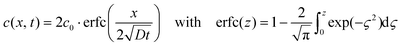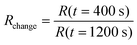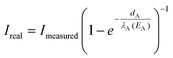Open Access Article
Open Access ArticleSelf-organized growth of graphene nanomesh with increased gas sensitivity
Matthias
König
*ab,
Günther
Ruhl
b,
Joerg-Martin
Batke
b and
Max C.
Lemme
 a
a
aUniversity of Siegen, Department of Electrical Engineering and Computer Science, Hölderlinstr. 3, 57076 Siegen, Germany
bInfineon Technologies AG, 93049 Regensburg, Germany. E-mail: Matthias.Koenig@infineon.com
First published on 8th August 2016
Abstract
A bottom-up chemical vapor deposition (CVD) process for the growth of graphene nanomesh films is demonstrated. The process relies on silicon nanospheres to block nucleation sites for graphene CVD on copper substrates. These spheres are formed in a self-organized way through silicon diffusion through a 5 μm copper layer on a silicon wafer coated with 400 nm of silicon nitride. The temperature during the growth process disintegrates the Si3N4 layer and silicon atoms diffuse to the copper surface, where they form the nanospheres. After graphene nanomesh growth, the Si nanospheres can be removed by a simple hydrofluoric acid etch, leaving holes in the graphene film. The nanomesh films have been successfully transferred to different substrates, including gas sensor test structures, and verified and characterized by Auger, TEM and SEM measurements. Electrical/gas-exposure measurements show a 2-fold increase in ammonia sensitivity compared to plain graphene sensors. This improvement can be explained by a higher adsorption site density (edge sites). This new method for nanopatterned graphene is scalable, inexpensive and can be carried out in standard semiconductor industry equipment. Furthermore, the substrates are reusable.
Graphene, a carbon allotrope of the two-dimensional material class, has attracted much interest because of its extraordinary intrinsic electronic,1 mechanical,2 optoelectronic3 and thermal4 properties. In addition, the two-dimensional nature of the material gives rise to extreme sensitivity to its environment, suggesting applications in gas and environmental sensing.5–8 Its remarkable properties can be tuned even further by modifying it at the nanoscale, e.g. nanoribbons that exhibit a band gap,9,10 improved contact resistance through local contact patterning11 or enhanced light absorption in nanostructures.12 There are several state-of-the-art methods for graphene patterning. A straight forward technique is conventional electron beam lithography,9,10 but there are also non-conventional methods based on lithographic processes like block-copolymer lithography,13–15 nanosphere lithography16–19 and nanowire lithography.20 Bottom-up techniques have been demonstrated to grow precise molecular-scale nanoribbons.21 Some of these methods cannot be scaled up for industrial use, and most of them suffer from contamination issues, like residues from polymeric and inorganic species from the process chemicals, which lead to degraded carrier mobilities and random Dirac-Point shifts.22 Independent of the technique, the resultant graphene nanostructures yield highly reactive graphene edges after patterning, with a number of possible chemical terminations. These edges may also be extremely defective or well-defined with a clear crystallographic orientation.23 Depending on the type of application, a high amount of edge defects can be detrimental (e.g. for generating a controlled bandgap24) or beneficial (e.g. for electrical contacts11). In particular, it has been shown that gas sensitivity can be enhanced considerably in defective graphene25 or patterned graphene meshes.13,19 Here, we present a new bottom-up method to synthesize patterned graphene in a simple, reproducible way. We further demonstrate superior gas sensing properties of devices made with these self-organized graphene nanomeshes.
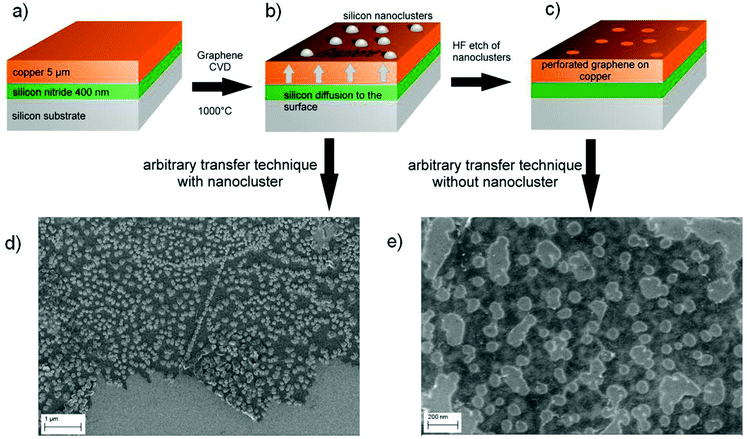
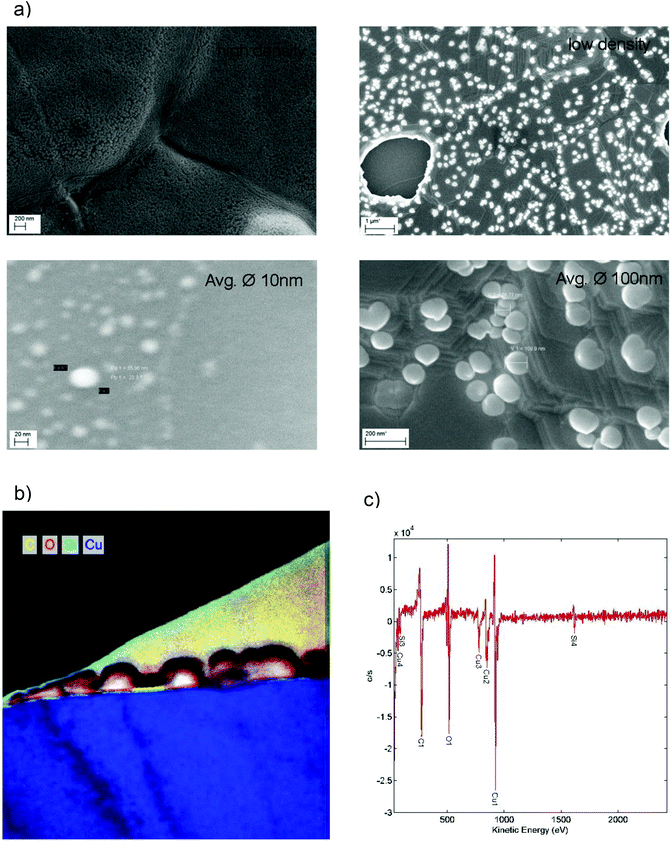
To fabricate the graphene nanomeshes, a 5 μm thick copper (Cu) film was sputter-deposited on a silicon (Si) wafer coated with 400 nm silicon nitride (Si3N4) (Fig. 1a). These samples were then placed in a CVD hot wall reactor and processed at 1000 °C for 10 min under hydrogen (H2) atmosphere, followed by graphene growth for 10 min in C2H4 atmosphere. During this process, Si3N4 starts to decompose and Si diffuses towards the Cu surface where it forms spherical aggregates in the nanometer scale. This is shown schematically in Fig. 1(b). It is important to note that the Si nanosphere growth takes place already in the annealing phase, prior to the start of the graphene film growth. The areas that become occupied by Si hence locally block the subsequent catalytic graphene growth. This leads to discontinuous graphene growth only between the Si nanospheres. A similar approach was reported by Yi et al.,26 who generated the blocking sites through self-assembled colloidal silica spheres. However, it is not clear what kind of contamination is introduced into the graphene films during the reported synthesis by the Stöber method27 and the Langmuir–Blodgett assembly. The method proposed here, in contrast, relies on standard semiconductor technology. This includes using copper-coated silicon wafers, as copper foil is quite unusual in semiconductor manufacturing, and reducing contamination issues (expected from the state of the art transfer) to a minimum. After 10 minutes of graphene growth time the samples were cooled down with a rate of 15 K min−1 to room temperature in hydrogen atmosphere. The Si-clusters were removed with hydrofluoric acid (HF), resulting in the graphene nanomesh structure shown schematically in Fig. 1(c). After HF etching, the graphene nanomesh was coated with polymethylmethacrylate (PMMA) and the Cu was underetched with 1 mol FeCl3 solution. The floating PMMA/graphene film was rinsed and picked up with a SiO2-coated Si wafer. At this stage, the Si/Si3N4 substrates are reusable, standard substrate cleaning procedures and new sputter deposition of Cu will re-establish the initial conditions. The sample was heated in a UHV furnace at 400 °C for 10 min to remove residual PMMA and HF. Scanning electron microscope images of transferred graphene meshes after nanosphere growth and after nanosphere removal are shown in Fig. 1(d) and (e), respectively. Some resultant copper surfaces with differently sized silicon nanospheres are shown in the SEM images in Fig. 2(a). The process conditions can clearly be tuned by growth temperature and time to adjust the nanosphere size and densities to the desired values (10–100 nm). The magnification of each image was optimized to visualize the nanospheres in each process condition. The as grown samples where investigated by SEM, TEM and Auger electron spectroscopy (Fig. 2b and c). Auger electron spectra (Fig. 2c) revealed a silicon surface concentration of 47 atomic%, corresponding to a Si/carbon surface concentration ratio of approximately 1. Details of the extraction procedure of the surface concentration are described in the Methods section. The element mapping (EFTEM) of a TEM cross section in Fig. 2b reveals that the Si nanospheres oxidize at their surface, which corresponds with the high oxygen amount seen in the Auger electron spectrum. This enables their wet chemical removal with HF. The graphene film between the Si nanospheres is clearly visibly (marked yellow), and is not present on the Si spheres.
The proposed mechanism for the nanosphere formation was verified experimentally by measuring the diffusion constant of silicon in copper. For this purpose the same substrates as for graphene nanomesh CVD were used (Si wafers coated with 400 nm of Si3N4 and 5 μm of Cu). A temperature treatment similar to the graphene growth experiment was performed in an RTP reactor under forming gas (4% H2 in N2). The samples were heated with a ramp of 25 K s−1, annealed at 850 °C for 1, 3, 5 and 7 min intervals, and then cooled down rapidly with a rate of 25 K s−1. The diffusion constant was calculated from the diffusion pair model
| Annealing time | 1 min | 3 min | 5 min | 7 min |
| Si-surface concentration | 0% | 0% | 16% | 28% |
The grown graphene nanomeshes can be transferred to arbitrary substrates after the silicon nanosphere removal by established transfer methods (as in Fig. 1d and e). In this case a common wet transfer method with a PMMA film as a support layer and FeCl3 as the Cu wet etchant was used.30–33 Fluorine residues from the HF treatment can still be detected after the transfer, but a 10 min anneal at 300 °C in an UHV furnace reduces the fluorine residues below the detection limit of Auger electron spectroscopy.
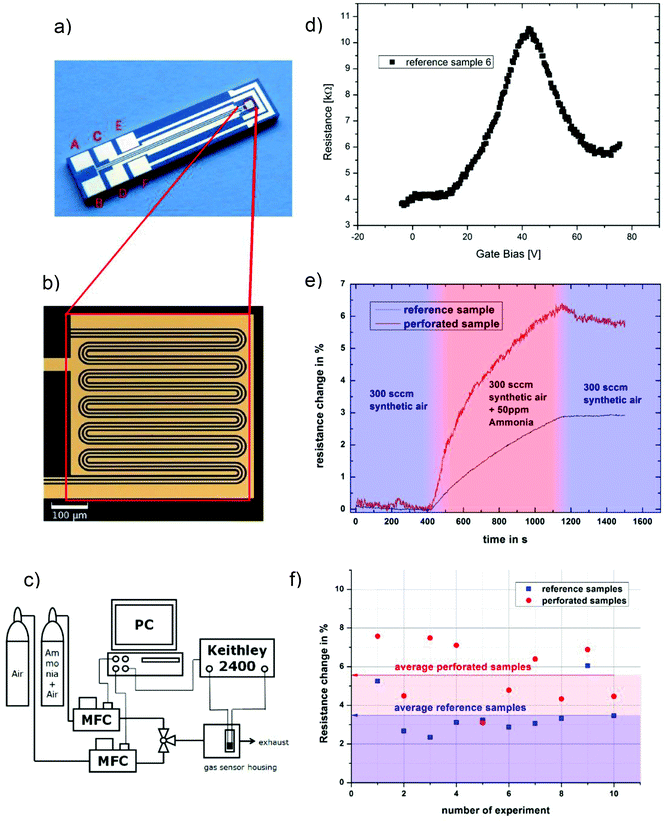
Defects and edges of graphene sheets are preferred adsorption sites for gas molecules. An important issue for manufacturing graphene devices is the sensitivity towards contamination, thus we investigated the effect of amines, which are typical gaseous contamination species in semiconductor manufacturing lines, e.g. from photoresist developers. In this study we used ammonia as model test gas: 20 samples were prepared: 10 samples with graphene nanomeshes grown according to the schematic process flow in Fig. 1a–c and 10 samples with homogeneous graphene films, produced at a lower temperature (800 °C) and growth time to avoid Si diffusion as described above. The films were transferred onto a gold meander electrode structure for electrical measurements (Fig. 3a and b). The layout allows two-point and 4-point I–V measurements, but the contact resistance proved to be negligible due to the extremely long contact length. Thus, only 2-point measurements were performed. The sheet resistance of several samples (both samples) was in the range of 10 kΩ to 1 MΩ, which is expected given the high defect density. Charge carrier mobility measurements are not meaningful due to the random device geometry and unknown current paths. A back gate sweep, where the Si substrates works as the gate electrode, indicates that the devices are working like typical graphene field effect transistors (Fig. 3d). In a flow-through gas exposure system (Fig. 3c), all samples were initially exposed to 200 sccm synthetic air flow at room temperature and pressure. After 400 s, 50 ppm of ammonia was added to the synthetic air flow for 900 s, before a final pure synthetic air purge. All measurements were done at constant measurement power (ID·VSD = 1 mW). Some measurements hence show a low S/N ratio due to the low measurement current. Fig. 3(e) compares measurements of one graphene nanomesh sensor and one graphene reference sensor. The resistance change of the devices was calculated by dividing the resistances before the start (at 400 s) and at the end (at 1200 s) of ammonia exposure:
All samples showed a resistance change between 2% and 8%. Generally the nanomesh samples show an increased sensitivity towards ammonia by an average factor of 1.6 (range: 0.85…2.14). Cagliani et al. reported a more drastic difference in resistance change for lithographically etched nanomesh devices,13 but under different measurement conditions. Under comparable measurement conditions Paul et al.19 found a sensitivity increase on lithographically etched nanomesh devices by a factor of 4.4. When analyzing the graphene egde/area ratio, which is mainly determining the gas sensitivity, by using SEM images our samples show a significantly lower ratio. Thus correcting our samples for this ratio, a sensitivity increase of factor 5.5 is found, which is in the same order of magnitude as in ref. 19. Additionally in this work the reference samples are grown at lower temperature which is known to yield very defective films.34 Thus the reference samples exhibit already increased gas sensitivity.
Furthermore, an incomplete recovery of the resistance values is observed after ammonia exposure. This can be attributed to the fact that the measurements were carried out at room temperature and ambient pressure, leading to incomplete gas desorption. The resistance changes of the entire set of samples, randomly chosen from different growth runs, are summarized in Fig. 3(f). The data was analyzed with a t-test to demonstrate the statistical significance of the difference between the two groups. The average value in the reference group is 3.54% with a standard deviation of 1.17%, while the graphene nanomesh sensors show an average of 5.66% with a standard deviation of 1.59%. The higher standard deviation in the nanomesh group can be explained by the fact that these samples have seen additional process steps with more influence sources. An F-test (α = 0.05) shows that there is no significant difference in the standard deviations. The two-tailed P value equals 0.33%, which means that the difference between the two groups is statistically very significant using conventional criteria (i.e. a 95% confidence interval).
We demonstrated the fabrication and performance of graphene nanomesh devices through a bottom-up growth method that blocks certain growth sites on copper substrates with silicon nanospheres. These spheres are generated by diffusion of Si through a copper film at high temperatures. The diffusion mechanism was investigated by diffusion experiments using Auger electron spectroscopy measurements of the Si concentration on the Cu surface. The experimentally measured Si diffusion constant is consistent with literature. The Si nanospheres oxidize in air, which is shown in TEM cross sections, and thus can be removed by a HF wet etch. The graphene nanomesh films were transferred to large-area sensor test structures. Exposure to ammonia gas showed a factor of 1.6 increase in sensitivity compared to non-perforated reference graphene films. A commercial ammonia ZnO gas sensor is not working at room temperature. At 400 °C the ZnO has a sensitivity of 1.7% per ppm35 compared to 0.16% per ppm of the perforated sensor at room temperature. The proposed bottom-up growth method is simple, scalable in size and was demonstrated with typical semiconductor manufacturing equipment. It can be used to manufacture low cost, large scale graphene nanomesh films e.g. for sensor applications. In addition, it may be utilized to improve metal–graphene contacts11 if it can be applied locally on pre-patterned substrates.
Methods
Nanomesh growth
5 μm Cu films on 400 nm Si3N4 were used as CVD substrates. Prior to the CVD process in a laboratory reactor, the samples were annealed in hydrogen at 1000 °C to remove copper oxides. The CVD process was done at 1000 °C using an ethane/hydrogen mixture at 1 Torr. After removing the Si spheres with 3% HF solution (semiconductor grade) the samples were spin-coated with PMMA. Subsequently the Cu film was underetched with 1 mol FeCl3 solution and the floating PMMA/graphene film was picked up with a SiO2 wafer. Heating in a UHV furnace at 400 °C for 10 min removed residual PMMA and HF. After the transfer the Si/Si3N4 substrates are reusable. Cleaning of the substrates and sputter deposition of Cu will lead to the initial conditions.Auger measurements
The Si surface concentration after annealing for 1, 3, 5 and 7 min was measured with Auger electron spectroscopy. As surfaces in ambient are covered with adventitious carbon, the signal attenuation by this carbon layer has to be eliminated to calculate the correct surface composition. The extraction procedure, including elimination of the natural carbon contamination, was as follows: the signal intensities of the Auger electron peaks from carbon (CKLL at 275 eV), copper (CuLMM at 922 eV) and silicon (SiKLL at 1621 eV) were multiplied with the individual sensitivity factors to calculate the surface concentrations. The CKLL/(CuLMM + SiKLL) intensity ratio leads to a virtual atomic concentration ratio of 50% Si on Cu which corresponds to an estimated carbon layer thickness of 1 nm. The electron energy dependent attenuation in the carbon layer can be eliminated by a correction factor depending on the mean free path λA(EA) of the element specific Auger electrons in the carbon layer and the thickness of the layer dA:The inelastic electron mean free path was calculated by the NIST electron mean-free-path database v1.1 software, yielding values of 1.53 nm for the CuLMM and 2.51 nm for the SiKLL Auger electrons. To obtain the real Cu/Si atomic ratio, the Si atomic concentration was corrected from the native SiO2 layer taking the ion radii of Si4+ (40 pm) and O2− (140 pm) into account.
Electric gas measurements
The graphene films were transferred to test devices with gold meander structures (Fig. 3a and b). The gas exposure was controlled by mass flow controllers. The samples were exposed to gas inside a small housing, allowing fast gas exchange. I–V characteristics were measured using a Keithley 2400 source meter. In this setup, the back gate cannot be controlled during the gas measurements. However, as the measurement chamber is closed and the chip area is rather large compared to the active area, we conclude that a floating back gate should have a negligible influence.Acknowledgements
The authors gratefully acknowledge funding from the German Federal Ministry of Education and Research (BMBF, NanoGraM, 03XP0006C). ML further acknowledges funding from the European Commission (ERC Starting Grant, InteGraDe, 307311) and the German Research Foundation (DFG, LE 2440/1-2).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- C. Chen and J. Hone, Proc. IEEE, 2013, 101, 1766–1779 CrossRef CAS.
- F. Bonaccorso, Z. Sun, T. Hasan and A. C. Ferrari, Nat. Photonics, 2010, 4, 611–622 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- K. R. Ratinac, W. Yang, S. P. Ringer and F. Braet, Environ. Sci. Technol., 2010, 44, 1167–1176 CrossRef CAS PubMed.
- Y. Dan, Y. Lu, N. J. Kybert, Z. Luo and A. T. C. Johnson, Nano Lett., 2009, 9, 1472–1475 CrossRef CAS PubMed.
- A. D. Smith, K. Elgammal, F. Niklaus, A. Delin, A. C. Fischer, S. Vaziri, F. Forsberg, M. Råsander, H. Hugosson, L. Bergqvist, S. Schröder, S. Kataria, M. Östling and M. C. Lemme, Nanoscale, 2015, 7, 19099–19109 RSC.
- Z. Chen, Y.-M. Lin, M. J. Rooks and P. Avouris, Phys. E, 2007, 40, 228–232 CrossRef CAS.
- M. Y. Han, B. Ozyilmaz, Y. Zhang and P. Kim, Phys. Rev. Lett., 2007, 98, 206805–206804 CrossRef PubMed.
- W. S. Leong, H. Gong and J. T. L. Thong, ACS Nano, 2014, 8, 994–1001 CrossRef CAS PubMed.
- S. Thongrattanasiri, F. H. L. Koppens and F. J. García de Abajo, Phys. Rev. Lett., 2012, 108, 047401 CrossRef PubMed.
- A. Cagliani, D. M. A. Mackenzie, L. K. Tschammer, F. Pizzocchero, K. Almdal and P. Bøggild, Nano Res., 2014, 7, 743–754 CrossRef CAS.
- J. Bai, X. Zhong, S. Jiang, Y. Huang and X. Duan, Nat. Nanotechnol., 2010, 5, 190–194 CrossRef CAS PubMed.
- J. G. Son, M. Son, K.-J. Moon, B. H. Lee, J.-M. Myoung, M. S. Strano, M.-H. Ham and C. A. Ross, Adv. Mater., 2013, 25, 4723–4728 CrossRef CAS PubMed.
- C. X. Cong, T. Yu, Z. H. Ni, L. Liu, Z. X. Shen and W. Huang, J. Phys. Chem. C, 2009, 113, 6529–6532 CAS.
- L. Liu, Y. Zhang, W. Wang, C. Gu, X. Bai and E. Wang, Adv. Mater., 2011, 23, 1246–1251 CrossRef CAS PubMed.
- M. Wang, L. Fu, L. Gan, C. Zhang, M. Rümmeli, A. Bachmatiuk, K. Huang, Y. Fang and Z. Liu, Sci. Rep., 2013, 3, 1238 Search PubMed.
- R. K. Paul, S. Badhulika, N. M. Saucedo and A. Mulchandani, Anal. Chem., 2012, 84, 8171–8178 CrossRef CAS PubMed.
- J. Bai, X. Duan and Y. Huang, Nano Lett., 2009, 9, 2083–2087 CrossRef CAS PubMed.
- J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Müllen and R. Fasel, Nature, 2010, 466, 470–473 CrossRef CAS PubMed.
- G. Lupina, J. Kitzmann, I. Costina, M. Lukosius, C. Wenger, A. Wolff, S. Vaziri, M. Ostling, I. Pasternak and A. Krajewska, et al. , ACS Nano, 2015, 9, 4776–4785 CrossRef CAS PubMed.
- M. Acik and Y. J. Chabal, Jpn. J. Appl. Phys., 2011, 50, 070101 CrossRef.
- M. Dvorak, W. Oswald and Z. Wu, Sci. Rep., 2013, 3, 2289 Search PubMed.
- Y. Hajati, T. Blom, S. H. M. Jafri, S. Haldar, S. Bhandary, M. Z. Shoushtari, O. Eriksson, B. Sanyal and K. Leifer, Nanotechnology, 2012, 23, 505501 CrossRef CAS PubMed.
- J. Yi, D. H. Lee, W. W. Lee and W. I. Park, J. Phys. Chem. Lett., 2013, 4, 2099–2104 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- G. Neumann and C. Tuijn, Self-diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data, Elsevier, 2011 Search PubMed.
- C. A. Howsare, X. Weng, V. Bojan, D. Snyder and J. A. Robinson, Nanotechnology, 2012, 23, 135601 CrossRef PubMed.
- A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- S. Kataria, S. Wagner, J. Ruhkopf, A. Gahoi, H. Pandey, R. Bornemann, S. Vaziri, A. D. Smith, M. Ostling and M. C. Lemme, Phys. Status Solidi A, 2014, 211, 2439–2449 CrossRef CAS.
- C. Mattevi, H. Kim and M. Chhowalla, J. Mater. Chem., 2011, 21, 3324–3334 RSC.
- L. A. Patil, L. S. Sonawane and D. G. Patil, J. Mod. Phys., 2011, 2, 1215–1221 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |