Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Three-minute synthesis of sp3 nanocrystalline carbon dots as non-toxic fluorescent platforms for intracellular delivery†
Stephen A.
Hill
a,
David.
Benito-Alifonso
a,
David J.
Morgan
b,
Sean A.
Davis
a,
Monica
Berry
a and
M. Carmen
Galan
 *a
*a
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: m.c.galan@bristol.ac.uk
bCardiff Catalysis Institute, School of Chemistry, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
First published on 21st October 2016
Abstract
A one-pot, three-minute, gram-scale synthesis of novel sp3-nanocrystalline, water-soluble, and fluorescent carbon dots (FCDs), from simple and cheap sugar starting materials is described. Mechanism studies showed that NH2-FCD formation proceeds via a crucial imine intermediate derived from reaction between a sugar hemiacetal and an amine. Moreover, we successfully demonstrate the utility of lactose functionalized FCDots (Lac-FCDots) as non-toxic fluorescent intracellular delivery vehicles.
The application of nanotechnology to biological and medical problems has seen an explosion of research in recent years.1 Functional nanomaterials that incorporate biomolecules as recognition motifs have become very useful for cargo delivery, sensing and catalysis.2 Nanomaterials with novel optical, electronic and surface properties that exhibit a low cytotoxicity profile are particularly valuable platforms for tracking biomolecules within the complex cellular environment, and in the development of novel therapies and in vivo diagnostics.3
Carbohydrate–protein recognition processes are mediated by multivalent interactions that help achieve higher affinity as well as higher specificity.4 Glycan-coated nanodots have been validated as multivalent tools to screen for protein–carbohydrate interactions associated with inter- and intracellular recognition processes.5 Our group recently demonstrated that the type of glycan presented on a fluorescent CdSe/ZnS quantum dot (QD) can enable control of nanodot uptake and intracellular localization in cervical cancer HeLa cells, and in immortalised corneal epithelial cells.6 Although we showed that glycan density mitigates the inherent toxicity of CdSe QDs, these QDs remain less than ideal for in vivo applications. In order to broaden the scope of functional nanomaterials for biomedical purposes, it is of the utmost importance to develop non-toxic and stable nanomaterials that can be used over long periods of exposure.
Carbon dots (CDs) are a relatively new material that have attracted significant interest since their serendipitous discovery in 2004 due to their unique and tuneable optical properties, which are comparable and sometimes superior to those of semiconductor QDs.7 Their photoluminescence (PL) properties, chemical inertness, excellent water solubility, low cost of fabrication, and general minimal toxicity suggest wide-ranging potential uses, including in vivo applications.8 Current CD fabrication methods can be either top-down (from bulk carbon sources) or bottom-up (seeding from molecular precursors) and generally result in CDs containing an amorphous or graphitic sp2 crystalline core with a range of polar, particularly, oxygenated functionality, i.e. alcohols and carboxylic acids, on an amorphous/sp2-embedded surface (Fig. 1).9 Bottom-up methods, which improve the photoluminescence (PL) properties of the material, involve either surface passivation or heteroatom doping strategies.8,10 The former provides uniform PL surface trap sites, and is generally achieved via amide conjugation of linear diamines (e.g. ethylene or polyethylene glycol (PEG)-type diamines) to surface-bound carboxylic acids in either the CD formation step or in a subsequent step.11 Similarly, the introduction of heteroatoms, especially N, has been shown to improve PL properties especially the quantum yield of fluorescence (QY).10,12 However, due to the intricate molecular structure of CDs and the lack of knowledge of the reaction mechanisms that govern nanoparticle formation and photoluminescence, most methods available are complex and time-consuming; and few large-scale, bottom-up protocols exist that exploit both strategies (surface passivation and N-heteroatom doping) simultaneously. A number of carbohydrates and different nucleation and growth conditions have been reported for the preparation of FCDs, which include glycerol, glycol, glucose, glucosamine, sucrose, dextran, chitosan, cellulose and ascorbic acid as common starting materials.11,13 However, most methods employ very harsh conditions (chemical or hydrothermal oxidation, pyrolysis, high acidic environments and long reactions times) and require complex post-modifications to improve the surface state of the CDs, followed by lengthy purifications.
As part of our ongoing interest in the development of non-toxic fluorescent nano-platforms for biological applications that are general, practical and accessible to the non-expert at the point of use (e.g. biologists and biochemists), we turned our attention to the preparation of water soluble FCDs from low-cost starting materials, that are easily functionalised by a given biomolecule. To that end, glucosamine HCl (GlcNH2·HCl) was chosen as the starting material, as it already contains the required N for nanoparticle doping, and 4,7,10-trioxa-1,13-tridecanediamine (TTDDA)11,14 as the diamine needed for surface passivation. In addition, the methylene protons in the propyl chain of TTDDA can be used as a 1H-NMR handle to help quantify surface ligands on the FCDs.
The gram-scale synthesis of FCDs was then investigated. An optimal QY of up to 18%, relative to quinine sulfate (Fig. S1A in ESI†),15 was obtained when GlcNH2·HCl (0.24 M) and TTDDA (1.1 eq.) were reacted in distilled water under microwave (MW) irradiation (domestic 700 W MW) for 3 minutes (Fig. 1, see ESI† for full details). After dissolution in water and sample filtration through a centrifugal concentrator filter with a 10 kDa molecular weight cut-off, amine-coated CDs (NH2-FCDs) with a strong blue fluorescence under 365 nm UV irradiation (Fig. 2B) and a hydrodynamic volume of 1–10 nm (as measured by dynamic light scattering (DLS)) were obtained (Fig. S2†). High-resolution transmission electron microscopy (HR-TEM) revealed that NH2-FCDs had an average diameter of 2.45 nm (Fig. 2A). Crystalline structure, with lattice spacings of 0.21 nm and 0.25 nm, was also observed (Fig. S3†). These spacings are consistent with both graphitic [(100), (020)] and diamond-like [(111), (110)] carbon preventing unambiguous structure assignment.8,19 However, the absence of graphitic (002) spacings of 0.33 nm indicates the NH2-FCDs may contain predominantly sp3 enriched carbon. Raman spectroscopy of NH2-FCDs exhibited two peaks at 660 cm−1, indicative of C–Cl bonds, and 1608 cm−1 which deconvoluted to two contributing peaks: a small shoulder centred at 1547 cm−1 is symptomatic of sp2-enriched areas, with the main peak, centred at 1608 cm−1. Being characteristic of strong sp3 character (Fig. S4A†).20 Interestingly, the observation of sp3-enriched crystallinity, instead of the commonly found graphitic or amorphous crystallinity, generated from similar syntheses,11,13a,21 is to our knowledge the first reported characterisation of a CD with a sp3-enriched crystalline core fabricated using a bottom-up synthesis.
 | ||
| Fig. 2 (A) HR-TEM image of NH2-FCDs (B) Left: NH2-FCDs aqueous dispersion in daylight. Right: Irradiated with 365 nm UV light (C) excitation and emission spectrum of NH2-FCDs. | ||
UV-vis physicochemical characterization of NH2-FCDs showed an absorbance profile characteristic of CDs from 200–450 nm, with a leading absorbance tail into the near UV region and a defined peak at 270 nm (Fig. S5†). This feature can be attributed to π–π* transition of aromatic/alkenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds or C
C bonds or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds.16 Moreover, the fluorescence spectroscopy emission profile (excitation 200–400 nm) exhibited the signature features of N-doped CDs, in which there is an excitation-dependent emission (Fig. 2C). The excitation spectrum shows three significant peaks at 200 nm (π–π* of aromatic/alkenyl C
N bonds.16 Moreover, the fluorescence spectroscopy emission profile (excitation 200–400 nm) exhibited the signature features of N-doped CDs, in which there is an excitation-dependent emission (Fig. 2C). The excitation spectrum shows three significant peaks at 200 nm (π–π* of aromatic/alkenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds),17 295 and 340 nm (n–π* transitions in C
C bonds),17 295 and 340 nm (n–π* transitions in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds).18 The excellent photostability of NH2-FCDs was evaluated and no decrease in emission intensity (at 440 nm) was observed over a 15 hour period of continuous irradiation at 340 nm, whereas an organic fluorophore such as Rhodamine 6G significantly photobleached under similar conditions after 30 min (Fig. S6†). Their chemical inertness was also assessed by monitoring emission over a range of pH values (0–13) (Fig. S7†). Emission intensity was maintained between pH 4–9, and above 70% between pH 1–3 and above pH 10. These results demonstrate the chemical robustness of the newly synthesized NH2-FCDs.
N bonds).18 The excellent photostability of NH2-FCDs was evaluated and no decrease in emission intensity (at 440 nm) was observed over a 15 hour period of continuous irradiation at 340 nm, whereas an organic fluorophore such as Rhodamine 6G significantly photobleached under similar conditions after 30 min (Fig. S6†). Their chemical inertness was also assessed by monitoring emission over a range of pH values (0–13) (Fig. S7†). Emission intensity was maintained between pH 4–9, and above 70% between pH 1–3 and above pH 10. These results demonstrate the chemical robustness of the newly synthesized NH2-FCDs.
The composition and chemical functionality present in NH2-FCDs was then assessed by elemental analysis, Fourier-transformed infra-red (FTIR) and X-ray photoelectron spectroscopy (XPS). Elemental analysis showed a composition of: C, 46.47%; H, 8.33%; N, 7.99%; Cl; 6.51%; and O, 30.70% (Table S1†). FTIR spectrum (Fig. S8†) showed characteristic peaks for the presence of amino or/and alcohol (N–H/O–H) functionalities (signals at 3345 cm−1). Bands at 2974 cm−1 indicate the presence of sp3 C–H, attributable either to amorphous carbon or the TTDDA linker. Importantly, surface passivation via amide formation is suggested by the presence of an absorption band at 1644 cm−1 (NHCO groups). Furthermore, an absorption peak at 1045 cm−1 is indicative of C–O ether vibrations, while the band at 625 cm−1 corroborates the presence of C–Cl bonds. The wide scan XPS spectrum indicated the presence of C, O, N and Cl (in proportions similar to elemental analysis) with peaks at ca. 285 eV (C 1s), 532 eV (O 1s), 400 eV (N 1s) and 197 eV (Cl 2p) (Fig. S9–S11†). Where applicable, high resolution scans of each region were fitted (Table S2†) to reveal further functional group detail. The fitted peaks highlighted the presence of aromatic moieties such as: aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and OH groups, aliphatic C
O and OH groups, aliphatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, imides, and N-heterocycles e.g. pyridines.
O, imides, and N-heterocycles e.g. pyridines.
The electrokinetic potential (zeta potential) of NH2-FCDs was measured as (−0.08 to +1.64) mV (Fig. S12†). Although a positive charge might be expected due to the presence of distal primary amines from the TTDDA linker, the observed net neutral surface charge can be attributed to other surface functional groups i.e. carboxylic acids, esters, alcohols, and bound chlorides. No particle flocculation or coagulation was observed, which suggests that stabilization of NH2-FCDs might be driven by linker steric effects or the high solubility afforded by the abundance of polar surface functionality.22
1H NMR structural characterization of NH2-FCDs (Fig. S13†) showed two sets of distinct signal intensities associated to FCD surface functionality. Large peaks at 3.6, 2.9 and 1.8 ppm could be assigned to surface bound TTDDA, whilst smaller peaks around 8.5 ppm and 1.5–4.0 ppm correspond to surface-bound aromatic and sp3-bound protons, respectively. Further analysis by 1H–13C HMBC (Fig. S14†) showed cross-peaks between these lower intensity aromatic and alkyl regions, indicating a patchwork of amorphous and aromatic moieties on the surface of NH2-FCDs. 1H–15N-HMBC NMR experiments further evidenced desymmetrisation/anchoring of TTDDA onto the surface via incorporation into N-containing heterocycles (−160 and −130 ppm) most likely imidazole, pyrazine or pyridine-type molecules (Fig. S15†). Presence of the distal amine was confirmed by the cross-peak at −350 ppm, indicating the presence of ammonium species. Finer NH2-FCD structural detail can be ascertained by the minor proton species in the 15N NMR spectrum (1.65 and 1.58 ppm) showing cross-peaks at −62 ppm (imine), −253 and −293 ppm (amides) (Fig. S16†).
In order to better understand the molecular mechanisms that lead to NH2-FCD formation, aliquots of the reaction at 30-second intervals were taken and analysed by FTIR, 1H, 13C, and elemental analysis (Fig. S17–S20†). Loss of the anomeric proton/carbon (1H, 13C NMR) as well as the formation of an aldehyde (FTIR) were observed over the first 90 seconds, after which time the aldehyde disappeared (FTIR) and formation of sp2-centres/aromatization was then observed (1H, 13C NMR). Additionally, amide formation occurred after 90 seconds (13C NMR, FTIR). Further mechanistic evidence was obtained from qualitative ReactIR studies, under hydrothermal conditions at 70 °C (Fig. S21†).23 Upon addition of GlcNH2·HCl, to the heated TTDDA solution, two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations appeared at 1625 and 1682 cm−1 which could be assigned to amide and imine species, respectively. While the imine signal at 1682 cm−1 quickly reaches a maximum intensity and then diminishes over time, the amide signal continues to increase before reaching a plateau. Based on these observations, we propose that initial reaction stages involve iminium formation, from reaction of an amine (TTDDA or GlcNH2) with the aldehyde that is generated from ring-opening of the hemiacetal in the carbohydrate moiety. Trapping of the iminium electrophile could allow oligomer formation which, when accompanied by dehydration (as confirmed by elemental analysis of the reaction aliquots at different time points, see Fig. S20†), leads to the formation of the sp3-enriched nanocrystalline core. In the second phase of the reaction, following the loss of bulk water, further carbonisation occurs and aromaticity is then generated on the outer layers of the sp3-enriched cores. Surface passivation by TTDDA can now take place via either incorporation of TTDDA into the surface heteroaromatics or amide bond formation. Amide formation can occur either through surface bound carboxylic acids reacting directly with an amine (e.g. TTDDA or sugar-derived amine) or through the nucleophilic attack of an alcohol to the iminium electrophile, followed by rearrangement of the resulting imidate (Scheme 1).
O vibrations appeared at 1625 and 1682 cm−1 which could be assigned to amide and imine species, respectively. While the imine signal at 1682 cm−1 quickly reaches a maximum intensity and then diminishes over time, the amide signal continues to increase before reaching a plateau. Based on these observations, we propose that initial reaction stages involve iminium formation, from reaction of an amine (TTDDA or GlcNH2) with the aldehyde that is generated from ring-opening of the hemiacetal in the carbohydrate moiety. Trapping of the iminium electrophile could allow oligomer formation which, when accompanied by dehydration (as confirmed by elemental analysis of the reaction aliquots at different time points, see Fig. S20†), leads to the formation of the sp3-enriched nanocrystalline core. In the second phase of the reaction, following the loss of bulk water, further carbonisation occurs and aromaticity is then generated on the outer layers of the sp3-enriched cores. Surface passivation by TTDDA can now take place via either incorporation of TTDDA into the surface heteroaromatics or amide bond formation. Amide formation can occur either through surface bound carboxylic acids reacting directly with an amine (e.g. TTDDA or sugar-derived amine) or through the nucleophilic attack of an alcohol to the iminium electrophile, followed by rearrangement of the resulting imidate (Scheme 1).
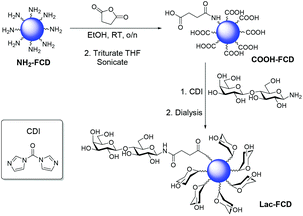
Functionalisation of NH2-FCDs with lactose disaccharide, was carried out to highlight the versatility of our FCDs (Scheme 2). Treatment of NH2-FCDs with succinic anhydride, proceeded by ring-opening, to yield carboxylic acid bearing FCDs (COOH-FCDs), which were then reacted with 1-aminolactose,6 in the presence of CDI, to afford lactose-coated CDs (Lac-FCDs). 1H-NMR confirmed the surface functionalization of COOH-FCDs and Lac-FCDs (Fig. S22 and S23†). Interestingly, it was found that the fluorescence properties for COOH/Lac-FCDs were not altered from those of NH2-FCDs (Fig. S29†).
Cell internalization, and toxicity studies using NH2-FCDs, COOH-FCDs and Lac-FCD were then performed in HeLa (human cervical) and MDA-MB-231 (human breast) cancer cells. Cultures were exposed to concentrations from 10−6 to 2000 μg mL−1 for 1 hour, 1, 3 and 7 days.24 Metabolic competence was assessed by Alamar Blue (AB), and the number of live cells with Calcein AM. The ratio of AB/Calcein affords the reductive metabolism per cell (RMPC), providing a metric to compare treated cells against untreated cells. In MDA (Fig. S30 and S31†), a distinct advantage for lactose-coated FCDs is observed at very high concentrations after 3 and 7 day exposures. NH2-FCDs and COOH-FCDs show elevated RMPC at concentrations above 500 μg mL−1 3 days and reduced RMPC above 250 μg mL−1 7 days, whereas Lac-FCDs show no significant change from control. In HeLa cells (Fig. S32 and S33†), differences can be seen between differentially functionalised FCDs. NH2-FCDs show elevated RMPC at 1 hour exposures, with no associated cell death, which is reduced after 1 and 3 days; with significant cell death being seen at concentrations over 250 μg mL−1 at 7 days. COOH-FCDs have little toxic effect except at 7 day exposures above 250 μg mL−1 where RMPC decreased significantly. Conversely, Lac-FCDs after 3 days, above concentrations of 10−1 μg mL−1, show elevated RMPC levels consistently. However, this is not associated with any change in the population size relative to control, and is reduced again after 7 days. These results highlight the utility of the glycan coating to decrease or obviate the toxicity of for FCDs at high concentrations.
Having confirmed the very low toxicity of our novel FCDs, confocal microscopy was used to visualise Lac-FCD's interaction with HeLa (Fig. S34†) and MDA cells (Fig. 3 and S35†). After 2 hour exposure, cells were imaged with 405 nm excitation.25 It was found that Lac-FCDs were indeed internalised by both cell lines, as determined by Z-stack analysis. Compression of a Z-stack, that is consecutive sections at different heights within a region of interest, allowed comparison of fluorescence per unit area in treated vs. untreated cells. It was found treated cells had a higher total fluorescence than untreated cells (Fig. S34D/35D†). Lac-FCDs generally display a diffuse localisation within the cell in either cell line, with some areas of bright aggregation.
 | ||
| Fig. 3 Confocal microscopy mages of Lac-FCD internalization in MDA cells. Left: Bright Field channel; Right: Fluorescent channel showing Lac-FCDs. | ||
Conclusions
In summary, the facile and expedient MW-assisted synthesis of a novel, sp3-enriched nanocrystalline and photostable amine-decorated FCD is described (NH2-FCD) from cheap and available starting materials. The novel NH2-FCDs consist of a sp3-enriched crystalline core with an amorphous surface studded with aromatic regions. Mechanistic studies on the formation of NH2-FCDs suggests the generation of an iminium species which is crucial for carbohydrate degradation and FCD surface passivation. We believe this key step could be exploited to further expand the emission profile of FCDs. Further FCD functionalization to afford non-toxic carboxylic acid and lactose-decorated FCDs (COOH/Lac-FCDs) showed their ease of functionalization. We also demonstrated that Lac-FCDs are readily internalised into human cancer cells with little toxic effect at high concentrations and long exposure times. The simplicity of the protocol and versatility of the FCDs makes this process a valuable addition to the toolbox of chemists and biologists with applications in, and beyond, the field of material chemistry. Moreover, the novel sp3 nanocrystallinity of these materials, which possess special chemical and physical properties such as high chemical inertness, diamond-like properties, and favorable tribological properties, offers unique opportunities in electrochemical applications and for probing the PL mechanism of FCDs that do not contain sp2-crystalline regions; these studies are ongoing.Acknowledgements
This research was supported by EPSRC CAF EP/L001926/1 (MCG), ERC-COG: 648239 (MCG) and EPSRC EP/G036764/1 (SAH). Many thanks to Dr D. Tiwari for Raman help, Dr D. Alibhai (Wolfson Imaging facility) for confocal assistance, and Jon Jones for assistance with TEM studies which were carried out in the Chemistry Imaging Facility with equipment funded by UoB and EPSRC (EP/K035746/1 and EP/M028216/1).Notes and references
- K. El-Boubbou and X. F. Huang, Curr. Med. Chem., 2011, 18, 2060–2078 CrossRef CAS PubMed.
- (a) E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS PubMed; (b) M. Marradi, M. Martin-Lomas and S. Penades, Adv. Carbohydr. Chem. Biochem., 2010, 64, 211 CrossRef CAS PubMed; (c) C. F. Wu and D. T. Chiu, Angew. Chem., Int. Ed., 2013, 52, 3086 CrossRef CAS PubMed; (d) R. Vacha, F. J. Martinez-Veracoechea and D. Frenkel, ACS Nano, 2012, 6, 10598 CAS; (e) I. Canton and G. Battaglia, Chem. Soc. Rev., 2012, 41, 2718 RSC.
- N. C. Reichardt, M. Martin-Lomas and S. Penades, Chem. Soc. Rev., 2013, 42, 4358 RSC.
- (a) Y. C. Lee and R. T. Lee, Acc. Chem. Res., 1995, 28, 321 CrossRef CAS; (b) C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357 CrossRef CAS PubMed; (c) J. J. Lundquist and E. J. Toone, Chem. Rev., 2002, 102, 555 CrossRef CAS PubMed; (d) A. Varki and J. B. Lowe, Essentials of glycobiology, ed. A. Varki, Cold Spring Harbor, NY, 2009 Search PubMed.
- (a) M. S. Hudlikar, X. R. Li, I. A. Gagarinov, N. Kolishetti, M. A. Wolfert and G. J. Boons, Chem. – Eur. J., 2016, 22, 1415 CrossRef CAS PubMed; (b) R. Kikkeri, B. Lepenies, A. Adibekian, P. Laurino and P. H. Seeberger, J. Am. Chem. Soc., 2009, 131, 2110 CrossRef CAS PubMed; (c) D. C. Kennedy, D. Grunstein, C. H. Lai and P. H. Seeberger, Chemistry, 2013, 19, 3794 CrossRef CAS PubMed; (d) Y. Yang, M. Yu, T. T. Yan, Z. H. Zhao, Y. L. Sha and Z. J. Li, Bioorg. Med. Chem., 2010, 18, 5234 CrossRef CAS PubMed; (e) J. Gallo, N. Genicio and S. Penades, Adv. Healthcare Mater., 2012, 1, 302 CrossRef CAS PubMed; (f) P. Di Gianvincenzo, F. Chiodo, M. Marradi and S. Penades, Methods Enzymol., 2012, 509, 21 CAS; (g) J. M. De la Fuente and S. Penades, Biochim. Biophys. Acta, Gen. Subj., 2006, 1760, 636 CrossRef CAS PubMed.
- D. Benito-Alifonso, S. Tremel, B. Hou, H. Lockyear, J. Mantell, D. J. Fermin, P. Verkade, M. Berry and M. C. Galan, Angew. Chem., Int. Ed., 2014, 53, 810 CrossRef CAS PubMed.
- R. Ray, X. Xu, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- S. J. Zhu, Y. B. Song, X. H. Zhao, J. R. Shao, J. H. Zhang and B. Yang, Nano Res., 2015, 8, 355 CrossRef CAS.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800 CrossRef CAS PubMed.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563 CrossRef CAS.
- (a) Q. Xu, Y. Liu, C. Gao, J. F. Wei, H. J. Zhou, Y. S. Chen, C. B. Dong, T. S. Sreeprasad, N. Li and Z. H. Xia, J. Mater. Chem. C, 2015, 3, 9885 RSC; (b) Y. L. Wang, Y. Q. Zhao, Y. Zhang, F. Zhang, X. T. Feng, L. Chen, Y. Z. Yang and X. G. Liu, RSC Adv., 2016, 6, 38761 RSC; (c) J. Hou, W. Wang, T. Zhou, B. Wang, H. Li and L. Ding, Nanoscale, 2016, 8, 11185 RSC.
- (a) H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC; (b) S. Liu, N. Zhao, Z. Cheng and H. Liu, Nanoscale, 2015, 7, 6836 RSC; (c) S. K. Bhunia, A. Saha, A. R. Maity, S. C. Ray and N. R. Jana, Sci. Rep., 2013, 3, 1473 Search PubMed; (d) J. Hou, J. Yan, Q. Zhao, Y. Li, H. Ding and L. Ding, Nanoscale, 2013, 5, 9558 RSC; (e) D. Y. Pan, J. C. Zhang, Z. Li, Z. W. Zhang, L. Guo and M. H. Wu, J. Mater. Chem., 2011, 21, 3565 RSC; (f) Y. Zhang, X. Liu, Y. Fan, X. Guo, L. Zhuo, Y. Liv and J. Lin, Nanoscale, 2016, 8, 15281 RSC; (g) Z.-C. Yang, X. Li and J. Wang, Carbon, 2011, 49, 5207 CrossRef CAS.
- (a) H. Peng, Y. Li, C. Jiang, C. Luo, R. Qi, R. Huang, C.-G. Duan and J. Travas-Sejdic, Carbon, 2016, 100, 386 CrossRef CAS; (b) Z. A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812 RSC; (c) C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163 RSC; (d) M. Tan, L. Zhang, R. Tang, X. Song, Y. Li, H. Wu, Y. Wang, G. Lv, W. Liu and X. Ma, Talanta, 2013, 115, 950 CrossRef CAS PubMed; (e) X. Ren, J. Liu, X. Meng, J. Wei, T. Liu and F. Tang, Chem. – Asian J., 2014, 9, 1054 CrossRef CAS PubMed.
- If free-based GlcNH2 is used under the described conditions, the QY of the NH2-FCDs drops to below 2% (Fig. S1B†), but the physical structure is unchanged (Raman spectroscopy, Fig. S4B†), suggesting Cl incorporation is paramount for PL properties, possibly acting in a crucial auxochromic manner.
- H. Ding, J. S. Wei and H. M. Xiong, Nanoscale, 2014, 6, 13817 RSC.
- J. Tan, R. Zou, J. Zhang, W. Li, L. Zhang and D. Yue, Nanoscale, 2016, 8, 4742 RSC.
- Y. H. Yuan, Z. X. Liu, R. S. Li, H. Y. Zou, M. Lin, H. Liu and C. Z. Huang, Nanoscale, 2016, 8, 6770 RSC.
- (a) A. Kumar, P. A. Lin, A. Xue, B. Hao, Y. K. Yap and R. M. Sankran, Nat. Commun., 2013, 4, 2618 Search PubMed; (b) H. Hirai and K. I. Kondo, Science, 1991, 253, 772 CAS.
- S. Prawer, K. W. Nugent, D. N. Jamieson, J. O. Orwa, L. A. Bursill and J. L. Peng, Chem. Phys. Lett., 2000, 332, 93 CrossRef CAS.
- Y. H. Yuan, R. S. Li, Q. Wang, Z. L. Wu, J. Wang, H. Liu and C. Z. Huang, Nanoscale, 2015, 7, 16841 RSC.
- G. Cao, Nanostructures and Nanomaterials: Synthesis, Properties and Applications, Imperial College Press, London, 2004 Search PubMed.
- Due to operational considerations, a TTDDA solution was heated to 70 °C, followed by addition of GlcNH2·HCl. Although, quantification is not possible, detection of imine species under these conditions, is highly suggestive of their presence under MW-treatment.
- It should be noted all FCDs were readily soluble in phosphate-buffered saline (PBS) and all cell media used in cellular experiments. No degradation or aggregation was observed over periods of 7 days at 37 °C or room temperature.
- Although this excitation is not optimal for Lac-FCDs, the average fluorescence intensity per treated cell was higher in both cell lines (Fig. S32D/S33D†).
Footnote |
| † Electronic supplementary information (ESI) available: Detailed protocols, supplementary figures and schemes, toxicity results and confocal microscopy images. See DOI: 10.1039/c6nr07336k |
| This journal is © The Royal Society of Chemistry 2016 |