single initial loading of Pd(PPh3)4, with 45 minutes at 120 °C under microwave irradiation for the borylation step, followed by addition of 3a and Na2CO3 with further heating to 120 °C for 30 min in the Suzuki step.
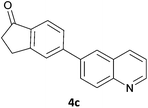
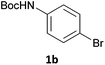
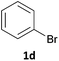
Next, we applied this optimised protocol to the synthesis of a small panel of putative kinase inhibitors. To explore the scope of the reaction and provide a set of hinge-binding fragments, we included in the reaction panel keto, boc-protected aniline, halo, aryl, indanone, pyridyl, pyrazole, azaindole and quinoline functional groups. To this end, a selection of eight commercially available, relatively undecorated halides was chosen: four heteroaromatic rings containing either a hydrogen bond acceptor, a hydrogen bond donor or both (3a–d) and four phenyl moieties (1a–d), to be coupled in combination to obtain 16 products (4a–p). Within this set is compound 4j, which is a precursor of the BRAF-selective inhibitor, vemurafenib. The isolated yields obtained were good to excellent with the single exception of 4b (Table 2). Pleasingly, regioselectivity in the coupling reaction was obtained for 1c (entries 9 to 12) and 3d (entries 4, 8, 12 and 6), which only reacted at the bromine and not at the chlorine.
| Entry | First halide | Second halide | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: first halide (1 equiv.), B2(pin)2 (1.2 equiv.), Pd(PPh3)4 (10 mol%) and KOAc (3 equiv.) in dioxane (0.5 M) followed by second halide (1 equiv.) and 2 M Na2CO3 (aq) (2 equiv.). b Isolated yield. | ||||
| 1 | 1a |
|
4a | 70 |
| 2 | 1a |

|

|
12 |
| 3 | 1a |

|
|
67 |
| 4 | 1a |

|

|
100 |
| 5 |
|
3a |

|
52 |
| 6 | 1b | 3b |

|
53 |
| 7 | 1b | 3c |

|
46 |
| 8 | 1b | 3d |

|
40 |
| 9 |

|
3a |

|
42 |
| 10 | 1c | 3b |

|
32 |
| 11 | 1c | 3c |

|
62 |
| 12 | 1c | 3d |

|
49 |
| 13 |
|
3a |

|
81 |
| 14 | 1d | 3b |
|
45 |
| 15 | 1d | 3c |

|
87 |
| 16 | 1d | 3d |

|
68 |
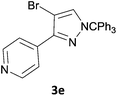
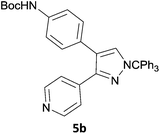
The new protocol was then evaluated for the synthesis of more complex scaffolds; coupling of halides 1a–d with the trityl-protected 4-(4-bromopyrazolyl)pyridine 3e provided GDC-087923 analogues 5a–d in moderate to good yields (Table 3) with a slightly modified protocol allowing for longer reaction times.
| Entry | First halide | Second halide | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: first halide (1 equiv.), B2(pin)2 (1.2 equiv.), Pd(PPh3)4 (10 mol%) and KOAc (3 equiv.) in dioxane (0.4 M) followed by second halide (1 equiv.) and 2 M Na2CO3 (aq) (2 equiv.). b Isolated yield. | ||||
| 1 |
|
1a |

|
57 |
| 2 | 3e | 1b |
|
33 |
| 3 | 3e | 1c |
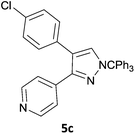
|
49 |
| 4 | 3e | 1d |

|
65 |
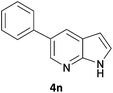
The 20 compounds synthesised were assessed on a small panel of kinases using the ProfilerPro® Selectivity Assay Kits (Caliper Life Sciences, Inc.). The inhibition of 24 kinases was determined at a compound concentration of 300 μM; of the 20 compounds, 11 showed greater than 50% inhibition of at least one kinase (Table 4) with compounds 4j, 4n, 4o and 4p showing greater than 90% inhibition for at least one kinase. Of these, 4o and 4p have a selective profile, inhibiting just ABL (entry 10, 4o) or both ABL and CK1 (entry 11, 4p) at the concentrations used. There is one commercially available ABL inhibitor which contains a quinoline moiety, Rebastinib (DCC-2036), but this has a binding mode in which the quinolone does not interact with the hinge region.24
| Entry | Compound | Kinase % inhibition | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AurA | RSK1 | PRAK | Erk1 | PKD2 | CK1δ | CHK1 | ABL | FYN | LYNα | CHK2 | MET | LCK | SRC | GSK3β | Erk2 | PKACα | INSR | MSK1 | ||
| 1 | 4a | 51 | 41 | 19 | 14 | 38 | 38 | 10 | 41 | −2 | 33 | 35 | 16 | 31 | 25 | 56 | 14 | 6 | 24 | 28 |
| 2 | 4b | 76 | −2 | 3 | 16 | 6 | 46 | 15 | 37 | 1 | 20 | 41 | 16 | 16 | 20 | 26 | 19 | −14 | 61 | 4 |
| 3 | 4f | 60 | 20 | 40 | 8 | 18 | 50 | 13 | 23 | −25 | 24 | 35 | 9 | 29 | 8 | 8 | −7 | 37 | 46 | 19 |
| 4 | 4i | 61 | 52 | 26 | 7 | 32 | 34 | 0 | 68 | 54 | 63 | 25 | 17 | 61 | 43 | 30 | 10 | 52 | 24 | 42 |
| 5 | 4j | 89 | 91 | 86 | 55 | 87 | 73 | 6 | 87 | 79 | 30 | 43 | 47 | 16 | 79 | 45 | 53 | 46 | 58 | 69 |
| 6 | 4k | 45 | 31 | 22 | 6 | 34 | 70 | −9 | 65 | −1 | 29 | 18 | 29 | 43 | 19 | 26 | 1 | 22 | 21 | 13 |
| 7 | 4l | 7 | 3 | −1 | 0 | −35 | 68 | −7 | 36 | 18 | −7 | 3 | 16 | 11 | 3 | 60 | 3 | 29 | 12 | 13 |
| 8 | 4m | 51 | 30 | 27 | 8 | 13 | 58 | −12 | 43 | 27 | 46 | 37 | 28 | 18 | 25 | 14 | 4 | 56 | 26 | 26 |
| 9 | 4n | 99 | 70 | 47 | 62 | 92 | 94 | 80 | 85 | 53 | 76 | 91 | 52 | 63 | 62 | 57 | 66 | 61 | 93 | 43 |
| 10 | 4o | 45 | 34 | 12 | 7 | 16 | 44 | 1 | 95 | 30 | 49 | 18 | 35 | 54 | 23 | 26 | 1 | 37 | 44 | 15 |
| 11 | 4p | 66 | 48 | 14 | 6 | 18 | 92 | −1 | 94 | 48 | 64 | 37 | 35 | 67 | 36 | 50 | −7 | 59 | 49 | 24 |
In order to validate hits 4o and 4p, ABL IC50s were determined (Invitrogen) at a starting compound concentration of 100 μM. Compound 4o inhibits ABL with an IC50 of 8 μM, corresponding to a high ligand efficiency of 0.45, as shown in Table 5 and, although it has a higher IC50, a similar ligand efficiency is observed for 4p with ABL. Conversely, the IC50 of 4p for CK1δ was >300 μM, further highlighting the selectivity of the scaffold.
| Entry | Compound | ABL IC50 (μM) | Ligand efficiency |
|---|---|---|---|
| 1 | 4o | 8.11 | 0.45 |
| 2 | 4p | 19.2 | 0.39 |
Conclusions
In conclusion, a novel, quick and robust microwave-assisted protocol for the one-pot borylation/Suzuki reaction has been developed to access a small panel of putative kinase inhibitors containing a variety of aryl and heteroaryl ring systems. These scaffolds have been assessed for inhibition of a panel of kinases, and a ligand efficiency of 0.45 is observed with the kinase ABL for compound 4o.Acknowledgements
We thank Wellcome Trust (grant WEL081X) for funding. We also thank Dr Jonathan Macdonald for assistance with the ProfilerPro Assay as well as Dr Maggie Liu, Dr Amin Mirza and Mr Meirion Richards for assistance with NMR and LCMS analysis.References
- D. Hanahan and R. A. Weinberg, Cell, 2000, 100, 57–70 CrossRef CAS PubMed
.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed
.
- P. Cohen, Nat. Rev. Drug Discovery, 2002, 1, 309–315 CrossRef CAS PubMed
.
- J. Zhang, P. L. Yang and N. S. Gray, Nat. Rev. Cancer, 2009, 9, 28–39 CrossRef CAS PubMed
.
- N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513–519 CrossRef CAS
.
- A. Suzuki, Acc. Chem. Res., 1982, 15, 178–184 CrossRef CAS
.
- S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS
.
- F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 2419–2440 CAS
.
- M. R. Biscoe, B. P. Fors and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 6686–6687 CrossRef CAS PubMed
.
- T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14073–14075 CrossRef CAS PubMed
.
- T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508–7510 CrossRef CAS
.
- O. Baudoin, D. Guenard and F. Gueritte, J. Org. Chem., 2000, 65, 9268–9271 CrossRef CAS PubMed
.
- O. Baudoin, M. Cesario, D. Guenard and F. Gueritte, J. Org. Chem., 2002, 67, 1199–1207 CrossRef CAS PubMed
.
- K. L. Billingsley, T. E. Barder and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 5359–5363 CrossRef CAS PubMed
.
- L. H. Wang, X. L. Cui, J. Y. Li, Y. S. Wu, Z. W. Zhu and Y. J. Wu, Eur. J. Org. Chem., 2012, 595–603 CrossRef CAS
.
- Y. Zhang, J. Gao, W. Li, H. Lee, B. Z. Lu and C. H. Senanayake, J. Org. Chem., 2011, 76, 6394–6400 CrossRef CAS PubMed
.
- D. P. Fernando, W. H. Jiao, J. Polivkova, J. Xiao, S. B. Coffey, C. Rose, A. Londregan, J. Saenz, R. Beveridge, Y. X. Zhang, G. E. Storer, D. Vrieze, N. Erasga, R. Jones, V. Khot, K. O. Cameron, K. F. McClure, S. K. Bhattacharya and S. T. M. Orr, Tetrahedron Lett., 2012, 53, 6351–6354 CrossRef CAS
.
- G. A. Molander, S. L. Trice and S. M. Kennedy, J. Org. Chem., 2012, 77, 8678–8688 CrossRef CAS PubMed
.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
- A. Suzuki, J. Organomet. Chem., 1999, 576, 147–168 CrossRef CAS
.
- A. A. Braga, N. H. Morgon, G. Ujaque and F. Maseras, J. Am. Chem. Soc., 2005, 127, 9298–9307 CrossRef CAS PubMed
.
- N. Miyaura, J. Organomet. Chem., 2002, 653, 54–57 CrossRef CAS
.
- J. D. Hansen, J. Grina, B. Newhouse, M. Welch, G. Topalov, N. Littman, M. Callejo, S. Gloor, M. Martinson, E. Laird, B. J. Brandhuber, G. Vigers, T. Morales, R. Woessner, N. Randolph, J. Lyssikatos and A. Olivero, Bioorg. Med. Chem. Lett., 2008, 18, 4692–4695 CrossRef CAS PubMed
.
- W. W. Chan, S. C. Wise, M. D. Kaufman, Y. M. Ahn, C. L. Ensinger, T. Haack, M. M. Hood, J. Jones, J. W. Lord, W. P. Lu, D. Miller, W. C. Patt, B. D. Smith, P. A. Petillo, T. J. Rutkoski, H. Telikepalli, L. Vogeti, T. Yao, L. Chun, R. Clark, P. Evangelista, L. C. Gavrilescu, K. Lazarides, V. M. Zaleskas, L. J. Stewart, R. A. Van Etten and D. L. Flynn, Cancer Cell, 2011, 19, 556–568 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob01915j |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2016 |