Open Access Article
Open Access ArticleMulti-conformer molecules in solutions: an NMR-based DFT/MP2 conformational study of two glucopyranosides of a vitamin E model compound†‡
Ryszard B.
Nazarski
*a,
Piotr
Wałejko
b and
Stanisław
Witkowski
b
aUniversity of Łódź, Faculty of Chemistry, Department of Theoretical and Structural Chemistry, Pomorska 163/165, 90-236 Łódź, Poland. E-mail: nazarski@uni.lodz.pl; Fax: (+48) 42635-5744; Tel: (+48) 42635-5615
bUniversity of Białystok, Institute of Chemistry, Ciołkowskiego 1K, 15-245 Białystok, Poland
First published on 17th February 2016
Abstract
Overall conformations of both anomeric per-O-acetylated glucosyl derivatives of 2,2,5,7,8-pentamethylchroman-6-ol were studied in the context of their high flexibility, on the basis of NMR spectra in CDCl3 solution and related DFT calculation results. A few computational protocols were used, including diverse density functional/basis set combinations with a special emphasis on accounting (at various steps of the study) for the impact of intramolecular London-dispersion (LD) effects on geometries and relative Gibbs free energies (ΔGs) of different conformers coexisting in solution. The solvent effect was simulated by an IEF-PCM approach with the UFF radii; its other variants, including the use of the recently introduced IDSCRF radii, were employed for a few compact B3LYP-GD3BJ optimized structures showing one small imaginary vibrational frequency. The advantage of using IDSCRF radii for such purposes was shown. Of the four tested DFT methods, only the application of the B3LYP/6-31+G(d,p) approximation afforded ensembles of 7–8 single forms for which population-average values of computed NMR parameters (δH, δC and some nJHH data) were in close agreement with those measured experimentally; binuclear (δH,C 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) correlations, rH,C2 = 0.9998. The associated individual ΔG values, corrected for LD interactions by applying Grimme's DFT-D3 terms, afforded relative contents of different contributors to the analyzed conformational families in much better agreement with pertinent DFT/NMR-derived populations (i.e., both data sets were found to be practically equal within the limits of estimated errors) than those calculated from dispersion uncorrected ΔGs. All these main findings were confirmed by additional results obtained at the MP2 level of theory. Various other aspects of the study such as the crystal vs. solution structure, gg/gt rotamer ratio, diagnostic (de)shielding effects, dihydrogen C–H⋯H–C contacts, and doubtful applicability of some specialized DFT functionals (M06-2X, ωB97X-D and B3LYP-GD3BJ) for the description of highly flexible molecules are also discussed in detail.
1) correlations, rH,C2 = 0.9998. The associated individual ΔG values, corrected for LD interactions by applying Grimme's DFT-D3 terms, afforded relative contents of different contributors to the analyzed conformational families in much better agreement with pertinent DFT/NMR-derived populations (i.e., both data sets were found to be practically equal within the limits of estimated errors) than those calculated from dispersion uncorrected ΔGs. All these main findings were confirmed by additional results obtained at the MP2 level of theory. Various other aspects of the study such as the crystal vs. solution structure, gg/gt rotamer ratio, diagnostic (de)shielding effects, dihydrogen C–H⋯H–C contacts, and doubtful applicability of some specialized DFT functionals (M06-2X, ωB97X-D and B3LYP-GD3BJ) for the description of highly flexible molecules are also discussed in detail.
Introduction
High-resolution nuclear magnetic resonance (NMR) spectroscopy is undoubtedly the most valuable experimental technique for the determination of the structure and dynamics of small- to medium-sized organic molecules, especially carbo- and heterocyclic ones, when elucidating a relative configuration and/or assessing the overall multi-conformer (composite) geometries1 of such species in solution. Among various isotropic NMR parameters, chemical shifts, δKs, and indirect spin–spin coupling constants (hereafter termed JKL couplings) are the most informative observables employed for such purposes. Nowadays, these possibilities have become considerably enhanced for common spin-1/2 magnetic active nuclei by two methods of the NMR-oriented density functional theory (DFT) calculations, i.e., gauge-including atomic orbital (GIAO)2 predictions of absolute shieldings σKs (and thus interrelated δK data), and computations of JKL values.3 The use of such approaches is crucial for structurally flexible systems affording only population-weighted averaged NMR spectra in solution. Indeed, reliable calculations of the above (not accessible in another way) NMR parameters for the major contributors to their conformational ensembles are usually necessary in all cases of this kind.12,2,5,7,8-Pentamethylchroman-6-ol (PMC) – the parent system of title compounds – is a potent phenolic free radical scavenger related to α-tocopherol (vitamin E),4 in which a long lipophilic phytyl side chain is replaced by a methyl (Me) substituent. It is the potent hydrophilic α-tocopherol derivative,5 but its biological activity is not always shared by its parent α-tocopherol (e.g., it acts as a potent anti-inflammatory agent).5b PMC shows over 5–10 times stronger dose-dependent inhibition of the agonist-induced platelet aggregation in human platelet-rich plasma, as compared to α-tocopherol.6 Among various α-tocopherol analogues, it is the most potent inhibitor of nuclear factor-kappa B (NF-κB) activity.7 Moreover, PMC has shown anti-androgen activity in prostate carcinoma cells and is considered a potent chemopreventive agent of androgen-dependent diseases, such as prostate cancer8 and other human cancers.9 Nevertheless, the potential therapeutic application of PMC is limited due to its relatively low water solubility. One of the most promising solutions is to convert PMC into its amphiphilic glycoconjugates.10 These derivatives as prodrugs would gain a favorable solubility in physiological fluids and a proper permeability through membranes and natural biological barriers e.g. blood to brain barriers. New data indicate that PMC can be helpful in designing such new potential medicinal compounds with a better clinical effectiveness.11 Some glycosides of vitamin E and its short-chain analogues were described earlier.10b,12 Also different structural aspects of this type and related model molecules, such as PMC and Trolox, were studied extensively in our laboratory by means of 13C NMR in solution13 and in the solid state (CP/MAS technique)14 as well as by ECD spectroscopy.15 It is obvious that for a complete understanding of the behavior of every system having potential biomedical activity, a good knowledge of its conformational properties (both structure and dynamics) is crucial. Therefore, a comprehensive 1H and 13C NMR data-based DFT conformational investigation of the two peracetylated glucosyl derivatives of PMC, i.e., compounds 1α and 1β (see Fig. 1), was undertaken.
 | ||
| Fig. 1 Structures of the studied compounds with the atom numbering and five relevant torsion angles concerning their mobile molecular units, where Ar means the chroman system.16 | ||
In view of the foregoing, the title highly structurally flexible 2,3,4,6-tetra-O-acetyl-D-glycopyranosides seemed to be particularly suitable entities for testing of a few calculational DFT-level protocols currently available for the analysis of composite shapes1 of small- to medium-sized multi-conformer systems. Indeed, such a mobility concerns even the aglycone (non-sugar) moiety of 1α in the solid state, as its 3,4-dihydro-2H-pyranyl (DHP) ring adopting two alternative half-chair (HC)-like forms was found disordered in the crystal structure at 100 K, along with related gem-dimethyl groups.18 Hence, 1H and 13C NMR spectra of both anomers of 1 in CDCl3 were fully interpreted and additionally analyzed in the light of σH and σC values GIAO-predicted for their preselected energetically reasonable forms. Some diagnostic JHH and JCH couplings were also calculated. The integral equation formalism (IEF)19 of an implicit solvation and UFF-radii cavities were mainly used within the polarizable continuum model (PCM)20 approach. Its other variants were also employed for some structures with one small imaginary harmonic vibrational (IHV) frequency, showing an advantage of using the recently introduced21 and applied22 IDSCRF radii in such cases. Moreover, an empirical post factum correction of the computed Gibbs free energy, G, data1c,23 for a proper account of long-range London dispersion (LD) forces of the van der Waals (vdW) type, which are neglected in conventional DFT approaches (with underestimation of LD),24,25 was inter alia tested with the use of pairwise DFT-D3 corrections of Grimme.25c
Thus, four inseparable points were especially addressed in this work: (i) a good representation of the overall solution shapes1 of glucopyranosides 1α and 1β, considered to be highly flexible molecules, (ii) testing of a few DFT model chemistries (functional and basis set) accessible today for the most reliable prediction of the structure and molecular, e.g. NMR spectroscopy, properties of the individual forms of 1 coexisting in real solutions at equilibrium, and, particularly, (iii) explicitly accounting for the impact of weak intramolecular LD attractions24,25 on separate geometries and/or (iv) post factum accounting for the influence of such interactions on their relative conformational energies (ΔG°). To the best of our knowledge, this kind of widespread NMR data- and dispersion-oriented DFT study of the multi-conformer systems, positively verified by additional results emerging from the much more expensive MP2 calculations, has not yet been published.
Results and discussion
NMR spectra of 1 and other related systems
The title O-glucopyranosides were synthesized from PMC26 according to a literature-reported procedure10b based on the Helferich glycosylation method,27 using peracetylated β-glucose as a donor and a mild Lewis acid (ZnCl2) as a glycosyl promoter, followed by deacetylation.28 The resulting deprotected α/β-anomers were separated chromatographically and then subjected to acetylation (Experimental‡). The isolated products 1α and 1β gave spectral data fully consistent with the literature.10b The molar α/β ratio of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 (by 1H NMR) was established when pure 1β was melted with ZnCl2 (1.2 equiv.) at 390 ± 5 K under diminished pressure (30 Torr), whereas 1α was decomposed with the liberation of PMC under the same conditions.
64 (by 1H NMR) was established when pure 1β was melted with ZnCl2 (1.2 equiv.) at 390 ± 5 K under diminished pressure (30 Torr), whereas 1α was decomposed with the liberation of PMC under the same conditions.
Analysis of various types of NMR spectra recorded for 1α and 1β in a CDCl3 solution at a 14.1 T magnetic field strength (for 1D spectra, see Fig. S1–S6‡) was performed as previously for the other multi-conformer systems.1,29 Thus, the δH, δC and 3JH,H values associated with their anomeric centers were found to be in agreement with those for D-glucopyranoses.30 Also all cross peaks due to expected C–H connectivities within both molecules were localized in 2D spectra, including correlations across the glucosidic linkage in 1H,13C HMBC plots. Moreover, diagnostic 1JC1′,H1′ couplings (of 172.1 and 163.4 Hz for 1α and 1β, respectively) fully compatible with the literature data (ca. 170–175 and 160–165 Hz for α- and β-forms, respectively)30b were derived from HMBC spectra. Only assignments of the two slightly differentiated NMR lines coming from protons/carbons in 2a/2b-gem-dimethyl groups and two 13C lines of the C3′/C6′ acetate methyl groups were not provided by an NMR experiment; however all these signals were unambiguously assigned in further calculations (vide infra). An observed chemical shift non-equivalence of these former Me groups indicated that the C6–O6 rotation is not (nearly) a free-energy process, because sharp 1H/13C resonance lines of the 2a/2b geminal groups are observed for PMC and its derivatives.13a,26b On the other hand, cross peaks of the four H1′/H5a (where H1′ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C1′–H, etc.), H1′/H7a, H5′/H5a and H5′/H7a pairs and the first two ones were found in ROESY spectra of 1α and 1β, respectively, as arising from related inter-residual H–H contacts. These nuclear Overhauser effect (NOE) data, well corroborated by broadening of a vast majority of the 1H signals of aglycone moieties of both anomers (Fig. S1 and S4‡), confirmed a high degree of rotameric flexibility around their C1′–O6 and/or neighboring C6–O6 bonds. In turn, conformational mobility of the constituent semi-unsaturated DHP rings is additionally indicated by motional averaging of the 2JH3,H4 values (Experimental‡).
C1′–H, etc.), H1′/H7a, H5′/H5a and H5′/H7a pairs and the first two ones were found in ROESY spectra of 1α and 1β, respectively, as arising from related inter-residual H–H contacts. These nuclear Overhauser effect (NOE) data, well corroborated by broadening of a vast majority of the 1H signals of aglycone moieties of both anomers (Fig. S1 and S4‡), confirmed a high degree of rotameric flexibility around their C1′–O6 and/or neighboring C6–O6 bonds. In turn, conformational mobility of the constituent semi-unsaturated DHP rings is additionally indicated by motional averaging of the 2JH3,H4 values (Experimental‡).
Interestingly, two long-range couplings, 4JH1′,H3′ = 0.40 and 4JH1′,H5′ = 0.52 Hz, were revealed for a sugar residue of 1α in 1H NMR spectra processed with resolution enhancement.31 Similar interactions (4JH1,H5 = 0.54 and 4JH1,H3 = 0.36 Hz) were also determined for methyl 2,3,4,6-tetra-O-acetyl-α-D-glucopyranoside 2α as the simplest aliphatic analogue of 1α (Fig. 2). The latter results are in good agreement with the corresponding heteronuclear couplings JC,Hs found for 2α in C6D6 (3JH1,C5 = 6.9 and 3JH1,C3 = 5.6 Hz).32 Moreover small couplings, 4JHH ∼ 0.45 Hz, between protons of the gem-dimethyl groups were found in both title systems. To the best of our knowledge, all these 4JHH couplings, whose existence was confirmed by our calculations (vide infra), were not reported before and were possibly unobserved.33 So, the sharp well-resolved multiplet consisting of 16 lines (dddd) due to an axial H5′ appeared in CDCl3 at 4.528 and 3.986 ppm for 1α and 2α, respectively (Fig. S2 and S8‡). Additionally, clear NOE interactions H4′/H6′R were observed in the ROESY spectra of both these α-anomers, confirming the configuration at C6′.32 Among other issues, the origin of a 0.54 ppm variation in the above 1H chemical shifts and especially a pronounced 1.00 ppm difference ΔH5′ = δH5′α − δH5′β found for compounds 1 was a particularly intriguing question. Such large ΔH5′ values were also determined for anomeric pairs of other O-glycosides of chroman-6-ol (3–8) possessing inter alia the mannoside, galactoside or 2-deoxyglucose residue; for details see Tables S1–S3.‡ Furthermore, it was found that ΔH5 diminished with the change of bulkiness of the aglycone moiety, but the impact of the pyranose ring structure is also evident – compare the ΔH5 values (1.00 vs. 0.70 ppm) for 1 and 8, respectively (Table S3‡). Nonetheless, one can conclude that ΔH5 is a much better determinant of stereochemistry at C1 than the usually considered difference ΔC1 = δC1α − δC1β, at least for glycopyranosides 1–8 (Table S2‡).
Conformational study
Owing to the complexity and great flexibility of both glucosides 1, their conformational analysis was done on the basis of structural information available from the NMR data, which was supplemented with computational results. Thus, a few standard approaches were applied in two inseparable steps of the study. An extensive HF/DFT modeling of the series of low-energy candidate conformational states of both anomers 1 was performed at beginning, by starting with huge amounts of their molecular-mechanics (MM) models found initially. This step was followed by predictions of relevant NMR spectral parameters (JKL and mainly δK values) of such DFT-optimized structures, carried out using different combinations of density functionals and basis sets (Calculational). Moreover, due to a fortunate lack of strong specific solvent–solute interactions, their solvation was simulated within the framework of an implicit solvation model, by using mainly an IEF-PCM19,20 method as implemented in the Gaussian 09 package of programs.34 Based on the GDFT 390 values computed in the standard way, it was found that 1β is more thermochemically stable than 1α, but the agreement with the equilibration experiment mentioned above was only qualitative (see however below).In order to determine the fully relaxed overall shapes1 of molecules 1α and 1β in the most general manner, a linear regression analysis of the measured δH and δC data vs. those values obtained from the σH/σC data GIAO predicted at the IEF-PCM(UFF,CHCl3)/mPW1PW91/6-311+G(2d,p)35//IEF-PCM(UFF,CHCl3)B3LYP/6-31+G(d,p)36 level was performed for some promising forms found at the beginning. The double-ζ (DZ) valence quality of the employed atomic basis sets was forced by the relatively large size of the molecules under study. Thus, the calculated data were plotted as usual1,23b,37 against experimental ones, but using the binuclear δcalcdH,C(DFT) vs. δobsdH,C correlation;1a,37,38 see Computational details. The thus obtained individual NMR data-derived populations pi were next confronted with pertinent results on energetics of different single forms of 1α (or 1β) coexisting in solution at equilibrium, i.e., relative total electronic-nuclear energies (0 K, ΔEtots) or relative standard Gibbs free energies (298.15 K, ΔG°s), computed for local minima on conformational energy hypersurfaces of the analyzed solutes immersed in a polarizable continuum, the relative permittivity of which matches that of CHCl3.
The above preliminary calculational vs. experimental data sets were subsequently analyzed in light of our previous results on the other non-rigid (flexible) molecules.1,23b In particular, the reliability of a standard approach concerning energy-weighted fractional populations39 and the reproduction of weak long-range attractive intramolecular LD forces of the vdW type,24,25 operative in two relative large systems 1, were considered. Thus, all available data were analyzed in terms of Boltzmann populations of potential contributors to the overall composite shapes of both of these molecules, based on the G values computed for their individual conformers in simulated solutions. The structure of glycosides is usually described40 by two torsion angles around the glycosidic linkage, i.e., φ (O5′–C1′–O6–C6) and ψ (C1′–O6–C6–C5), and the ω angle (O5′–C5′–C6′–O6′) within the exocyclic acetoxymethyl group (Fig. 1 and 3). Hence, great rotameric flexibility is generally possible, but only some of the above rotamers of 1α and 1β really exist in solution. In other words, their conformational freedom was found to be restricted to only a few (nearly) freely rotating bonds, as described later.
 | ||
| Fig. 3 Newman projections outlining the nomenclature used throughout for the discussed C5′–C6′ rotamers. | ||
Fortunately, the first of the angles mentioned above was found at the same magnitude (φ = 127° and ca. −73° for 1α and 1β, respectively) in all our initial B3LYP-optimized structures, i.e., 8 forms of 1α and 7 forms of 1β, derived from the respective starting MMX geometries (Computational details). The D-glucopyranose ring of both systems was consistently computed to be a unit adopting the relatively rigid 4C1 chair conformation.41 Also the three consecutive equatorial acetoxy groups in positions 2′, 3′ and 4′ were always found situated in the planes approximately perpendicular to an average sugar plane, in line with such arrangements determined in the crystals of 1α18 and 1β.42 Moreover, one of the three rotamers (each separated by ∼120° dihedral rotation) around the exocyclic C5′–C6′ bond in a pyranose ring, i.e., the tg form43 with ω ≈ 180°, was not found within the used 25.1 kJ mol−1 MMX energy cutoff. This finding was in agreement with the assumption that little or no contribution would be expected from the tg rotamer of 1, because of unfavorable steric interactions between the acetoxy groups borne by C4′ and C6′.32,43a Indeed, its participation for anomers 2α and 2β having an identical glucose residue was suggested32,43c to be only 4–11 and 1–8%, respectively, and so practically within an estimated uncertainty of 5–10% in the NMR data-based conformer population.43c Thus, the other three staggered rotamers [namely gg (ω ≈ −60°),43gt (ω ≈ 60°, χ ≈ ±180°)43 and an unusual ‘bent’ form denoted hereafter as gt90 (ω ≈ 60°, χ ≈ 90°), all shown schematically in Fig. 3 and characterized by the angles ω and χ (≡ C5′–C6′–O6′–CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) given in parentheses], four O6–C6 rotamers [referred to as Rα− (ψ ≈ −62°) and Rα+ (ψ ≈ 123°) for 1α as well as Rβ− (ψ ≈ −80.5°) and Rβ + (ψ ≈ 104.5°) for 1β, with the ψ values stated above] and two half-chairs arising from the ring-puckering deformation of a DHP moiety,18i.e., HC− (θ = −58.5°) and an opposite form HC+ (θ ≈ 58.5°) with the angle θ = C1–C2–C3–C4, were analyzed in detail. Hence, the twelve most promising candidate structures with all possible combinations of the local atom arrangements (geometric motifs) mentioned above, which were originally found by applying the GMMX random subroutine of PCMODEL44 (above 15 forms), constructed from incomplete geometries of two crystallographically independent molecules coexisting in the crystal structure of 1α (2 forms)18 and additionally built with the MM+ force field45 of Hyperchem46 by adequate modification of the geometry of other forms in our hands (7 remaining forms),47 were taken into account in all further studies for every two molecules (for full details, see Tables S4 and S5‡). In both structures found in the crystal of 1α, the CH2OAc unit adopts the gt90 form. As far as we know, the presence of such ‘bent’ rotamers in solution was not considered before.
O) given in parentheses], four O6–C6 rotamers [referred to as Rα− (ψ ≈ −62°) and Rα+ (ψ ≈ 123°) for 1α as well as Rβ− (ψ ≈ −80.5°) and Rβ + (ψ ≈ 104.5°) for 1β, with the ψ values stated above] and two half-chairs arising from the ring-puckering deformation of a DHP moiety,18i.e., HC− (θ = −58.5°) and an opposite form HC+ (θ ≈ 58.5°) with the angle θ = C1–C2–C3–C4, were analyzed in detail. Hence, the twelve most promising candidate structures with all possible combinations of the local atom arrangements (geometric motifs) mentioned above, which were originally found by applying the GMMX random subroutine of PCMODEL44 (above 15 forms), constructed from incomplete geometries of two crystallographically independent molecules coexisting in the crystal structure of 1α (2 forms)18 and additionally built with the MM+ force field45 of Hyperchem46 by adequate modification of the geometry of other forms in our hands (7 remaining forms),47 were taken into account in all further studies for every two molecules (for full details, see Tables S4 and S5‡). In both structures found in the crystal of 1α, the CH2OAc unit adopts the gt90 form. As far as we know, the presence of such ‘bent’ rotamers in solution was not considered before.
However, the rather highly incoherent conformational landscape was found in a general manner outlined above. Indeed, several trial structures of 1α and 1β proposed as privileged on the basis of standard ΔG data (and for which all GIAO-based δH and δC values were a priori predicted) were ‘not visible’ in the measured NMR spectra. More precisely, simulated 1H and 13C chemical shifts, obtained as Boltzmann-population-weighted sums of such NMR parameters computed for these individual forms of 1α and 1β, did not match the related values found experimentally. An occasional failure of such a common approach39 for flexible molecules is poorly documented in the literature dealing with NMR1,49 and infrared vibrational circular dichroism (VCD)50 spectroscopy studies in solutions. The usage of a ‘solution-phase environment (spectroscopic) match criterion’ instead of an ‘energetic criterion’ was suggested in some cases.1 These discrepancies most likely originate from known imperfections of the used theoretical approaches, e.g., not adequately mimicking the influence of surrounding media1c,49,50 and/or accounting for LD effects1c for multi-conformer systems, i.e., geometries, relative energies (ΔGs) and spectral responses of single contributors to their conformational families in solution. But we must also keep in mind that in certain physico-chemical and biophysical events, wide energy basins associated with ensembles of many structurally similar, highly flexible conformers (‘flat’ local minima) may be preferred over narrow energy wells of comparable depth and representing individual rigid forms (global minima), owing to the entropy factor.51
In view of the foregoing, the B3LYP-GD3BJ25c,e,34 flavor of DFT corrected for dispersion energy was applied in additional geometry reoptimizations carried out ‘in CHCl3’ starting with the 24 most promising B3LYP structures discussed earlier. However, all of these computational efforts, performed again using the standard IEF-PCM approach with UFF radii-cavities, led to very disappointing results. Indeed, much worse agreement between the values of predicted and observed NMR parameters was generally found for the structures of 1α and 1β optimized in this way (data not given). Analogous effects were also obtained with two other specialized DFT functionals, namely, M06-2X52 and ωB97X-D.53 Thus, the M06-2X structures were similar to extended B3LYP geometries, while more compact shapes predicted with ωB97X-D were close to those B3LYP-GD3BJ optimized (for views of selected forms, see Fig. 4, S12 and S13‡). These new geometries of 1α and 1β, described in terms of five torsion angles (Fig. 1), are collected in Tables S4 and S5‡ together with those of initial B3LYP structures. Also pertinent Gibbs free energies are given there, with the exception of such values for some B3LYP-GD3BJ optimized geometries having one small IHV frequency (up to 10i cm−1). For the latter structures some uncertainties in their G° values are expected, because the constituent zero-point vibrational energy (ZPVE) term is calculated only from non-imaginary frequencies.54
 | ||
| Fig. 4 Chemcraft 3D drawings of two types of non-physical structures found at the IEF-PCM(UFF,CHCl3)/B3LYP-GD3BJ/6-31+G(d,p) level: 14599comp (top) and 12272comp (bottom), see the text for details. | ||
The main modifications of the geometry of 1α and 1β concern the angle φ, which increases from ca. 125° to 156° for 1α, and χ, which decreases from ca. −180° to −123° or even −107° for 1α and 1β, respectively. Particularly large rotational freedom, manifested by a relatively wide low-energy valley, exists for the C1′–O6 rotation in 1α (Tables S4 and S5‡). The greatest changes are observed on going from B3LYP to compact structures ωB97X-D and especially B3LYP-GD3BJ (cf. Fig. S10 and S11 vs. S12 and S13‡). Thus, a large movement of the aromatic part of aglycone to the C2′ acetoxy groups primarily takes place for most forms of 1α (arrangement of the type I, changes in φ), while the C6′ acetoxy units in all their gg rotamers move strongly towards the C4′ acetoxy groups with unexpected formation of tg forms via a C5′–C6′ rotation (type II, χ → ca. −123°); the latter displacement is less pronounced for M06-2X (χ → ca. −148°). In turn, Me groups of C6′ acetoxy units in gt rotamers of 1β move strongly towards the 2a-Me group in a DHP ring of aglycone with the formation of stabilizing C–H⋯H–C attractions55 (type III, χ → ca. −108°); this change is marginal for M06-2X (χ → ca. −167°). Two B3LYP-GD3BJ-optimized non-physical geometries of the structures 14599comp and 12272comp56 with LD effects of the type I/II and III (the latter exemplified in 12272comp by two short dihydrogen CH⋯HC contacts of 2.327 and 2.753 Å), respectively, are shown in Fig. 4. The displacements of type I are a little similar to stabilizing intramolecular attractions originating from LD forces between aromatic rings and π-electron containing groups recognized in high-level correlated ab initio calculations concerning some oligopeptides and isolated small proteins in the gas-phase.25a,b,57
The aforementioned atom displacements and especially the presence of tg forms instead of gg ones in analyzed solution mixtures (contrary to the observation vide supra) were perhaps the reason for the lack of consistency between the computed and measured NMR data. Hence, one can suppose that use of these specialized DFT functionals (ωB97X-D and B3LYP-GD3BJ, in particular) is rather unsuitable for modeling the ground-state geometry of the title and most likely also other floppy molecules with small barriers to conformational changes owing to an overestimation of LD attractions. Some recent examples of transition-state structures optimized by these or other similar methods – and for which also too strong intramolecular LD effects (and so not wholly reliable ΔGs) were computed – were reported for B3LYP-D358 and M06-2X and ωB97X-D.59 Problems with the description of LD interactions in biologically relevant conformers (including sugars) by such a class of DFT methods were also identified by Goerigk.25j Therefore, it was obvious that only B3LYP optimized geometries of glucopyranosides 1 should be further considered. Our choice was in line with the conclusion of Roy et al.25h that the density functionals specifically designed to address dispersion behave rather erratically for some systems (but with a tendency to overestimate the strength of LD effects), while B3LYP can describe these interactions as well as or better than some specialized functionals.
As to small IHV frequencies found analytically for eight B3LYP-GD3BJ geometries of 1α and 1β, we decided to check whether the use of a standard IEF-PCM model of solvation was the most probable cause of such findings (as was suggested by one reviewer). Indeed, the IHV modes resulting from too short vdW radii of the lithium atom were found in the DFT study on some Li-containing species.60 Accordingly, all eight ‘wrong’ B3LYP-GD3BJ structures were recalculated using the three other vdW atomic radii, namely, UA0 and Bondi (both available in Gaussian 09) and IDSCRF.61 The latter, isodensity-based SCRF radii were recently evaluated61 and applied by the Fang group in mechanistic considerations,59,62 as a correction of the default IEF-PCM approach implemented within Gaussian 09. The new results thus obtained are collected in Table S6,‡ together with those concerning the precursor UFF radii-based structures. Inspection of this table revealed that the gradual change from UFF to IDSCRF via UA0 and Bondi radii gave good results in the majority of cases. Indeed, four positive or two slightly negative ωe values were computed using the IDSCRF radii but a lack of improvement is also found (2 forms). Especially erratic results were obtained for the structure 13787 including an outstanding ωe value of 12.5i cm−1 found by the use of the radii of Bondi. It should be noted that a high-quality integration grid and a convergence threshold were applied in all calculations;60,63 see Computational details. In conclusion, our results strongly suggest an imperfection of the IEF-PCM/B3LYP-GD3BJ approach. Indeed, all of these ‘wrong’ geometries are undoubtedly genuine energy minima because they are very similar to their ωB97X-D counterparts (or B3LYP-GD3BJ structures obtained with other vdW radii) showing real vibrational frequencies.64 Moreover, only the use of the B3LYP-GD3BJ functional provides such wrong results for various radii. Hence, all of the above-discussed IHV frequencies, being well within the range of accepted computational accuracies [∼±20 (ref. 65) or even ∼±50 (ref. 60) cm−1] arising from errors of the numerical integration procedures used in DFT calculations,63c are considered to be artificial. Our findings indicate, on the other hand, that further improvement of the existing implicit solvation models is possible.
To circumvent the whole problem concerning the title compounds 1, a non-classical ‘method of gradual exclusion’ had to be used to make the analysis tractable. Thus, it was realized that (i) an unusual gt90 rotamer, which was originally only found for two forms of 1α in our extensive MM search, can be safely discarded as a critical determinant of related δH data. Indeed, the δH values predicted for two anisochronous methylene protons at C6′ in the CHCH2OAc molecular unit, adopting such ‘bent’ gt90 forms, strongly deviate from the observed values by ca. −0.7 and +0.7 ppm for the prochiral H6′R and H6′S protons, respectively. In turn, two vicinal time-averaged J couplings within these units, measured for glucopyranosides 1α and 1β as 3JH5′,H6′S = 2.5 ± 0.2 Hz and 3JH5′,H6′R = 4.7 Hz,66 indicated, in view of the above assumption and our predicted JHH data given below in parentheses, that (ii) the contribution of gg forms (3JH5′,H6′S ≈ 2.3 Hz, 3JH5′,H6′R ≈ 2.2 Hz) to equilibrated mixtures must be approximately twice that of related gt forms (3JH5′,H6′S ≈ 1.4 Hz, 3JH5′,H6′R ≈ 9.1 Hz), because the measured 3JHHs are mainly due to motional averaging of such rotamers in solution. This finding was qualitatively consistent with the gg/gt/tg ratio of 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 49
9 and 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 proposed, respectively, for 2α and 2β having an identical sugar part, on the basis of 3JC5,H6s measured in C6D6 solution.32 Moreover, (iii) the participation of the puckers HC+ and HC− of a flexible DHP ring is most likely comparable, as very similar values of δH and δC were found for the 2a/2bgem-dimethyl groups. An analogous conclusion can also be drawn from the X-ray analysis of 1α showing the coexistence of two different half-chair forms in the crystal state.18 It should be also noted that all the above guidelines (i)–(iii) were fully in line with considerations of the effect of magnetic anisotropy of the 6′-O-acetyl carbonyl group43a and an aromatic core of the aglycone (diamagnetic ring current), respectively.
4 proposed, respectively, for 2α and 2β having an identical sugar part, on the basis of 3JC5,H6s measured in C6D6 solution.32 Moreover, (iii) the participation of the puckers HC+ and HC− of a flexible DHP ring is most likely comparable, as very similar values of δH and δC were found for the 2a/2bgem-dimethyl groups. An analogous conclusion can also be drawn from the X-ray analysis of 1α showing the coexistence of two different half-chair forms in the crystal state.18 It should be also noted that all the above guidelines (i)–(iii) were fully in line with considerations of the effect of magnetic anisotropy of the 6′-O-acetyl carbonyl group43a and an aromatic core of the aglycone (diamagnetic ring current), respectively.
As a result, only eight structures of every anomer of 1 optimized by the IEF-PCM(UFF,CHCl3)/B3LYP//6-31+G(d,p) method and denoted as forms 1αA to 1βH were further studied; their geometries and atomic coordinates are listed in Tables S4, S5, S15 and S16.‡ At this stage, Grimme's D3 scheme25c was post factum applied to account for the impact of weak intramolecular LD effects on related energetic data. More precisely, the total standard Gibbs free energy 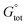 of every single form was approximated by a dispersion-corrected
of every single form was approximated by a dispersion-corrected  value considered as including a harmonic DFT contribution,
value considered as including a harmonic DFT contribution,  , plus a (negative) pairwise interatomic LD correction term Edisp,
, plus a (negative) pairwise interatomic LD correction term Edisp,
 (
( ), atomic pairwise vdW dispersion terms (DFT-D V3 data),67 corrected
), atomic pairwise vdW dispersion terms (DFT-D V3 data),67 corrected  data and contributions p2i (where i = A, B, C…H) of the forms 1αA–1βH to their equilibrium mixtures in simulated CHCl3 solutions are collected in Tables S7 and S9‡ together with the p1i values calculated, according to the Boltzmann distribution law, from the uncorrected
data and contributions p2i (where i = A, B, C…H) of the forms 1αA–1βH to their equilibrium mixtures in simulated CHCl3 solutions are collected in Tables S7 and S9‡ together with the p1i values calculated, according to the Boltzmann distribution law, from the uncorrected  (‘Boltzmann 1’ data). For completeness, the initial code names of all 16 finally selected conformers of 1 are also included. Because of the inherent limited accuracy of conventional DFT approaches, the differences in energies (Etots or G°s) being less than the ‘chemical accuracy’ of 4 kJ mol−1 means comparable thermochemical stability of the predicted structures.38c,49b This opinion is consistent with our findings on relative stability of both anomers of 1. Indeed, the difference in values of GDFT 390 and GDFT-D3 390 estimated ‘in CHCl3’ for their lowest-energy forms 1αA and 1βB amounts to 9.11 and 2.41 kJ mol−1, respectively, whereas ΔG390 = 1.87 kJ mol−1 follows from an experimental α/β ratio of 36
(‘Boltzmann 1’ data). For completeness, the initial code names of all 16 finally selected conformers of 1 are also included. Because of the inherent limited accuracy of conventional DFT approaches, the differences in energies (Etots or G°s) being less than the ‘chemical accuracy’ of 4 kJ mol−1 means comparable thermochemical stability of the predicted structures.38c,49b This opinion is consistent with our findings on relative stability of both anomers of 1. Indeed, the difference in values of GDFT 390 and GDFT-D3 390 estimated ‘in CHCl3’ for their lowest-energy forms 1αA and 1βB amounts to 9.11 and 2.41 kJ mol−1, respectively, whereas ΔG390 = 1.87 kJ mol−1 follows from an experimental α/β ratio of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 (vide supra). These results strongly indicate the need for the usage of dispersion corrections and suggest that the discrepancy in our GDFT-D3s is only 0.5 kJ mol−1. Consequently, energetic ordering gathered in Tables S7 and S9‡ that resulted from similar thermodynamic data were considered relatively good indicators.
64 (vide supra). These results strongly indicate the need for the usage of dispersion corrections and suggest that the discrepancy in our GDFT-D3s is only 0.5 kJ mol−1. Consequently, energetic ordering gathered in Tables S7 and S9‡ that resulted from similar thermodynamic data were considered relatively good indicators.
In the final stage of this research, the GIAO/DFT-based values of δH,Cs and a few J couplings predicted for the individual forms A–H of 1α and 1β were confronted with respective parameters of NMR spectra measured in solution by using a linear regression analysis (Computational details). Relative populations p3i of these conformers, roughly known from the foregoing discussion rooted in an NMR experiment, were used as our supplementary and complementary guidelines. The analysis of all of the structural information indicated that a simultaneous fitting of chemical-shift values and some diagnostic nJHH data regarding, respectively, the gem-dimethyl and CHCH2OAc units in both glucosides 1 was of crucial importance. The findings from such a combined experimental–theoretical approach supported by the statistical treatment are shown graphically in Fig. 5 and summarized in Table 1. All three relevant statistical indicators (r2, CRMSE, and CMAE; see Computational details) are given in the plots as estimates of the reliability of the results.
 | ||
Fig. 5 Scatter plots of DFT computed vs. experimental (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) δH,C data sets for the overall multi-component solution conformation of 1α (left) and 1β (right); for additional information see the text, Table 1 and Computational details. 1) δH,C data sets for the overall multi-component solution conformation of 1α (left) and 1β (right); for additional information see the text, Table 1 and Computational details. | ||
| i | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| a The greatest divergence in the pi populations is shown in bold type. b Corresponding HC pairs of DHP rings (with the ± sign of θ) for the same gg or gt form. c For full details, see Tables S7–S10. d Without the dispersion correction. e With the dispersion correction. f Without the correction for ZPVEDFT. g With the correction for ZPVEDFT. h For cut-off subsets of the σH,C data (see text). | ||||||||
| α-Anomer (1α) | ||||||||
| C5–C6 rotamer | gg | gt | gt | gg | gg | gt | gt | gg |
| HC pairb | I− | II+ | III− | IV− | I+ | II− | III+ | IV+ |
| p1i × 100, Boltzmann 1, %c,d | 18.8 |
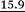
|
14.85 | 13.8 | 13.7 | 9.1 | 7.9 |

|
| p2i × 100, Boltzmann 2, %c,e | 24.6 5 |

|
11.1 | 17.2 | 17.7 5 | 4.7 5 | 6.4 |
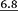
|
| p3i × 100, Boltzmann 3, %c,f | 21.9 |

|
4.6 | 22.2 | 17.2 | 4.85 | 3.0 |

|
| p4i × 100, Boltzmann 4, %c,g | 21.4 |

|
4.5 | 21.3 | 19.85 | 5.0 | 3.3 |

|
| p5i × 100, DFT/NMR data, % | 19 | 10 | 4 | 15 | 19 | 8 | 10 | 15 |
| p6i × 100, MP2/NMR data, %h | 19.5 | 10 | 5 | 15 | 18 | 7 | 10 | 15.5 |
| β-Anomer (1β) | ||||||||
| C5–C6 rotamer | gt | gg | gg | gg | gt | gt | gt | gg |
| HC pairb | I− | II− | II+ | III+ | I+ | IV− | IV+ | III− |
| p1i × 100, Boltzmann 1, %c,d |
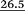
|
21.0 | 12.8 | 10.45 | 9.7 | 7.5 | 6.5 |

|
| p2i × 100, Boltzmann 2, %c,e |

|
27.0 | 15.6 | 13.6 5 | 7.6 | 5.6 | 4.6 |

|
| p3i × 100, Boltzmann 3, %c,f |

|
18.3 | 15.6 | 22.3 | 4.6 | 5.2 | 5.6 |

|
| p4i × 100, Boltzmann 4, %c,g |

|
20.15 | 16.7 | 21.7 | 5.4 | 5.6 | 5.6 |

|
| p5i × 100, DFT/NMR data, % | 14 | 14.5 | 10.5 | 20 | 16 | 0 | 5 | 20 |
| p6i × 100, MP2/NMR data, %h | 14 | 14.5 | 10.5 | 20 | 16 | 0 | 5 | 20 |
Inspection of Table 1 (and Tables S11 and S12,‡ with the p1 and p2-based values of selected NMR data, respectively) reveals that the use of dispersion corrected G°s really led to much better agreement between populations of single species accessed from the energy vs. DFT/NMR data, at least for the forms 1αA–1αF and 1βA–1βD; see italicized figures relating to p2 and p5 data. This result for the studied seven/eight-conformer objects is in full accord with a similar conclusion drawn from our previous study limited to the three-component systems.1c The 3D shapes of the most privileged gg forms, i.e., 1αA and 1αE (p3 = 0.19) as well as 1βD and 1βH (p3 = 0.20), are depicted in Fig. 6. This finding seems to indicate that the aglycone part of both anomers adopts mainly the same orientation with respect to their glycone moieties (the Me group at C5 close to O5′). All conformers of 1 with percentage populations are shown in Fig. S10 and S11.‡ It should be noted that the magnitude of r2 was not decisive in the analysis, because only very small changes in the magnitude of this correlation indicator were found for 1αA-1βH on going from the p1 (or p2) to p5 data (Tables 2, S11, and S12‡). In sharp contrast, a great change (from ∼1.0 to ∼1.8) in the gg/gt rotamer ratio was observed on coming from the p1 to p2 results, strongly suggesting that structures with the CHCH2OAc unit in the gg conformation are favored by LD forces.
| α-Anomer (1α) | β-Anomer (1β) | |||||||
|---|---|---|---|---|---|---|---|---|
| Nucleus j | Exp. | Calcda | Scaledb | −(δscaled − δobsd) | Exp. | Calcda | Scaledb | −(δscaled − δobsd) |
| H2a | 1.298 | 1.231c | 1.231 | 0.067 | 1.293 | 1.227c | 1.303 | −0.010 |
| H2b | 1.268 | 1.199 | 1.201 | 0.067 | 1.281 | 1.215 | 1.292 | −0.011 |
| C2a | 27.04 | 26.85 | 25.45 | 1.59 | 26.92 | 26.69 | 25.33 | 1.59 |
| C2b | 26.48 | 26.25 | 24.88 | 1.60 | 26.70 | 26.46 | 25.12 | 1.58 |
| H2a | 1.298 | 1.342d | 1.056 | 0.242 | 1.293 | 1.343d | 1.004 | 0.289 |
| H2b | 1.268 | 1.312 | 1.029 | 0.239 | 1.281 | 1.332 | 0.994 | 0.287 |
| C2a | 27.04 | 29.34 | 27.02 | 0.02 | 26.92 | 29.20 | 26.93 | −0.01 |
| C2b | 26.48 | 28.77 | 26.49 | −0.01 | 26.70 | 28.95 | 26.69 | 0.01 |
| Coupling H,H | Exp. | Calcde | — | −(δscaled − δobsd) | Exp. | Calcde | — | −(δscaled − δobsd) |
|---|---|---|---|---|---|---|---|---|
a
δ
calcdK,j = σK,TMS − (p3A·σK,A,j + p3B·σK,B,j + ⋯ + p3H·σK,H,j), K = H or C.
b
δ
scaledK,j = (δcalcdK,j − b)/a, for the least squares linear fitting values of the slope a and intercept b, see below and Fig. 5.
c GIAO/DFT data-based results.
d GIAO/MP2 data-based results.
e
J
HH = p3A·JA,HH + p3B·JB,HH + ⋯ + p3H·JH,HH (found at both theory levels for the DFT-level J-data); see Computational details.
f A binuclear (δH,C 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) regression analysis was applied, see also Computational details. 1) regression analysis was applied, see also Computational details.
|
||||||||
| 3 J H5′,H6′S | 2.34 | 2.06 | — | 0.28 | 2.74 | 1.98 | — | 0.76 |
| 3 J H5′,H6′R | 4.68 | 4.46 | — | 0.22 | 4.72 | 4.59 | — | 0.13 |
| 2 J H6′R,H6′S | (−)12.37 | −12.63 | — | 0.26 | (−)12.18 | −12.51 | — | 0.33 |
| DFT/NMRf | r C/H 2 = 0.99983, gg/gt = 2.13, CRMSE = 0.75, CMAE = 0.51, δcalcd = 1.0579 · δobsd − 0.0707 | r C/H 2 = 0.99977, gg/gt = 1.86, CRMSE = 0.87, CMAE = 0.61, δcalcd = 1.0566 · δobsd − 0.0765 | ||||||
| MP2/NMRf | r C/H 2 = 0.99930, gg/gt = 2.13, CRMSE = 0.73, CMAE = 0.58, δcalcd = 1.0784 · δobsd + 0.2031 | r C/H 2 = 0.99922, gg/gt = 1.86, CRMSE = 0.79, CMAE = 0.67, δcalcd = 1.0746 · δobsd + 0.2641 | ||||||
Overall, only a slightly weaker correlation between the predicted and experimental δH,C sets was found for 1β. Indeed, the greatest discrepancy in p2s/p5s (∼8%) was obtained for 1βE (Table 1). Nevertheless, only for these NMR-based populations very small differences in the simultaneously analyzed data of δH, δC and nJHH were found in a laborious but critical step in achieving very good reproduction of the observed values of chemical shifts of 2a/2bgem-dimethyl groups and JHHs in the CHCH2OAc unit. Additionally, population-averaged values of the other computed J data discussed in the text, i.e., 2JH3,H4 = 6.60, 4JH1′,H3′ = −0.45, 4JH1′,H5′ = −0.72, 4JH2a,H2b = 0.46 and 1JC1′,H1′ = 168.79 Hz for 1α as well as 2JH3,H4 = 6.59, 4JH2a,H2b = 0.47 and 1JC1′,H1′ = 161.87 Hz for 1β, were obtained in good agreement with those found experimentally; for a scatter plot of selected relationships Jcalcd avHHvs. JobsdHH (r2 = 0.99900) see Fig. S14.‡
Moreover, the structure 1βA, observed as its enantiomeric form in the crystal of 1β,42 was relatively strongly privileged in CHCl3 solution (p5 = 0.14), in sharp contrast to the case of the α-anomer. Indeed, close inspection of the crystal structure of 1α18 suggests that the coexistence of the four species with a ‘bent’ gt90 rotamer of the CHCH2OAc segment (different from those located in our MM search) in the unit cell is due to crystal packing effects largely dominated by intermolecular LD contacts of the CH⋯HC type,55 involving inter alia the Me group of this unit interacting with 2a/2b-gem-dimethyl groups of a neighboring molecule (see also above). A great similarity between angles φ, ψ and χ in both these main gt90 conformers and their related non-physical solution M06-2X structures optimized with allowance for LD forces supports this conception (Table S4,‡ the forms 14229 and 14913). As a result, ‘extended’ gt and especially gg rotamers of this molecular unit in both glucosides 1 under study are favored in the solution state.
On the other hand, a definitive and unambiguous assignment of the 1H/13C NMR signals of 2a/2bgem-dimethyl groups was simultaneously acquired in our analysis. Pertinent chemical-shift values are only slightly more differentiated for 1α, but a downward Me substituent labeled 2a was always found to resonate downfield of its upward counterpart 2b (Fig. 1 and Table 2); the spatial relationship between these groups adopted throughout this paper is the same as that used before.13a Therefore, one cannot speak about an equatorial and axial position of the 2a and 2b methyl group, respectively, as was considered previously.13a In this case each of these two Me groups occupies both such orientations during low-energy interconversions (rapid in the NMR timescale) between two different half-chair forms of a DHP ring.13a,68 Interestingly, an experimental ΔδC/ΔδH ratio amounts to 18.5 ± 0.2 for both anomers and also the average values of δ are very similar, δH = 1.285 ± 0.002 ppm and δC = 26.785 ± 0.025 ppm. All the above facts indicate that the structural features and dynamics of the DHP part of both anomeric glucosides 1 in solution are comparable.
It should also be noted that the gg/gt rotamer ratio determined for 1α is slightly greater than that found for 1β [the α/β ratio (of both gg/gt ratios) ∼1.15], see Table 2, similarly to that estimated32 for the pair 2α and 2β having the same sugar moiety (α/β ∼ 1.3). In light of these results, more recent literature data43c suggesting the gg/gt ratio of 0.61 and 0.52 for 2α and 2β, respectively, are questionable, but the associated α/β ratio ∼1.2 is correct.
The inherent uncertainty of the finest GIAO/DFT-based p5i values is difficult to estimate, due to possible summation and/or cancellation of errors in two subsequent computations of geometries and chemical shifts (or  ). The differences between the p2i and p5i results found for 1 (Table 1) suggest that such an uncertainty is of the order of 4–7%, under the assumption of perfect correctness of p2i data. But one should remember a modest accuracy of typical ΔGs and so the relatively large errors in calculations of p1is and perhaps also, to some extent, p2is. Thus, it seems that the uncertainty in question is comparable with that reported previously for the best example of three-component systems studied analogously (most likely <5%).1c So it was concluded that the values of p2is and p5is are consistent with each other within their errors; however, very good agreement with the NMR experimental observations was found for the latter data set only. Hence, one can invoke again the concept of superiority of the ‘solution match criterion’ over the ‘thermodynamic criterion’, stressing simultaneously that accounting for weak LD forces in calculations of ΔGs and thus Boltzmann distributions is mandatory in all such cases. A very similar conclusion was drawn earlier.1c
). The differences between the p2i and p5i results found for 1 (Table 1) suggest that such an uncertainty is of the order of 4–7%, under the assumption of perfect correctness of p2i data. But one should remember a modest accuracy of typical ΔGs and so the relatively large errors in calculations of p1is and perhaps also, to some extent, p2is. Thus, it seems that the uncertainty in question is comparable with that reported previously for the best example of three-component systems studied analogously (most likely <5%).1c So it was concluded that the values of p2is and p5is are consistent with each other within their errors; however, very good agreement with the NMR experimental observations was found for the latter data set only. Hence, one can invoke again the concept of superiority of the ‘solution match criterion’ over the ‘thermodynamic criterion’, stressing simultaneously that accounting for weak LD forces in calculations of ΔGs and thus Boltzmann distributions is mandatory in all such cases. A very similar conclusion was drawn earlier.1c
The foregoing results based on the DFT data were finally compared with those arising from the total electronic-nuclear energies, Etots, and GIAO predictions of σKs obtained for solutions of 1αA–1βH at the MP269/6-311+G(2d,p) and MP2/6-311G(d,p) levels, respectively. As to energy data and thus conformer populations p3 and p4 found from relative energies ΔEtot and ΔE0 (Tables 1, S8 and S10‡),70 the new results are generally consistent with DFT findings, with the exception of cases of 1αB, 1αH, 1βA, and 1βH (Table 1, numbers underlined). But also in these instances, the MP2 data-derived results are in agreement with the trends observed on going from p1s to p2s within the limits of inherent errors of both theoretical models.71,72 Also ‘mean’ populations found for related p2/p4 pairs, namely 8.1, 13.35, 12.0 and 13.4%, are in line with the p5 DFT/NMR data. As previously stated, the greatest discrepancy between p4 and p6 values is found for 1βE. Therefore, one can then consider, based on such new data (Table 1, p3s and p4s), that the results of MP2 calculations are qualitatively consistent with the DFT-D3-type intramolecular LD attractions in the systems 1α and 1β estimated here. Moreover, according to the aforementioned data, it seems that the inclusion of DFT-level ZPVE terms in calculations of ΔE0s and so p6 values was fully justified, despite some warnings on this topic concerning systems with the relatively flat potential energy hypersurfaces.73
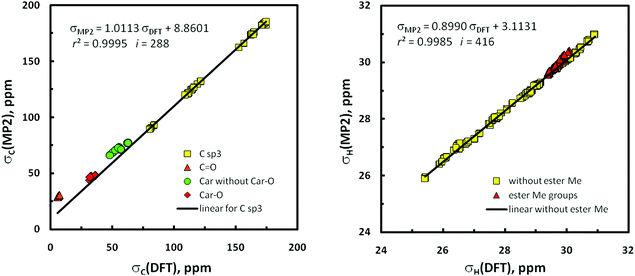
The situation with the results of GIAO/MP2 calculations74 is more complicated. Generally, these data seem to be by far less exact than related DFT-level findings concerning the same geometries and being in good agreement with the empirical observations. Instead of very good correlation between σCs computed at the MP2 vs. DFT level, awaited in light of the pioneering results of Wiberg (r2C = 0.9994),75 only a good relationship was found for all 13C nuclei in the 16 conformers under study (r2C = 0.9977 for i = 16 × 28 unique nuclei);76 the correlation between all σHs is still weaker (r2H = 0.9946, i = 16 × 38). Evidently, both models of chemistry provided different GIAO predictions for 13C nuclei involved in π-systems (especially in the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds). Indeed, exclusion of all sp2 hybridized carbons in the σC set gives r2C = 0.99953 (Fig. 7, right); four different clusters of data points due to sp2 carbons are worth mentioning. The same is also true to some degree with σHs, and omission of all protons of the methyl ester groups experiencing an anisotropic effect of neighboring C
O bonds). Indeed, exclusion of all sp2 hybridized carbons in the σC set gives r2C = 0.99953 (Fig. 7, right); four different clusters of data points due to sp2 carbons are worth mentioning. The same is also true to some degree with σHs, and omission of all protons of the methyl ester groups experiencing an anisotropic effect of neighboring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups leads to r2H = 0.99845 (Fig. 7, left). Therefore, only the use of two such cut-off subsets of the σ values in subsequent binuclear δcalcdH,C(MP2) vs. δobsdH,C correlations important for this investigation was fully legitimate (for related plots, see Fig. S15‡). But the MP2/NMR populations thus obtained (p6i values, Table 1) are slightly less reliable owing to a lack of some data points – as previously stated, the best reproduction of ‘diagnostic’ patterns of δKs concerning the gem-dimethyl groups at C2 and nJHHs around C6′ was of crucial importance. Slightly changed p6is were thus obtained for 1α, but all attempts to correct the p5i data used as tentative starting values for 1β were unsuccessful.
O groups leads to r2H = 0.99845 (Fig. 7, left). Therefore, only the use of two such cut-off subsets of the σ values in subsequent binuclear δcalcdH,C(MP2) vs. δobsdH,C correlations important for this investigation was fully legitimate (for related plots, see Fig. S15‡). But the MP2/NMR populations thus obtained (p6i values, Table 1) are slightly less reliable owing to a lack of some data points – as previously stated, the best reproduction of ‘diagnostic’ patterns of δKs concerning the gem-dimethyl groups at C2 and nJHHs around C6′ was of crucial importance. Slightly changed p6is were thus obtained for 1α, but all attempts to correct the p5i data used as tentative starting values for 1β were unsuccessful.
On the whole, satisfying agreement with the earlier DFT/NMR results was found (Table 1). The discrepancies between the DFT and MP2-derived values of Δδ (= δscaled − δobsd) concerning the 2a/2b Me groups arise mainly from different slopes of related best-fit lines (Table 2; cf.Fig. 5 and S15‡). Such Δδ data obtained for p3 and p4 abundances (Tables 2, S13 and S14‡) are less consistent, but those found for the p4s are better. Also the gg/gt ratios improve on going from p3 to p4 values (1α 5.0 → 4.7, 1β 4.1 → 3.7). However, what must be emphasized here is that all these data are incompatible with the NMR spectroscopic observations (gg/gt ∼ 2, vide supra). Interestingly, the reverse trend in gg/gt is observed on coming from p1 to p2 data evaluated from the DFT results (1α 1.1 → 2.0, 1β 1.0 → 1.7; Tables S11 and S12‡). Thus, is it possible that dispersive attractions (?) between H5′ and the two H6′ atoms in gg rotamers of the CHCH2OAc fragment of systems 1 (see Fig. 3) are favored too much in MP2 and underestimated in B3LYP treatments? In summary, one can consider that the results emerging from MP2 calculations confirm the earlier DFT results, though certain disagreements between them (and with the experiment) were also identified. Particularly interesting are the foregoing discrepancies in σCs predicted at both levels of theory.
For some other findings, it was recognized that the large differences ΔH5 = δH5α − δH5β, observed for anomeric pairs of several O-glycosides of PMC (vide supra, see also Table S2‡), must arise from an aromatic ring-current effect of the constituent chroman system. Indeed, inspection of molecular representations of all forms A–H of 1α (Fig. S10‡) revealed that their hydrogen atoms at C5′ are situated within the deshielding cone produced by circulating π-electrons. By contrast, a relatively small shielding of both H6′ protons (in relation to those occurring in 1α) is suggested on the basis of 3D drawings of all forms of 1β (Fig. S11‡), in full agreement with the experiment.
Conclusions
In this combined theoretical/experimental study, two highly flexible glucoside derivatives of PMC (a model compound of α-tocopherol) were used to test several current calculational protocols accessible for predicting the overall shapes of multi-conformer systems and population-weighted averages of their NMR parameters based on high-quality spectroscopic data. A special emphasis was given to accounting for the impact of intramolecular LD effects on the geometries and relative Gibbs free energies (ΔGs) of various forms coexisting in solution. Detection of a few small 4JHH coupling constants in both molecules is also worth mentioning.Of the many possible single conformers of glucopyranosides 1α and 1β localized in initial Monte Carlo MM searches, only twelve of them were finally recognized in quantum-chemical calculations to contribute significantly (≥10%) to related conformational mixtures in solution, where solvent effects on geometries and NMR spectral properties of the analyzed solutes were mainly simulated with an IEF-PCM(UFF,CHCl3) approach of implicit solvation. Simultaneous matching of computed vs. observed NMR chemical-shift sets by applying the binuclear (δH,C 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) linear regression analysis was considered the best procedure for disentangling the conformational preferences of these systems. The presence of their 2a/2b-gem-dimethyl and CHCH2OAc structural units, as sensitive intrinsic magnetic probes for detecting time-averaged spatial arrangements of the atom arrays in their nearest electronic environments (local solute geometries), was recognized to be of crucial importance for achieving good reproduction of solution NMR spectra of both anomers.
1) linear regression analysis was considered the best procedure for disentangling the conformational preferences of these systems. The presence of their 2a/2b-gem-dimethyl and CHCH2OAc structural units, as sensitive intrinsic magnetic probes for detecting time-averaged spatial arrangements of the atom arrays in their nearest electronic environments (local solute geometries), was recognized to be of crucial importance for achieving good reproduction of solution NMR spectra of both anomers.
Regarding the molecular structure of 1α and 1β, the four DFT functionals including three with a priori corrections for attractive LD forces (M06-2X, ωB97X-D and B3LYP-GD3BJ) gave different geometries. The best results were found with B3LYP, while the two last specialized DFT methods afforded tg rotamers of the CHCH2OAc fragment instead of related gg forms in contradiction with the experiment. The advantage of the application of IDSCRF over default UFF radii in the IEF-PCM simulations of solvation was simultaneously shown for several B3LYP-GD3BJ optimized structures having one small imaginary vibrational frequency. All these findings strongly suggest that functional ωB97X-D and especially B3LYP-GD3BJ are rather not suitable for modeling the ground-state geometry of highly flexible molecules. Moreover, some serious problems with the IEF-PCM/B3LYP-GD3BJ approach were found.
The Gibbs free energies of individual forms of 1α and 1β optimized by the B3LYP/6-31+G(d,p) method were subjected to vdW (DFT-D3) corrections for LD effects to give the respective GDFT-D3s. The latter values gave (via Boltzmann statistics) estimated populations of single forms in the solution mixtures (p2is) being in much better agreement with NMR data-based populations (p5is) than those calculated for uncorrected GDFTs (p1is). Related p2 and p5 values were found to be practically equivalent within their error limits, but only the latter values showed very good agreement with the observation. Very similar conformer populations were also derived from the MP2/NMR data (p6is). These findings confirm the need to post factum perform LD corrections in DFT studies of this kind.
A gg/gt rotamer ratio of ∼2.0 was established for the CHCH2OAc fragment of both glucosides on the basis of DFT data (the MP2 energetic results give a considerably overestimated value of ∼4.2). Also such a ratio, estimated from the GDFT-D3 data, was much better than that found from the initial GDFTs (∼1.8 vs. ∼1.0). The more compact gt90 rotamer of this unit was not recognized in solution and so its presence in the crystal structure of 1α originates evidently from packing effects. In contrast, its gt rotamer identified in the crystal of 1β was found to be one of the five predominant forms in solution. It was also established that the differences ΔH5 = δH5α − δH5β, observed for anomeric pairs of some O-glycopyranosides of PMC, are due to the aromatic ring-current effect of a chroman skeleton. Hence, this parameter is proposed as a determinant of stereochemistry at anomeric centers in molecules of this kind.
All the main findings of this work were confirmed by additional calculations performed at the MP2 level. Simultaneously, some interesting discrepancies in the values of σCs predicted at both theory levels were recognized. One can suppose that with the applied (or equivalent)77 MM/DFT methodology and a careful analysis of the results, it is possible to find all, or at least the huge majority, of the low-energy conformers of various other small- to medium-sized flexible molecules. Hence, we believe that our results prove useful for guiding similar joint NMR spectroscopic/DFT computational studies on further multi-conformer systems in solution, especially those having the sugar moiety as a structural motif.
Computational details
Geometry optimization, vibrational frequency and energy calculations
A stochastic conformational search for minima on the potential energy hypersurfaces of the objects 1α and β was performed with the Global-MMX (GMMX) subroutine built into the PCMODEL 8.5 package.44,78 Specifically, a mixed MM protocol,79 based on Monte-Carlo (MC) procedures used originally in the BAKMDL program,80 was employed in which randomly selected atoms of the semi- and saturated (hetero)cyclic rings and all of the seven rotatable bonds were randomly moved in the Cartesian70,81 and dihedral angle82 space and energies of such formed species were subsequently minimized within the MMX (1986) force field.83 About 40 cycles of GMMX calculations, each embracing 5000 MC searching steps, were performed for every molecule with the bulk relative permittivity (dielectric constant, ε) of 4.71 (ref. 34) used for a rough simulation of the CHCl3 environment. A search was continued until ∼180 unique energetically lowest energy lying structures of each anomer were generated within an arbitrarily chosen 25.1 kJ mol−1 energy window. The thus-obtained MMX models were then subjected to a gradient geometry optimization, initially at HF/3-21G84 and then (after sorting and removing duplicates) at HF/6-31G(d) levels, by using the Gaussian 09 suite of electronic structure programs.34 All types of geometric motifs of various occurring rotameric forms were recognized in this way. Initial MMX structures of the seven not originally located conformers were built without any changes in atom numbering through adequate modifications of the partially (or fully) optimized geometries of the relevant closely related forms,47 by using Hyperchem46 (MM+ force field);45 for full details see footnotes to Tables S4 and S5.‡ The MM+ calculations were followed by MMX optimizations in these additional cases. It should be noted that very large differences in energetic ordering of the input MM models of 1α and β (established via their MMX energies) and pertinent HF/3-21G optimized structures (via the ΔEtot data) were generally found; a similar situation was observed previously.23bFinal geometry refinement of the ‘best’ structures was carried out at the double-ζ (DZ) valence quality level of theory using the hybrid B3LYP36a–c exchange–correlation functional, as implemented in the Gaussian code,2c,36d in conjunction with the 6-31+G(d,p) basis set recommended for DFT calculations of energy data,36f especially for the systems with lone electron pairs on heteroatoms.85 For the sake of accuracy, the ‘Tight’ SCF and Opt convergence criteria were used in all computations.1c,60,63 Moreover, a fine-pruned (150,590)86 numerical integration grid having 150 radial shells and 590 angular points per shell was always selected applying the Int(Grid=150590) keyword,63d–f because of soft modes coming from dynamic phenomena of methyl group rotations.34 Simultaneously, an attempt to evaluate solvent influences on the solute structures and properties was made within an equilibrium solvation protocol20b of an integral equation formalism-polarizable continuum model (IEF-PCM)19,20 of solvation, by employing the UFF atomic radii when constructing the solvent cavity and other default parameters. Analogous optimizations in the implicit CHCl3 solvent were also carried out with the use of a 6-31+G(d,p) basis set and three specialized DFT functionals a priori corrected for the contributions of LD effects, namely, B3LYP-GD3BJ [i.e., B3LYP with the addition of the D3 version of Grimme's dispersion25c with Becke–Johnson damping25e (Gaussian keyword: B3LYP/base EmpiricalDispersion=GD3BJ)],34 M06-2X,52 and ωB97X-D.53 Some additional structures were also optimized with IEF-PCM/B3LYP-GD3BJ applying three other atomic radii, i.e., UA0 and Bondi (both available in Gaussian 09) and IDSCRF61 (see also text). Fully-relaxed geometries of 16 finally considered forms of 1 found at the IEF-PCM(UFF,CHCl3)/B3LYP/6-31+G(d,p) level are given in Tables S15 and S16,‡ while their 3D shapes are depicted in Fig. S10 and S11,‡ using graphical representations created with the ChemCraft program.87
Moreover, vibrational wavenumbers ωe were always evaluated in the rigid rotor-harmonic oscillator-ideal gas approximation of vibrational modes that was used in the frame of the same DFT method, to verify whether the located stationary points represented true minima (Nimag = 0) on the Born–Oppenheimer ground-state energy hypersurfaces of analyzed structures and to determine their unscaled ZPVE corrections and Gibbs free energies, 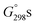 , at standard ambient temperature and pressure (298.15 K, p = 1 atm), i.e., close to the NMR recording temperature of 302 ± 2 K. Finally, all of these
, at standard ambient temperature and pressure (298.15 K, p = 1 atm), i.e., close to the NMR recording temperature of 302 ± 2 K. Finally, all of these  data were corrected for vdW dispersion effects (LD forces)1c,24,25 as was explained in the text, by using respective B3LYP(G) specific D3 Grimme's DFT-D V3 correcting terms25c computed with the ORCA package.67
data were corrected for vdW dispersion effects (LD forces)1c,24,25 as was explained in the text, by using respective B3LYP(G) specific D3 Grimme's DFT-D V3 correcting terms25c computed with the ORCA package.67
In addition, individual total energies, Etots, of all the forms 1αA–1βH were single-point calculated74 by the second-order Møller–Plesset (MP2) perturbation method69 with the 6-311+G(2d,p) basis set of triple-ζ (TZ) valence quality.70 These computations were additional jobs in the MP2 runs carried out as is described below in the section on NMR spectra predictions.
For assessing relative abundances of individual forms in the conformational equilibria in solution, the fractional Boltzmann population (mole fraction, pi) of each entity was found using the Boltzmann distribution function,  , where j is the number of species in thermal equilibrium, R is the ideal gas constant, T is the system absolute temperature set at 298.15 K, and ΔGi° is the ΔG value of the ith form relative to the energy of the most stable conformer. For the MP2-level results, ΔE0s were used instead of ΔG values in the calculation of p3 and p4 data.70
, where j is the number of species in thermal equilibrium, R is the ideal gas constant, T is the system absolute temperature set at 298.15 K, and ΔGi° is the ΔG value of the ith form relative to the energy of the most stable conformer. For the MP2-level results, ΔE0s were used instead of ΔG values in the calculation of p3 and p4 data.70
Prediction of NMR spectra
Single-point GIAO2 formalism-based computations of isotropic NMR chemical shielding constants, σKs, were carried out at the IEF-PCM(UFF,CHCl3)/mPW1PW91/6-311+G(2d,p)35 level on the IEF-PCM(UFF,CHCl3)/B3LYP/6-31+G(d,p) computed structures, by using Gaussian 09. Our approach88 was similar to that used by the Tantillo research group;3c,35b,c however, these authors applied another solvent continuum model and used the gas-phase instead of (probably much better)89 the solution-phase optimized solute structures used here. According to the classical tetramethylsilane (TMS) based protocol, the relative chemical shift, δK, of a given nucleus K in each molecular entity is defined as δcalcdK [ppm] = σrefK − σcalcdK. For the thus predicted 1H and 13C NMR spectra, σrefK is equal to 31.7023 and 186.9100 ppm, respectively, as was computed in simulated CHCl3 solution – analogously to that mentioned above – for the exact Td symmetry90 molecule of TMS as a dual-reference δK standard. Several other combinations of functional [B3LYP-GD3BJ,25c,e,34 M06-2X52 or ωB97X-D53 (first step) and mPW1PW9135a (second step)] and basis set [6-31+G(d,p) (first step) or 6-311+G(2d,p) (second step)] were used in additional GIAO NMR predictions. All of these solution-state calculations were performed on the corresponding structures fully pre-optimized at the DZ quality level, see also text. Moreover, supplementary74 time-consuming GIAO predictions of σKs were performed at the IEF-PCM(UFF,CHCl3)/MP2/6-311G(d,p)//IEF-PCM(UFF,CHCl3)/B3LYP/6-31+G(d,p) level for all 16 forms 1αA–1βH in order to verify the correctness and internal consistency of the GIAO/mPW1PW91 results mentioned above, see text and also Fig. 7 and S15.‡ These MP2 runs were computationally very demanding tasks. After several initial tests, we were able to perform a single GIAO NMR calculation in 8–9 days, by using 24 processors (2.50 GHz), 128 GB of memory, and at least 7.2 TB of scratch disk space for temporary storage of data. The σcalcdKs obtained in all of these cases were, as above, referred to TMS applying σrefK terms evaluated at the same computational level: σrefK (MP2) of 31.8587 and 198.8873 ppm, respectively.In addition, some indirect couplings, nJKL, were single-point computed for CDCl3 solutions of 1 at the IEF-PCM(UFF,CHCl3)/B3LYP/IGLO-II level1c,91 with Gaussian 09. An extended NMR property-oriented IGLO-II basis set of Huzinaga modified next by Kutzelnigg and coworkers (also known as the HII or BII set)92 and widely used in predicting JKL data91,93 was downloaded from the EMSL Basis Set Library.94 The five so-called pure d basis functions were employed for non-hydrogen atoms in all the NMR calculations mentioned above.
The GIAO computed σHs of each of the three mutually exchanging hydrogen atoms in the Me groups were arithmetically averaged to produce a single σH (or δH) value for each Me group as a whole; the same concerns also the two methylene groups of the highly flexible DHP rings. A linear regression analysis of the relationships between the predicted and observed NMR parameters (δKs, in particular) was achieved by a least-squares method; see also footnote b to Table 2. More precisely, the calculated data were plotted as usual1,23b,37 against experimental ones; however binuclear1a,37,38 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 correlations, δcalcdH,Cvs. δobsdH,C, were applied instead of two separate classical mononuclear relationships. Such an associated H,C approach was strongly suggested by the analysis of the problems entailed inter alia in our previous study dealing with multiple (>2) conformers,1c in which the application of δCs for assessing populations of the single forms in solution was unsuccessful. The case of the superiority of structural results coming from the GIAO-derived δHs over those from related δC data was reported by Koskowich et al.95
1 correlations, δcalcdH,Cvs. δobsdH,C, were applied instead of two separate classical mononuclear relationships. Such an associated H,C approach was strongly suggested by the analysis of the problems entailed inter alia in our previous study dealing with multiple (>2) conformers,1c in which the application of δCs for assessing populations of the single forms in solution was unsuccessful. The case of the superiority of structural results coming from the GIAO-derived δHs over those from related δC data was reported by Koskowich et al.95
The three relevant statistical metrics, i.e., a square of the Pearson correlation coefficient (r2), the corrected root-mean-square error [CRMSE equal to RMSE49b,95,96 with the valuescaled data applied instead of the valuecalcd ones] and the corrected mean absolute error [CMAE,97 defined as (Σi|valuescaled − valueobsd|)/number of comparisons (i)], were used throughout the paper as estimates of uncertainties of the results. The greater value of r2 (also called coefficient of determination and showing correlation significance) was considered as an indication of better adjustment of correlated data sets. All of the statistical analysis was performed using an MS Excel 2010 spreadsheet.
Acknowledgements
R. B. N. is grateful to Prof. Laurence A. Nafie (Syracuse University, USA) for the stimulating exchange of letters regarding the VCD spectra. He also thanks Prof. De-Cai Fang (Beijing Normal University, China) for providing the SCRF-RADII program and Dr Piotr Matczak (University of Łódź) for help in installing this software. This work was supported by the computer facilities and the Gaussian 09 software in the Academic Computer Centre CYFRONET (AGH – University of Science and Technology, Kraków) through computational grant No. MNiSW/SGI3700/UŁódzki/057/2010 and MNiSW/Zeus_lokalnie/UŁódzki/016/2013 (to R. B. N.). R. B. N. is also indebted to the staff of CYFRONET for their assistance in conducting MP2 calculations on the Prometheus supercomputer (within PL-Grid). The authors also thank two anonymous reviewers for their criticism, valuable comments and suggestion of some additional calculations, which helped us to improve the paper.Notes and references
- (a) E. Michalik and R. B. Nazarski, Tetrahedron, 2004, 60, 9213–9222 CrossRef CAS; (b) R. B. Nazarski, J. Phys. Org. Chem., 2009, 22, 834–844 CrossRef CAS; (c) R. B. Nazarski, B. Pasternak and S. Leśniak, Tetrahedron, 2011, 67, 6901–6916 CrossRef CAS.
- (a) R. Ditchfield, Mol. Phys., 1974, 27, 789–807 CrossRef CAS and references cited therein; ; (b) K. Wolinski, J. F. Hilton and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS; (c) J. R. Cheeseman, G. W. Trucks, T. A. Keith and M. J. Frisch, J. Chem. Phys., 1996, 104, 5497–5509 CrossRef CAS.
- (a) T. Helgaker, M. Jaszuński and K. Ruud, Chem. Rev., 1999, 99, 293–352 CrossRef CAS PubMed; (b) See also some chapters in Calculation of NMR and EPR Parameters. Theory and Applications, ed. M. Kaupp, M. Bühl and V. G. Malkin, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004 Search PubMed (c) M. W. Lodewyk, M. R. Siebert and D. J. Tantillo, J. Chem. Rev., 2012, 112, 1839–1862 CrossRef CAS PubMed.
- G. W. Burton and K. U. Ingold, Acc. Chem. Res., 1986, 19, 194–201 CrossRef CAS.
- (a) P. B. Nielsen, A. Müllertz, T. Norling and H. G. Kristensen, Int. J. Pharm., 2001, 222, 217–224 CrossRef CAS PubMed; (b) C.-C. Chang, J.-J. Lee, C.-W. Chiang, T. Jayakumar, G. Hsiao, C.-Y. Hsieh and J.-R. Sheu, Pharm. Biol., 2010, 48, 938–946 CrossRef CAS PubMed.
- (a) J. R. Sheu, C. R. Lee, G. Hsiao, W. C. Hung, Y. M. Lee, Y. C. Chen and M. H. Yen, Life Sci., 1999, 65, 197–206 CrossRef CAS PubMed; (b) J. R. Sheu, C. R. Lee, C. C. Lin, Y. C. Kan, C. H. Lin, W. C. Hung, Y. M. Lee and M. H. Yen, Br. J. Pharmacol., 1999, 127, 1206–1212 CrossRef CAS PubMed.
- Y. J. Suzuki and L. Packer, Biochem. Biophys. Res. Commun., 1993, 193, 277–283 CrossRef CAS PubMed.
- T. A. Thompson and G. Wilding, Mol. Cancer Ther., 2003, 2, 797–803 CAS.
- D. Liang, J. Lin, H. B. Grossman, J. Ma, B. Wei, C. P. Dinney and X. Wu, Cancer, Causes Control, Pap. Symp., 2008, 19, 981–992 CrossRef PubMed.
- (a) S. Witkowski and P. Wałejko, Z. Naturforsch., B: Chem. Sci., 2001, 56, 411–415 CAS; (b) S. Witkowski and P. Wałejko, Z. Naturforsch., B: Chem. Sci., 2002, 57, 571–578 CAS; (c) A. Hryniewicka, P. Wałejko, J. Morzycki and S. Witkowski, Pol. J. Chem., 2009, 83, 78–80 Search PubMed.
- T. Parman, D. I. Bunin, H. H. Ng, J. E. McDunn, J. E. Wulff, A. Wang, R. Swezey, L. Rasay, D. G. Fairchild, I. M. Kapetanovic and C. E. Green, Toxicol. Sci., 2011, 124, 487–501 CrossRef CAS PubMed.
- (a) K. Shimoda, Y. Kondo, K. Abe, H. Hamada and H. Hamada, Tetrahedron Lett., 2006, 47, 2695–2698 CrossRef CAS; (b) R. K. Uhrig, M. A. Picard, K. Beyreuther and M. Wiessler, Carbohydr. Res., 2000, 325, 72–80 CrossRef CAS PubMed; (c) T. Satoh, H. Miyataka, K. Yamamoto and T. Hirano, Chem. Pharm. Bull., 2001, 49, 948–953 CrossRef CAS PubMed.
- (a) S. Witkowski, D. Maciejewska and I. Wawer, J. Chem. Soc., Perkin Trans. 2, 2000, 1471–1476 RSC and references cited therein; ; (b) S. Witkowski and I. Wawer, J. Chem. Soc., Perkin Trans. 2, 2002, 433–436 RSC.
- (a) I. Wawer and S. Witkowski, Curr. Org. Chem., 2001, 5, 987–999 CrossRef CAS; (b) S. Witkowski, K. Paradowska and I. Wawer, Magn. Reson. Chem., 2004, 42, 863–869 CrossRef CAS PubMed; (c) D. K. Stępień, M. K. Cyrański, Ł. Dobrzycki, P. Wałejko, A. Baj, S. Witkowski, K. Paradowska and I. Wawer, J. Mol. Struct., 2014, 1076, 512–517 CrossRef.
- M. Górecki, A. Suszczyńska, M. Woźnica, A. Baj, M. Wolniak, M. K. Cyrański, S. Witkowski and J. Frelek, Org. Biomol. Chem., 2014, 12, 2235–2254 Search PubMed.
- The numbering of carbon atoms and the Nomenclature of Tocopherol and Related Compounds as proposed by the IUPAC-IUB Joint Commission on Biochemical Nomenclature (Recommendations 1981)17 was used. In the sugar part of both the examined molecules, the numbering with primes (1′, 2′, 3′ etc.) was applied throughout this work.
- See, e.g., P. Karlson, H. B. F. Dixon, C. Liébecq, K. L. Loening, G. P. Moss, J. Reedijk, S. F. Velick and J. F. G. Vliegenthar, Pure Appl. Chem., 1982, 54, 1507–1510 Search PubMed.
- K. Brzezinski, P. Wałejko, A. Baj, S. Witkowski and Z. Dauter, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, o718–o718 CAS.
- J. Tomasi, B. Mennucci and E. Cancès, J. Mol. Struct. (THEOCHEM), 1999, 464, 211–226 CrossRef CAS and references cited therein.
- (a) J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed; (b) G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 114110 CrossRef PubMed.
- (a) J.-Y. Tao, W.-H. Mu, G. A. Chass, T.-H. Tang and D.-C. Fang, Int. J. Quantum Chem., 2013, 113, 975–984 CrossRef CAS; (b) D.-C. Fang, IDSCRF-RADII, Beijing Normal University, Beijing, China Search PubMed.
- For some very recent papers, see: (a) Y.-M. Xing, L. Zhang and D.-C. Fang, Organometallics, 2015, 34, 770–777 CrossRef CAS; (b) L. Zhao and D.-C. Fang, Eur. J. Org. Chem., 2015, 4772–4781 CrossRef CAS; (c) L. Zhang and D.-C. Fang, Org. Biomol. Chem., 2015, 13, 7950–7960 RSC; (d) W.-H. Mu, S.-Y. Xia, Y. Li, D.-C. Fang, G. Wei and G. A. Chass, J. Org. Chem., 2015, 80, 9108–9117 CrossRef CAS PubMed.
- (a) E. A. Skorupska, R. B. Nazarski, M. Ciechańska, A. Jóźwiak and A. Kłys, Tetrahedron, 2013, 69, 8147–8154 CrossRef CAS; (b) R. B. Nazarski, J. Inclusion Phenom. Macrocyclic Chem., 2014, 78, 299–310 CrossRef CAS PubMed.
- See ref. 45a–k cited in ref. 1c.
- See, e.g., (a) J. Vondrášek, L. Bendová, V. Klusák and P. Hobza, J. Am. Chem. Soc., 2005, 127, 2615–2619 CrossRef PubMed; (b) T. van Mourik, J. Chem. Theory Comput., 2008, 4, 1610–1619 CrossRef CAS PubMed; (c) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (d) S. Grimme, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 211–228 CrossRef CAS; (e) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed; (f) N. Marom, A. Tkatchenko, M. Rossi, V. V. Gobre, O. Hod, M. Scheffler and L. Kronik, J. Chem. Theory Comput., 2011, 7, 3944–3951 CrossRef CAS PubMed; (g) J. Klimeš and A. Michaelides, J. Chem. Phys., 2012, 137, 120901 CrossRef PubMed; (h) D. Roy, M. Marianski, N. T. Maitra and J. J. Dannenberg, J. Chem. Phys., 2012, 137, 134109 CrossRef PubMed; (i) S. Grimme and M. Steinmetz, Phys. Chem. Chem. Phys., 2013, 15, 16031–16042 RSC; (j) L. Goerigk, J. Chem. Theory Comput., 2014, 10, 968–980 CrossRef CAS PubMed.
- (a) L. I. Smith, H. E. Ungnade, H. H. Hoehn and S. Wawzonek, J. Org. Chem., 1939, 4, 311–317 CrossRef CAS; (b) Y. Yamamoto and K. Itonaga, Org. Lett., 2009, 11, 717–720 CrossRef CAS PubMed.
- B. Helferich and E. Schmitz-Hillebrecht, Berichte, 1933, 66, 378–383 CrossRef.
- J. Herzig, A. Nudelman, H. E. Gottlieb and B. Fischer, J. Org. Chem., 1986, 51, 727–730 CrossRef CAS.
- R. B. Nazarski, Magn. Reson. Chem., 2003, 41, 70–74 CrossRef CAS.
- (a) J. F. Stoddart, Stereochemistry of Carbohydrates, Wiley-Interscience, New York, 1971, pp. 129–145 Search PubMed; (b) D. R. Bundle and R. U. Lemieux, Methods Carbohydr. Chem., 1976, 7, 79–86 CAS.
- See, e.g., (a) J. C. Lindon and A. G. Ferrige, Prog. Nucl. Magn. Reson. Spectrosc., 1980, 14, 27–66 CrossRef CAS; (b) T. Kupka and J. O. Dzięgielewski, Magn. Reson. Chem., 1988, 26, 353–357 CrossRef CAS; (c) F. A. Anet and D. J. O'Leary, Tetrahedron Lett., 1989, 30, 2755–2758 CrossRef CAS; (d) L. Griffiths, Magn. Reson. Chem., 2001, 39, 194–202 CrossRef CAS.
- C. Morat, F. R. Taravel and M. R. Vignon, Magn. Reson. Chem., 1988, 26, 264–270 CrossRef CAS.
- See, e.g., papers with the 400 MHz NMR spectra of pure 2α: (a) R. M. van Well, K. P. Ravindranathan Kartha and R. A. Field, J. Carbohydr. Chem., 2005, 24, 463–474 CrossRef CAS; (b) L. Shi, G. Zhang and F. Pan, Tetrahedron, 2008, 64, 2572–2575 CrossRef CAS; (c) A. Kumar, Y. Geng and R. R. Schmidt, Eur. J. Org. Chem., 2012, 6846–6851 CrossRef CAS; (d) D. Chatterjee, A. Paul, R. Yadav and S. Yadav, RSC Adv., 2015, 5, 29669–29674 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT 06492, USA, 2013 Search PubMed.
- (a) C. Adamo and V. Barone, J. Chem. Phys., 1998, 108, 664–675 CrossRef CAS; (b) M. W. Lodewyk and D. J. Tantillo, J. Nat. Prod., 2011, 74, 1339–1343 CrossRef CAS PubMed; (c) M. W. Lodewyk, C. Soldi, P. B. Jones, M. M. Olmstead, J. Rita, J. T. Shaw and D. J. Tantillo, J. Am. Chem. Soc., 2012, 134, 18550–18553 CrossRef CAS PubMed.
- (a) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789 CrossRef CAS; (b) A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed; (c) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS; (d) P. J. Stephenes, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef; (e) M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS; (f) B. J. Lynch, Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2003, 107, 1384–1388 CrossRef CAS; (g) G. I. Csonka, A. D. French, G. P. Johnson and C. A. Stortz, J. Chem. Theory Comput., 2009, 5, 679–692 CrossRef CAS PubMed.
- R. B. Nazarski, J. Phys. Org. Chem., 2007, 20, 422–430 CrossRef CAS.
- (a) I. Alkorta and J. Elguero, New J. Chem., 1998, 22, 381–385 RSC; (b) A. Perczel and A. G. Császár, Eur. Phys. J. D, 2002, 20, 513–530 CrossRef CAS and their previous papers; ; (c) A. Buczek, M. Makowski, M. Jewgiński, R. Latajka, T. Kupka and M. A. Broda, Biopolymers, 2014, 101, 28–40 CrossRef CAS PubMed; (d) R. Wałęsa, T. Ptak, D. Siodłak, T. Kupka and M. A. Broda, Magn. Reson. Chem., 2014, 52, 298–305 CrossRef PubMed; (e) R. Wałęsa, T. Kupka and M. A. Broda, Struct. Chem., 2015, 26, 1083–1093 CrossRef.
- See, e.g., ref. 2b, 4e–h, j, 12, 15e, 18d, e and 34c cited in ref. 1c.
- Symbols for Specifying the Conformation of Polysaccharide Chains [The IUPAC-IUB Joint Commission on Biochemical Nomenclature (Recommendations 1981)] – P. Karlson, H. B. F. Dixon, A. Cornish-Bowden, C. Liébecq, K. L. Loening, G. P. Moss, J. Reedijk, S. F. Velick and J. F. G. Vliegenthar, Pure Appl. Chem., 1983, 55, 1269–1272 Search PubMed.
- Nomenclature of Carbohydrates [The IUPAC-IUB Joint Commission on Biochemical Nomenclature (Recommendations 1996)] prepared for publication by A. D. McNaught, Pure Appl. Chem., 1996, 68, 1919–2008 CrossRef CAS.
- P. Wałejko and M. K. Cyrański, in preparation.
- (a) V. S. Rao and A. S. Perlin, Can. J. Chem., 1983, 61, 2688–2694 CrossRef CAS; (b) H. Ohrui, Y. Nishida, H. Itoh and H. Meguro, J. Org. Chem., 1991, 56, 1726–1731 CrossRef CAS; (c) I. Tvaroška and J. Gajdoš, Carbohydr. Res., 1995, 271, 151–162 CrossRef; (d) G. D. Rockwell, T. B. Grindley and J.-P. Lepoittevin, J. Carbohydr. Chem., 1999, 18, 51–56 CrossRef CAS; (e) M. Appell, G. Strati, J. L. Willett and F. A. Momany, Carbohydr. Res., 2004, 339, 537–551 CrossRef CAS PubMed.
- (a) PCMODEL V 8.5, Molecular Modeling Software for Windows Operating System, Apple Macintosh OS, Linux and Unix, Serena Software, Bloomington, IN 47402-3076, USA, 2003 Search PubMed.
- A. Hocquet and M. Langgård, J. Mol. Model., 1998, 4, 94–112 CrossRef CAS.
- HyperChem–Molecular Modeling System, Release 8.0.10 for Windows, Hypercube, Inc., 1115 NW, 4th Street, Gainesville, FL 32601, USA, 2011 Search PubMed.
- It was taking a shortcut to reduce the number of steps of an available GMMX searching protocol needed to find all the energy minima, because ‘the number of the conformations found is proportional to the time spent conducting the search’.48.
- W. Schepens, PhD thesis, Ghent University, Belgium, 2000 (as was cited in L. Piela, Ideas of Quantum Chemistry, Elsevier, Amsterdam, 2007, p. 292 Search PubMed).
- (a) A. F. C. Alcântara, D. Piló-Veloso, W. B. De Almeida, C. R. A. Maltha and L. C. A. Barbosa, J. Mol. Struct., 2006, 791, 180–185 CrossRef; (b) A. M. Belostotskii, J. Org. Chem., 2008, 73, 5723–5731 CrossRef CAS PubMed; (c) G. P. Souza, C. Konzen, T. R. G. Simões, B. L. Rodrigues, A. F. C. Alcântara and H. O. Stumpf, J. Mol. Struct., 2012, 1016, 13–21 CrossRef CAS.
- (a) L. A. Nafie and T. B. Feedman, Biological and Pharmaceutical Applications of Vibrational Optical Activity, in Infrared and Raman Spectroscopy of Biological Materials, ed. H.-U. Gremlich and B. Yan, Marcel Dekker, Inc., New York, 2001, pp. 15–54 Search PubMed; (b) Y. He, B. Wang, R. K. Dukor and L. A. Nafie, Appl. Spectrosc., 2011, 65, 699–723 CrossRef CAS PubMed; (c) L. A. Nafie , personal communication (August 14, 2015).
- (a) L. Piela, J. Kostrowicki and H. A. Scheraga, J. Phys. Chem., 1989, 93, 3339–3346 CrossRef CAS; (b) J. Kostrowicki, L. Piela, B. J. Cherayil and H. A. Scheraga, J. Phys. Chem., 1991, 95, 4113–4119 CrossRef CAS; (c) S. I. Sukharev, W. J. Sigurdson, C. Kung and F. Sachs, J. Gen. Physiol., 1999, 113, 525–539 CrossRef CAS PubMed; (d) Z. Xiang, C. S. Soto and B. Honig, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 7432–7437 CrossRef CAS PubMed.
- (a) Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS; (b) Y. Zhao and D. G. Truhlar, J. Phys. Chem. C, 2008, 112, 4061–4067 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- J. W. Ochterski, Thermochemistry in Gaussian; http://www.gaussian.com/g_whitepap/thermo/thermo.pdf; accessed August 10, 2015.
- (a) J. Echeverría, G. Aullón, D. Danovich, S. Shaik and S. Alvarez, Nat. Chem., 2011, 3, 323–330 CrossRef PubMed; (b) D. Danovich, S. Shaik, F. Neese, J. Echeverría, G. Aullón and S. Alvarez, J. Chem. Theory Comput., 2013, 9, 1977–1991 CrossRef CAS PubMed; (c) W. C. McKee and P. v. R. Schleyer, J. Am. Chem. Soc., 2013, 135, 13008–13014 CrossRef CAS PubMed and references cited therein.
- Initial code names xxxxxx of all of the structures of 1α and 1β originate from the MMX energies (xx.xxx in kcal mol−1) of their simple MMX models, after omission of the decimal point.
- (a) D. Ŕeha, H. Valdés, J. Vondrášek, P. Hobza, A. Abu-Riziq, B. Crews and M. S. de Vries, Chem. – Eur. J., 2005, 11, 6803–6817 CrossRef PubMed; (b) L. F. Holroyd and T. van Mourik, Chem. Phys. Lett., 2007, 442, 42–46 CrossRef CAS; (c) H. Valdes, K. Pluháčková, M. Pitonák, J. Řezáč and P. Hobza, Phys. Chem. Chem. Phys., 2008, 10, 2747–2757 RSC; (d) H. Valdes, V. Spivok, J. Řezáč, D. Reha, A. Abu-Riziq, M. S. de Vries and P. Hobza, Chem. – Eur. J., 2008, 14, 4886–4898 CrossRef CAS PubMed.
- L. Ding, N. Ishida, M. Murakami and K. Morokuma, J. Am. Chem. Soc., 2014, 136, 169–178 CrossRef CAS PubMed.
- Y. Li and D.-C. Fang, Phys. Chem. Chem. Phys., 2014, 16, 15224–15230 RSC.
- W.-H. Mu, G. A. Chass and D.-C. Fang, Int. J. Quantum Chem., 2008, 108, 1422–1434 CrossRef CAS and references cited therein.
- (a) J.-Y. Tao, W.-H. Mu, G. A. Chass, T.-H. Tang and D.-C. Fang, Int. J. Quantum Chem., 2013, 113, 975–984 CrossRef CAS; (b) D.-C. Fang, IDSCRF-RADII, Beijing Normal University, Beijing, China Search PubMed.
- For some very recent papers, see: (a) Y.-M. Xing, L. Zhang and D.-C. Fang, Organometallics, 2015, 34, 770–777 CrossRef CAS; (b) L. Zhao and D.-C. Fang, Eur. J. Org. Chem., 2015, 4772–4781 CrossRef CAS; (c) L. Zhang and D.-C. Fang, Org. Biomol. Chem., 2015, 13, 7950–7960 RSC; (d) W.-H. Mu, S.-Y. Xia, Y. Li, D.-C. Fang, G. Wei and G. A. Chass, J. Org. Chem., 2015, 80, 9108–9117 CrossRef CAS PubMed.
- (a) P. Tähtinen, A. Bagno, K. D. Klika and K. Pihlaja, J. Am. Chem. Soc., 2003, 125, 4609–4618 CrossRef PubMed; (b) J. N. Woodford and G. S. Harbison, J. Chem. Theory Comput., 2006, 2, 1464–1475 CrossRef CAS PubMed; (c) Z. Zhang, Q.-s. Li, Y. Xie, R. B. King and H. F. Schaefer III, New J. Chem., 2010, 34, 92–102 RSC; (d) A. Buczek, T. Kupka and M. A. Broda, J. Mol. Model., 2011, 17, 2029–2040 CrossRef CAS PubMed; (e) M. A. Broda, A. Buczek and T. Kupka, Vib. Spectrosc., 2012, 63, 432–439 CrossRef CAS; (f) T. Kupka, M. Stachów, E. Chełmecka, K. Pasterny, M. Stobińska, L. Stobiński and J. Kaminský, J. Chem. Theor. Comput., 2013, 9, 4275–4286 CrossRef CAS PubMed.
- A very similar approach was presented in ref. 63c.
- D. C. Young, Computational Chemistry: A Practical Guide for Applying Techniques to Real-World Problems, John Wiley & Sons, New York, 2001, p. 94 Search PubMed.
- These are very similar to those reported43a for methyl 2,3,4,6-tetra-O-acetyl-D-glucopyranoside in CDCl3 (2α: 3JH5,H6S = 2.4 and 3JH5,H6R = 4.6 Hz, 2β: 3JH5,H6S = 2.5 and 3JH5,H6R = 4.6 Hz).
- (a) F. Neese, ORCA – An ab initio, DFT and semiempirical SCF-MO package. Version 2.8.0.1, University of Bonn, Bonn, Germany, 2010; http://www.thch.uni-bonn.de/tc/orca Search PubMed; (b) F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CrossRef CAS.
- S. Sofue, T. Yamasaki, H. Morita and Y. Kitahama, Polym. J., 1998, 30, 891–896 CrossRef CAS.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618–622 CrossRef.
- Individual zero-point corrected total electronic-nuclear energies, E0,is, were approximated here as E0,i ≅ Etot,i + ZPVEDFT,i where ZPVEDFT,i is an unscaled DFT-level zero-point vibrational energy correction (see Computational details). Undoubtedly, the use of ΔE0s (0 K) instead of ΔGs (288.15 K) introduced additional errors in Boltzmann populations.
- The problem of MP2 calculations is the inaccurate description (overestimation in most cases) of long-range LD forces when an extended basis set is used. Reliable energies are thus frequently obtained with the medium size basis sets. However, this is evidently due to a compensation of errors.57c.
- For the limitations of MP2 methods in the description of conformational energies, see also: (a) M. Goldey, A. Dutoi and M. Head-Gordon, Phys. Chem. Chem. Phys., 2013, 15, 15869–15875 RSC; (b) Y. K. Kang and H. S. Park, Chem. Phys. Lett., 2014, 600, 112–117 CrossRef CAS.
- P. Schreiner, P. v. R. Schleyer and H. F. Schaefer III, J. Org. Chem., 1997, 62, 4216–4228 CrossRef CAS PubMed.
- Such calculations were suggested by one of the referees.
- K. B. Wiberg, J. Comput. Chem., 1999, 20, 1299–1303 CrossRef CAS.
- It should be noted that the MP2/6-311+G(d,p) optimized geometries were used in ref. 75 and that the GIAO predictions (MP2 vs. DFT-mPW1PW91) of σCs were performed applying the 6-311+G(2d,p) basis set. Moreover, the GIAO/MP2 procedure was not as successful; mPW1PW91 gave smaller errors.75 The lack of diffuse functions was most likely critical for our GIAO/MP2 results, but their use was computationally prohibitively expensive.
- It is obvious that adequate scripting and data-management techniques used as a screening tool in the initial step of this study and similar studies (as suggested by one reviewer) would make such endeavors manageable for many other multi-conformer systems.
- See also: (a) E. Kolehmainen, K. Laihia, M. Heinänen, K. Rissanen, R. Fröhlich, J. Korvola, P. Mänttäri and R. Kauppinen, J. Chem. Soc., Perkin Trans. 2, 1993, 641–648 RSC; (b) Ref. 22 cited in M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer, M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845–11854 CrossRef CAS.
- See, e.g., (a) M. Saunders, J. Am. Chem. Soc., 1987, 109, 3150–3152 CrossRef CAS; (b) M. Saunders, J. Comput. Chem., 1989, 10, 203–208 CrossRef CAS; (c) M. Saunders, K. N. Houk, Y.-D. Wu, W. C. Still, M. Lipton, G. Chang and W. C. Guida, J. Am. Chem. Soc., 1990, 112, 1419–1427 CrossRef CAS.
- (a) K. S. Steliou, BAKMDL, based on the original program by W. C. Still, 1989 Search PubMed; (b) Y. Murakami, J.-i. Kikuchi, T. Ohno, T. Hirayama, Y. Hisaeda, H. Nishimura, J. P. Snyder and K. Steliou, J. Am. Chem. Soc., 1991, 113, 8229–8242 CrossRef CAS and references therein; ; (c) M. M. Midland, G. Asirwatham, J. C. Cheng, J. A. Miller and L. A. Morell, J. Org. Chem., 1994, 59, 4438–4442 CrossRef CAS and references cited therein.
- D. M. Ferguson and D. J. Raber, J. Am. Chem. Soc., 1989, 111, 4379–4386 CrossRef.
- (a) S. R. Wilson, W. Cui, J. W. Moskowitz and K. E. Schmidt, Tetrahedron Lett., 1988, 29, 4373–4376 CrossRef CAS; (b) G. Chang, W. C. Guida and W. C. Still, J. Am. Chem. Soc., 1989, 111, 4371–4378 CrossRef.
- J. J. Gajewski, K. E. Gillbert and J. McKelvey, MMX: An enhanced version of MM2 in Advances in Molecular Modeling, ed. D. Liotta, JAI Press, Inc., London, 1990, vol. 2, pp. 65–92 Search PubMed.
- D. Jiao, M. Barfield and V. J. Hruby, J. Am. Chem. Soc., 1993, 115, 10883–10887 CrossRef CAS.
- W. Migda and B. Rys, Magn. Reson. Chem., 2004, 42, 459–466 CrossRef CAS PubMed and ref. 10–15 cited therein.
- J. Gräfenstein and D. Cremer, J. Chem. Phys., 2007, 127, 164113 CrossRef PubMed.
- ChemCraft, Version 1.7 (built 375); http://www.chemcraftprog.com Search PubMed.
- (a) R. B. Nazarski, unpublished results, 2013; ; (b) A. Marciniak, Master's Thesis, University of Łódź, Faculty of Chemistry, Łódź, 2014; (c) R. B. Nazarski and P. Wałejko, VIII Symposium on: Nuclear Magnetic Resonance in Chemistry, Physics and Biological Sciences, Warsaw, 24–26 September, 2014, poster; Programme & Book of Abstracts, page P-21.
- See, e.g., (a) K. W. Wiitala, T. R. Hoye and C. J. Cramer, J. Chem. Theory Comput., 2006, 2, 1085–1092 CrossRef CAS PubMed; (b) K. Dybiec and A. Gryff-Keller, Magn. Reson. Chem., 2009, 47, 63–66 CrossRef CAS PubMed; (c) R. F. Ribeiro, A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2011, 115, 14556–14562 CrossRef CAS PubMed.
- (a) J. B. Foresman and Æ. Frisch, Exploring Chemistry with Electronic Structure Methods, Gaussian, Inc., Pittsburgh, PA 15106, USA, 2nd edn, 1996 Search PubMed, ch. 4 + Errata; ; (b) R. Jain, T. Bally and P. R. Rablen, J. Org. Chem., 2009, 74, 4017–4023 CrossRef CAS PubMed.
- See, e.g., (a) H. Dodziuk, M. Jaszuński and W. Schilf, Magn. Reson. Chem., 2005, 43, 639–646 CrossRef CAS PubMed; (b) S. Leśniak, A. Chrostowska, D. Kuc, M. Maciejczyk, S. Khayar, R. B. Nazarski and Ł. Urbaniak, Tetrahedron, 2009, 65, 10581–10589 CrossRef; (c) R. B. Nazarski and W. Makulski, Phys. Chem. Chem. Phys., 2014, 16, 15699–15708 RSC.
- (a) S. Huzinaga, J. Chem. Phys., 1965, 42, 1293–1302 CrossRef; (b) S. Huzinaga, Approximate Atomic Functions. Technical Report, University of Alberta, Edmonton, Canada, 1971; (c) M. Schindler and W. Kutzelnigg, J. Chem. Phys., 1982, 76, 1919–1933 CrossRef CAS; (d) W. Kutzelnigg, U. Fleischer and M. Schindler, The IGLO-Method: Ab initio Calculation and Interpretation of NMR Chemical Shifts and Magnetic Susceptibilities in NMR – Basic Principles and Progress (Deuterium and Shift Calculation), ed. P. Diehl, E. Fluck, H. Günther, R. Kosfeld and J. Seelig, Springer-Verlag, Berlin, 1991, vol. 23, pp. 165–262 Search PubMed.
- See ref. 38–43 cited in ref. 91c.
- (a) EMSL Basis Set Library; https://bse.pnl.gov/bse/portal; ; (b) D. Feller, J. Comput. Chem., 1996, 17, 1571–1586 CrossRef CAS; (c) K. L. Schuchardt, B. T. Didier, T. Elsethagen, L. Sun, V. Gurumoorthi, J. Chase, J. Li and T. L. Windus, J. Chem. Inf. Model., 2007, 47, 1045–1052 CrossRef CAS PubMed.
- S. M. Koskowich, W. C. Johnson, R. S. Paley and P. R. Rablen, J. Org. Chem., 2008, 73, 3492–3496 CrossRef CAS PubMed.
- T. Chai and R. R. Draxler, Geosci. Model Dev., 2014, 7, 1247–1250 CrossRef.
- S. G. Smith and J. M. Goodman, J. Org. Chem., 2009, 74, 4597–4607 CrossRef CAS PubMed.
Footnotes |
| † Physical image vs. molecular structure relation, Part 19. For Part 18, see ref. 91c. |
| ‡ Electronic supplementary information (ESI) available: Experimental – general information, synthesis and spectroscopic data, NMR spectra of 1α, 1β and 2α, ΔH5 values for selected glucosides; calculation results for 1α and 1β – structural parameters, energetics and populations, molecular representations, scatter plots of JcaldHHs vs. JobsdHHs and δcalcdH,C(MP2) vs. δobsdH,C relationships, and Cartesian coordinates of DFT structures. See DOI: 10.1039/c5ob01865j |
| This journal is © The Royal Society of Chemistry 2016 |