 Open Access Article
Open Access ArticleInfluence of lipid bilayer properties on nanodisc formation mediated by styrene/maleic acid copolymers†
Rodrigo
Cuevas Arenas
,
Johannes
Klingler
,
Carolyn
Vargas
and
Sandro
Keller
*
Molecular Biophysics, University of Kaiserslautern, Erwin-Schrödinger-Str. 13, 67663 Kaiserslautern, Germany. E-mail: mail@sandrokeller.com
First published on 8th July 2016
Abstract
Copolymers of styrene and maleic acid (SMA) have gained great attention as alternatives to conventional detergents, as they offer decisive advantages for studying membrane proteins and lipids in vitro. These polymers self-insert into artificial and biological membranes and, at sufficiently high concentrations, solubilise them into disc-shaped nanostructures containing a lipid bilayer core surrounded by a polymer belt. We have used 31P nuclear magnetic resonance spectroscopy and dynamic light scattering to systematically study the solubilisation of vesicles composed of saturated or unsaturated phospholipids by an SMA copolymer with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 styrene/maleic acid molar ratio at different temperatures. Solubilisation was thermodynamically rationalised in terms of a three-stage model that treats various lipid/polymer aggregates as pseudophases. The solubilising capacity of SMA(3
1 styrene/maleic acid molar ratio at different temperatures. Solubilisation was thermodynamically rationalised in terms of a three-stage model that treats various lipid/polymer aggregates as pseudophases. The solubilising capacity of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) towards a saturated lipid is higher in the gel than in the liquid–crystalline state of the membrane even though solubilisation is slower. Although the solubilisation of mixed fluid membranes is non-selective, the presence of a non-bilayer phospholipid lowers the threshold at which the membrane becomes saturated with SMA(3
1) towards a saturated lipid is higher in the gel than in the liquid–crystalline state of the membrane even though solubilisation is slower. Although the solubilisation of mixed fluid membranes is non-selective, the presence of a non-bilayer phospholipid lowers the threshold at which the membrane becomes saturated with SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) but raises the polymer concentration required for complete solubilisation. Both of these trends can be explained by considering the vesicle-to-nanodisc transfer free energies of the lipid and the polymer. On the basis of the phase diagrams thus obtained, re-association of polymer-solubilised lipids with vesicles is possible under mild conditions, which has implications for the reconstitution of proteins and lipids from nanodiscs into vesicular membranes. Finally, the phase diagrams provide evidence for the absence of free SMA(3
1) but raises the polymer concentration required for complete solubilisation. Both of these trends can be explained by considering the vesicle-to-nanodisc transfer free energies of the lipid and the polymer. On the basis of the phase diagrams thus obtained, re-association of polymer-solubilised lipids with vesicles is possible under mild conditions, which has implications for the reconstitution of proteins and lipids from nanodiscs into vesicular membranes. Finally, the phase diagrams provide evidence for the absence of free SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in vesicular lipid suspensions.
1) in vesicular lipid suspensions.
Introduction
A recent development in the study of membrane proteins and lipid bilayers is the use of amphiphilic copolymers composed of styrene and maleic acid (SMA) units. SMA copolymers of different lengths and styrene/maleic acid ratios are able to directly solubilise phospholipids and membrane proteins from artificial1,2 and natural bilayers3–5 to form discoidal SMA/lipid particles (SMALPs). These nanodiscs contain a lipid bilayer core surrounded by a polymer belt2,6 and have diameters of 10–30 nm,2,6–8 which renders them suitable for optical spectroscopy,1,2,4,5,9–12 nuclear magnetic resonance (NMR) spectroscopy,2,7,13 and other techniques requiring relatively small particles with a unimodal size distribution such as protein chromatography.8,14–17We have recently shown7 that solubilisation of large unilamellar vesicles (LUVs) formed from the unsaturated zwitterionic phospholipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) by an SMA copolymer having a styrene/maleic acid molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (SMA(3
1 (SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) can be conceptualised with the aid of a three-stage equilibrium model. This model, which originally was developed to explain the solubilisation of lipid bilayers by surfactants such as typical head-and-tail detergents,18 treats the different supramolecular aggregates formed during solubilisation as distinct pseudophases dispersed in an aqueous solution.7,19,20 Accordingly, titration of a lipid (L) membrane with surfactant (S) initially results in the accommodation of surfactant molecules in the bilayer (b), thereby inducing increasingly positive curvature stress. At a critical surfactant/lipid molar ratio denoted Rb,SATS (cf. eqn (1) in ESI†), the bilayer becomes saturated (SAT) with surfactant. Addition of more surfactant initiates solubilisation, that is, extraction of phospholipids from the membrane in the form of mixed lipid/surfactant micelles (m). According to Gibbs’ phase rule, this leads to surfactant-saturated bilayer vesicles coexisting with lipid-saturated micelles until a second critical surfactant/lipid molar ratio Rm,SOLS (eqn (2)†) is reached, where solubilisation (SOL) is complete. By determining the surfactant concentrations at the onset and completion of solubilisation at several lipid concentrations, it is possible to construct the corresponding pseudophase diagram, with the coexistence range separating the purely vesicular range from the purely micellar range. The slopes of the phase boundaries yield Rb,SATS and Rm,SOLS and, through some simple relationships (eqn (3)–(8)†), the Gibbs free energies of lipid and surfactant transfer from vesicular bilayers into micelles, ΔGb→m,oL and ΔGb→m,oS, respectively. These values provide a quantitative, thermodynamic basis for rationalising the surfactant's detergency as reflected in its solubilising power towards the lipid membrane system under study.7,18–20
1)) can be conceptualised with the aid of a three-stage equilibrium model. This model, which originally was developed to explain the solubilisation of lipid bilayers by surfactants such as typical head-and-tail detergents,18 treats the different supramolecular aggregates formed during solubilisation as distinct pseudophases dispersed in an aqueous solution.7,19,20 Accordingly, titration of a lipid (L) membrane with surfactant (S) initially results in the accommodation of surfactant molecules in the bilayer (b), thereby inducing increasingly positive curvature stress. At a critical surfactant/lipid molar ratio denoted Rb,SATS (cf. eqn (1) in ESI†), the bilayer becomes saturated (SAT) with surfactant. Addition of more surfactant initiates solubilisation, that is, extraction of phospholipids from the membrane in the form of mixed lipid/surfactant micelles (m). According to Gibbs’ phase rule, this leads to surfactant-saturated bilayer vesicles coexisting with lipid-saturated micelles until a second critical surfactant/lipid molar ratio Rm,SOLS (eqn (2)†) is reached, where solubilisation (SOL) is complete. By determining the surfactant concentrations at the onset and completion of solubilisation at several lipid concentrations, it is possible to construct the corresponding pseudophase diagram, with the coexistence range separating the purely vesicular range from the purely micellar range. The slopes of the phase boundaries yield Rb,SATS and Rm,SOLS and, through some simple relationships (eqn (3)–(8)†), the Gibbs free energies of lipid and surfactant transfer from vesicular bilayers into micelles, ΔGb→m,oL and ΔGb→m,oS, respectively. These values provide a quantitative, thermodynamic basis for rationalising the surfactant's detergency as reflected in its solubilising power towards the lipid membrane system under study.7,18–20
It has been shown21 that membrane solubilisation by SMA copolymers is faster (i) for short-chain phospholipids, (ii) at elevated temperatures and, particularly, above the main phase transition temperature (Tm), and (iii) at higher ionic strengths. However, it remains unclear if and to what extent the kinetics of solubilisation reflects the solubilising power of SMA from a thermodynamic perspective, that is, if faster solubilisation correlates with lower Rb,SATS and Rm,SOLS boundaries and less endergonic or more exergonic values of ΔGb→m,oL and ΔGb→m,oS, respectively. Here, we address this question by following the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-mediated solubilisation of LUVs composed of the zwitterionic saturated phospholipid 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) both above and below its Tm to quantify the polymer's solubilising capacity towards membranes in the liquid–crystalline (i.e., fluid) and gel phases, respectively. Importantly, thermodynamic efficiency was found not to correlate with kinetic efficiency, as slow solubilisation of gel-phase membranes requires less SMA(3
1)-mediated solubilisation of LUVs composed of the zwitterionic saturated phospholipid 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) both above and below its Tm to quantify the polymer's solubilising capacity towards membranes in the liquid–crystalline (i.e., fluid) and gel phases, respectively. Importantly, thermodynamic efficiency was found not to correlate with kinetic efficiency, as slow solubilisation of gel-phase membranes requires less SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) than does faster solubilisation of fluid-phase membranes. On the basis of the phase diagrams thus obtained, we could demonstrate re-association of polymer-solubilised lipid with vesicular bilayer membranes both above and below Tm. Finally, we used mixed LUVs composed of the singly unsaturated phospholipids POPC and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) to examine the influence of membrane composition on the thermodynamics of solubilisation. While the biophysical properties of the membrane such as lipid packing and phospholipid order were found to play an important role in determining the solubilising capacity of SMA(3
1) than does faster solubilisation of fluid-phase membranes. On the basis of the phase diagrams thus obtained, we could demonstrate re-association of polymer-solubilised lipid with vesicular bilayer membranes both above and below Tm. Finally, we used mixed LUVs composed of the singly unsaturated phospholipids POPC and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) to examine the influence of membrane composition on the thermodynamics of solubilisation. While the biophysical properties of the membrane such as lipid packing and phospholipid order were found to play an important role in determining the solubilising capacity of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), different lipid species residing in the same membrane were extracted non-preferentially throughout all stages of solubilisation.
1), different lipid species residing in the same membrane were extracted non-preferentially throughout all stages of solubilisation.
Results
Solubilisation of fluid-phase DMPC vesicles
We first examined the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-driven solubilisation of unilamellar vesicles composed of the saturated phospholipid DMPC at 30 °C, that is, 6 °C above its Tm. For this purpose, we used 31P NMR spectroscopy22–24 to quantify the amount of lipid solubilised in SMALPs.7 In the absence of polymer and at SMA(3
1)-driven solubilisation of unilamellar vesicles composed of the saturated phospholipid DMPC at 30 °C, that is, 6 °C above its Tm. For this purpose, we used 31P NMR spectroscopy22–24 to quantify the amount of lipid solubilised in SMALPs.7 In the absence of polymer and at SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations below the SAT boundary, the signal arising from vesicular phospholipids was broadened beyond detection (Fig. 1a). At SMA(3
1) concentrations below the SAT boundary, the signal arising from vesicular phospholipids was broadened beyond detection (Fig. 1a). At SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations greater than SAT, an isotropic, Lorentzian-shaped peak appeared at −0.3 ppm, whose area increased linearly upon further addition of polymer until plateauing at the SOL boundary, where all lipid was solubilised into SMALPs (Fig. 1b). While the peak area remained constant beyond SOL, there was a slight downfield shift of the peak centre with increasing SMA(3
1) concentrations greater than SAT, an isotropic, Lorentzian-shaped peak appeared at −0.3 ppm, whose area increased linearly upon further addition of polymer until plateauing at the SOL boundary, where all lipid was solubilised into SMALPs (Fig. 1b). While the peak area remained constant beyond SOL, there was a slight downfield shift of the peak centre with increasing SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Such a shift reflects a change in the average chemical environment of the 31P nuclei, which we attribute to a rise in the fraction of phospholipid molecules found close to the nanodisc rim, that is, in the vicinity of the polymer. Plotting the SMA(3
1) concentration. Such a shift reflects a change in the average chemical environment of the 31P nuclei, which we attribute to a rise in the fraction of phospholipid molecules found close to the nanodisc rim, that is, in the vicinity of the polymer. Plotting the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations at SAT and SOL for different DMPC concentrations yielded a phase diagram (Fig. 1c). From the slopes of the phase boundaries, we derived saturating and solubilising SMA(3
1) concentrations at SAT and SOL for different DMPC concentrations yielded a phase diagram (Fig. 1c). From the slopes of the phase boundaries, we derived saturating and solubilising SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/DMPC molar ratios of Rb,SATS = 0.078 ± 0.008 and Rm,SOLS = 0.144 ± 0.014, respectively. These values translate into vesicle-to-SMALP transfer free energies of ΔGb→m,oL = (0.15 ± 0.05) kJ mol−1 and ΔGb→m,oS = −(1.36 ± 0.45) kJ mol−1 for DMPC and SMA(3
1)/DMPC molar ratios of Rb,SATS = 0.078 ± 0.008 and Rm,SOLS = 0.144 ± 0.014, respectively. These values translate into vesicle-to-SMALP transfer free energies of ΔGb→m,oL = (0.15 ± 0.05) kJ mol−1 and ΔGb→m,oS = −(1.36 ± 0.45) kJ mol−1 for DMPC and SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively (Table S1†). Both the SAT and the SOL boundaries in the phase diagram (Fig. 1c) revealed a negligible ordinate intercept, confirming the absence of “free” SMA(3
1), respectively (Table S1†). Both the SAT and the SOL boundaries in the phase diagram (Fig. 1c) revealed a negligible ordinate intercept, confirming the absence of “free” SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in solution throughout the vesicular and coexistence ranges.7
1) in solution throughout the vesicular and coexistence ranges.7
 | ||
Fig. 1 Solubilisation of fluid-phase DMPC vesicles by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C as monitored by 31P NMR. (a) NMR spectra of 10 mM DMPC initially present in the form of LUVs upon exposure to increasing concentrations of SMA(3 1) at 30 °C as monitored by 31P NMR. (a) NMR spectra of 10 mM DMPC initially present in the form of LUVs upon exposure to increasing concentrations of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (b) Peak areas, A, at four DMPC concentrations as functions of SMA(3 1). (b) Peak areas, A, at four DMPC concentrations as functions of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Experimental data (circles) derived from titrations such as that in (a) and linear fits (lines) according to eqn (9)–(11).† (c) Phase diagram of DMPC/SMA(3 1) concentration. Experimental data (circles) derived from titrations such as that in (a) and linear fits (lines) according to eqn (9)–(11).† (c) Phase diagram of DMPC/SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints derived from local fits in (b) and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of local fits based on eqn (9)–(11).† (d) NMR spectra of 2.5 mM DMPC and 0.4 mM SMA(3 1) at 30 °C. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints derived from local fits in (b) and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of local fits based on eqn (9)–(11).† (d) NMR spectra of 2.5 mM DMPC and 0.4 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) initially present in the form of SMALPs upon addition of DMPC LUVs. Inset: Phase diagram of DMPC/SMA(3 1) initially present in the form of SMALPs upon addition of DMPC LUVs. Inset: Phase diagram of DMPC/SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C indicating the SAT (red) and SOL (blue) boundaries from (c) and the DMPC concentrations tested in the reconstitution assay (triangles). 1) at 30 °C indicating the SAT (red) and SOL (blue) boundaries from (c) and the DMPC concentrations tested in the reconstitution assay (triangles). | ||
We also tested for reversibility of the solubilisation process, that is, whether lipids residing in preformed SMALPs are able to interact with and become re-associated with lipid vesicles, which might suggest a novel route for reconstituting SMALP-solubilised lipids and proteins into artificial vesicles. Guided by the phase diagram (Fig. 1c), we first solubilised 2.5 mM vesicular DMPC by adding 0.4 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to form SMALPs. As expected, these preformed SMALPs produced a sharp NMR signal (Fig. 1d). Upon titration with increasing concentrations of DMPC in the form of LUVs, the signal intensity decreased as the SMA(3
1) to form SMALPs. As expected, these preformed SMALPs produced a sharp NMR signal (Fig. 1d). Upon titration with increasing concentrations of DMPC in the form of LUVs, the signal intensity decreased as the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration dropped below the SOL boundary and was broadened beyond detection when the SAT threshold was reached (Fig. 1d). Thus, re-association of phospholipids residing in SMALPs with vesicles can be triggered by addition of excess lipid, implying that the formation of freely diffusing nanodiscs is a dynamic and reversible process.
1) concentration dropped below the SOL boundary and was broadened beyond detection when the SAT threshold was reached (Fig. 1d). Thus, re-association of phospholipids residing in SMALPs with vesicles can be triggered by addition of excess lipid, implying that the formation of freely diffusing nanodiscs is a dynamic and reversible process.
In the absence of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), dynamic light scattering (DLS) yielded a monoexponential autocorrelation function for DMPC LUVs (Fig. 2a), from which we derived a unimodal vesicle size distribution with a z-average size and peak width of (121 ± 12) nm (Fig. 2b and c). Complete solubilisation with SMA(3
1), dynamic light scattering (DLS) yielded a monoexponential autocorrelation function for DMPC LUVs (Fig. 2a), from which we derived a unimodal vesicle size distribution with a z-average size and peak width of (121 ± 12) nm (Fig. 2b and c). Complete solubilisation with SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave rise to autocorrelation functions typical of small particles undergoing rapid Brownian motion (Fig. 2a–c). Importantly, however, the size of the SMALPs considerably decreased with increasing SMA(3
1) gave rise to autocorrelation functions typical of small particles undergoing rapid Brownian motion (Fig. 2a–c). Importantly, however, the size of the SMALPs considerably decreased with increasing SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Irrespective of the lipid concentration tested, the nanodiscs displayed a z-average size and peak width of (29 ± 13) nm at an SMA(3
1) concentration. Irrespective of the lipid concentration tested, the nanodiscs displayed a z-average size and peak width of (29 ± 13) nm at an SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/DMPC ratio of 0.17, that is, slightly above Rm,SOLS, but addition of excess SMA(3
1)/DMPC ratio of 0.17, that is, slightly above Rm,SOLS, but addition of excess SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) further reduced the particle size to (13 ± 6) nm at an SMA(3
1) further reduced the particle size to (13 ± 6) nm at an SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/DMPC ratio of 0.5 (Fig. 2d). The latter value is in good agreement with SMALP sizes reported for various combinations of phospholipids with excess SMA.1,6,11,21 Our findings demonstrate that the average size of SMALPs can be tuned by controlling the polymer/lipid molar ratio. DLS also showed that subsolubilising SMA(3
1)/DMPC ratio of 0.5 (Fig. 2d). The latter value is in good agreement with SMALP sizes reported for various combinations of phospholipids with excess SMA.1,6,11,21 Our findings demonstrate that the average size of SMALPs can be tuned by controlling the polymer/lipid molar ratio. DLS also showed that subsolubilising SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations led to the formation of larger particles in suspension (Fig. 2b and c), which have been speculated7 to represent vesicle aggregates or agglomerates cross-linked by polymer chains.
1) concentrations led to the formation of larger particles in suspension (Fig. 2b and c), which have been speculated7 to represent vesicle aggregates or agglomerates cross-linked by polymer chains.
 | ||
Fig. 2 Solubilisation of fluid-phase DMPC LUVs by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C as monitored by DLS. (a) Normalised autocorrelation functions, C(τ), versus delay time, τ, as determined for 4 mM DMPC LUVs (red) and after complete solubilisation with 2 mM SMA(3 1) at 30 °C as monitored by DLS. (a) Normalised autocorrelation functions, C(τ), versus delay time, τ, as determined for 4 mM DMPC LUVs (red) and after complete solubilisation with 2 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (black). Note that the low ordinate intercept of the LUV autocorrelation function is due to excessive scattering resulting from the large number of vesicles initially present in the sample. Virtually identical size distribution functions and z-average values were obtained from diluted LUV suspensions (data not shown). (b) Intensity-weighted particle size distribution functions, f(d), versus hydrodynamic diameter, d, obtained upon exposure of 4 mM DMPC LUVs to increasing concentrations of SMA(3 1) (black). Note that the low ordinate intercept of the LUV autocorrelation function is due to excessive scattering resulting from the large number of vesicles initially present in the sample. Virtually identical size distribution functions and z-average values were obtained from diluted LUV suspensions (data not shown). (b) Intensity-weighted particle size distribution functions, f(d), versus hydrodynamic diameter, d, obtained upon exposure of 4 mM DMPC LUVs to increasing concentrations of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (c) Total light scattering intensity at 90°, I, and z-average particle diameter, z, upon solubilisation of 4 mM DMPC LUVs with SMA(3 1). (c) Total light scattering intensity at 90°, I, and z-average particle diameter, z, upon solubilisation of 4 mM DMPC LUVs with SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Also indicated are the SAT and SOL boundaries (dashed lines) derived from 31P NMR experiments (Fig. 1). Vertical bars denote peak widths as given by the corresponding PDI values. (d) z-Average particle diameters, z, at four DMPC concentrations as functions of the SMA(3 1). Also indicated are the SAT and SOL boundaries (dashed lines) derived from 31P NMR experiments (Fig. 1). Vertical bars denote peak widths as given by the corresponding PDI values. (d) z-Average particle diameters, z, at four DMPC concentrations as functions of the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/DMPC molar ratio, RSMA(3:1). Vertical bars denote peak widths as given by the corresponding PDI values. 1)/DMPC molar ratio, RSMA(3:1). Vertical bars denote peak widths as given by the corresponding PDI values. | ||
Solubilisation of gel-phase DMPC vesicles
Similarly, we followed the solubilisation of DMPC LUVs at 10 °C, that is, 14 °C below Tm. Challenging LUVs with increasing SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations resulted in the appearance of a sharp, isotropic 31P NMR peak (Fig. 3a). With saturating and solubilising SMA(3
1) concentrations resulted in the appearance of a sharp, isotropic 31P NMR peak (Fig. 3a). With saturating and solubilising SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/DMPC molar ratios of Rb,SATS = 0.039 ± 0.010 and Rm,SOLS = 0.095 ± 0.011, respectively (Fig. 3b and c), the onset and completion of gel-phase DMPC solubilisation were found to occur at even lower SMA(3
1)/DMPC molar ratios of Rb,SATS = 0.039 ± 0.010 and Rm,SOLS = 0.095 ± 0.011, respectively (Fig. 3b and c), the onset and completion of gel-phase DMPC solubilisation were found to occur at even lower SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations as compared with fluid-phase DMPC (Fig. 1b and c). This was reflected in vesicle-to-SMALP transfer free energies of ΔGb→m,oL = (0.12 ± 0.05) kJ mol−1 and ΔGb→m,oS = −(1.94 ± 0.79) kJ mol−1 at 10 °C. These values were, respectively, less endergonic for the lipid and more exergonic for the polymer than the corresponding free energies at 30 °C (Table S1†). Thus, although solubilisation of DMPC vesicles is slower at temperatures below than above Tm,21 less polymer is required to initiate and complete the extraction of DMPC molecules from vesicles at 10 °C than at 30 °C. Re-association of SMALP-solubilised lipids with vesicles was possible also below Tm (Fig. S1d†).
1) concentrations as compared with fluid-phase DMPC (Fig. 1b and c). This was reflected in vesicle-to-SMALP transfer free energies of ΔGb→m,oL = (0.12 ± 0.05) kJ mol−1 and ΔGb→m,oS = −(1.94 ± 0.79) kJ mol−1 at 10 °C. These values were, respectively, less endergonic for the lipid and more exergonic for the polymer than the corresponding free energies at 30 °C (Table S1†). Thus, although solubilisation of DMPC vesicles is slower at temperatures below than above Tm,21 less polymer is required to initiate and complete the extraction of DMPC molecules from vesicles at 10 °C than at 30 °C. Re-association of SMALP-solubilised lipids with vesicles was possible also below Tm (Fig. S1d†).
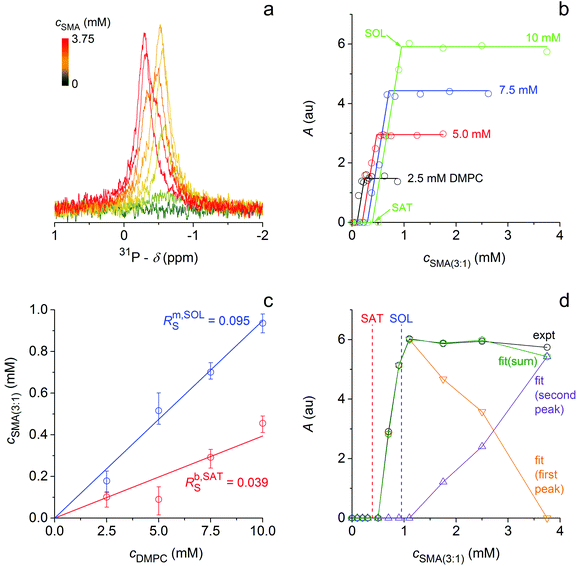 | ||
Fig. 3 Solubilisation of gel-phase DMPC vesicles by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 10 °C as monitored by 31P NMR. (a) NMR spectra of 10 mM DMPC initially present in the form of LUVs upon exposure to increasing concentrations of SMA(3 1) at 10 °C as monitored by 31P NMR. (a) NMR spectra of 10 mM DMPC initially present in the form of LUVs upon exposure to increasing concentrations of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (b) Peak areas, A, at four DMPC concentrations as functions of SMA(3 1). (b) Peak areas, A, at four DMPC concentrations as functions of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Experimental data (circles) derived from titrations such as that in (a) and linear fits (lines) according to eqn (9)–(11).† (c) Phase diagram of DMPC/SMA(3 1) concentration. Experimental data (circles) derived from titrations such as that in (a) and linear fits (lines) according to eqn (9)–(11).† (c) Phase diagram of DMPC/SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 10 °C. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints of fits in (b) and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of fits based on eqn (9)–(11).† (d) Peak areas, A, at 10 mM DMPC as functions of SMA(3 1) at 10 °C. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints of fits in (b) and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of fits based on eqn (9)–(11).† (d) Peak areas, A, at 10 mM DMPC as functions of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Experimental data (black) obtained from numerical peak integration and fitted values derived from a double-Lorentzian fit according to eqn (13)† for the first, upfield peak (orange), the second, downfield peak (purple), and the sum of both (green). Also indicated are the SAT and SOL boundaries (dashed lines) obtained from (c). 1) concentration. Experimental data (black) obtained from numerical peak integration and fitted values derived from a double-Lorentzian fit according to eqn (13)† for the first, upfield peak (orange), the second, downfield peak (purple), and the sum of both (green). Also indicated are the SAT and SOL boundaries (dashed lines) obtained from (c). | ||
A conspicuous difference between the experiments above and those below Tm was the shape of the 31P NMR peak. Throughout the coexistence range at 10 °C, a single Lorentzian-shaped peak (Fig. 3a) was observed at −0.6 ppm, that is, −0.3 ppm upfield of the corresponding peak at 30 °C (Fig. 1a). At 10 °C, addition of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) beyond Rm,SOLS caused a gradual reduction in the area of the original peak and a concomitant rise of a second Lorentzian-shaped peak at −0.3 ppm. The position of the latter peak was comparable to that of the single peak found at 30 °C, which changed only slightly in the course of the titration with SMA(3
1) beyond Rm,SOLS caused a gradual reduction in the area of the original peak and a concomitant rise of a second Lorentzian-shaped peak at −0.3 ppm. The position of the latter peak was comparable to that of the single peak found at 30 °C, which changed only slightly in the course of the titration with SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This suggests that, at 10 °C, the two peaks at −0.6 ppm and −0.3 ppm represent gel-phase and fluid-phase DMPC, respectively. Thus, by fitting a double-Lorentzian function (eqn (13)†) to the NMR spectra, we found that the fraction of lipid tentatively assigned to the fluid phase increased linearly with the concentration of SMA(3
1). This suggests that, at 10 °C, the two peaks at −0.6 ppm and −0.3 ppm represent gel-phase and fluid-phase DMPC, respectively. Thus, by fitting a double-Lorentzian function (eqn (13)†) to the NMR spectra, we found that the fraction of lipid tentatively assigned to the fluid phase increased linearly with the concentration of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) once solubilisation was complete (Fig. 3d). Another interesting difference with respect to solubilisation experiments performed at 30 °C was that the gel-phase nanodiscs existing at Rm,SOLS were already very small, with a z-average diameter of (11 ± 4) nm, which decreased only marginally upon further addition of SMA(3
1) once solubilisation was complete (Fig. 3d). Another interesting difference with respect to solubilisation experiments performed at 30 °C was that the gel-phase nanodiscs existing at Rm,SOLS were already very small, with a z-average diameter of (11 ± 4) nm, which decreased only marginally upon further addition of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S1a and b†).
1) (Fig. S1a and b†).
Solubilisation of mixed POPC/POPE vesicles
Finally, we probed the solubilising activity of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) towards mixed membranes composed of POPC and POPE, the latter of which does not form bilayers on its own. To this end, we systematically increased the mole fraction of POPE in a POPC matrix from 0 mol mol−1 (POPC/POPE(1
1) towards mixed membranes composed of POPC and POPE, the latter of which does not form bilayers on its own. To this end, we systematically increased the mole fraction of POPE in a POPC matrix from 0 mol mol−1 (POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)) to 0.75 mol mol−1 (POPC/POPE(1
0)) to 0.75 mol mol−1 (POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)). As exemplified by a representative 31P NMR spectrum of a POPC/POPE(1
3)). As exemplified by a representative 31P NMR spectrum of a POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (Fig. 4a), solubilisation with SMA(3
1) mixture (Fig. 4a), solubilisation with SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to the appearance of two peaks centred at −0.16 ppm and 0.27 ppm corresponding to the signals arising from POPC and POPE, respectively.25 The contribution of each phospholipid was quantified with the aid of a double-Lorentzian fit (eqn (13)†). For an equimolar lipid composition, the peak areas of the signals arising from POPC and POPE were identical across the entire range of SMA(3
1) led to the appearance of two peaks centred at −0.16 ppm and 0.27 ppm corresponding to the signals arising from POPC and POPE, respectively.25 The contribution of each phospholipid was quantified with the aid of a double-Lorentzian fit (eqn (13)†). For an equimolar lipid composition, the peak areas of the signals arising from POPC and POPE were identical across the entire range of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations (Fig. 4b), demonstrating that the polymer extracted both phospholipids non-preferentially from the vesicular bilayer not only upon complete solubilisation in the presence of excess SMA(3
1) concentrations (Fig. 4b), demonstrating that the polymer extracted both phospholipids non-preferentially from the vesicular bilayer not only upon complete solubilisation in the presence of excess SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)3,12,21,26 but throughout all solubilisation stages.
1)3,12,21,26 but throughout all solubilisation stages.
 | ||
Fig. 4 Solubilisation of an equimolar POPC/POPE mixture by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C as monitored by 31P NMR. (a) NMR spectrum of 5 mM POPC/POPE(1 1) at 30 °C as monitored by 31P NMR. (a) NMR spectrum of 5 mM POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) completely solubilised into SMALPs with 3 mM SMA(3 1) completely solubilised into SMALPs with 3 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (black solid line). Lorentzian fits to signals arising from POPE (green dashed line) and POPC (orange dashed line) and the sum of both (red dashed line) according to eqn (13).† (b) Peak areas, A, at 5 mM POPC/POPE(1 1) (black solid line). Lorentzian fits to signals arising from POPE (green dashed line) and POPC (orange dashed line) and the sum of both (red dashed line) according to eqn (13).† (b) Peak areas, A, at 5 mM POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) derived from spectra such as that in (a) as functions of SMA(3 1) derived from spectra such as that in (a) as functions of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration. Experimental data (black) obtained from numerical peak integration and fitted values derived from a double-Lorentzian fit according to eqn (13)† for POPE (green), POPC (orange), and the sum of both (red). Also indicated are the SAT and SOL boundaries (dashed lines) obtained from (c). (c) Phase diagram of POPC/POPE(1 1) concentration. Experimental data (black) obtained from numerical peak integration and fitted values derived from a double-Lorentzian fit according to eqn (13)† for POPE (green), POPC (orange), and the sum of both (red). Also indicated are the SAT and SOL boundaries (dashed lines) obtained from (c). (c) Phase diagram of POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and SMA(3 1) and SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints of linear fits at individual lipid concentrations according to eqn (9)–(11)† and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of fits based on eqn (9)–(11).† 1) mixtures. Pairs of cb,SATS and cm,SOLS (circles) obtained from breakpoints of linear fits at individual lipid concentrations according to eqn (9)–(11)† and global fits (solid lines) according to eqn (9), (10), and (12)† indicating the onset (SAT; red) and completion (SOL; blue) of solubilisation, respectively. Error bars are 95% confidence intervals of fits based on eqn (9)–(11).† | ||
The phase diagram obtained for POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is characterised by saturating and solubilising polymer/lipid ratios of Rb,SATS = 0.057 ± 0.009 and Rm,SOLS = 0.211 ± 0.008, respectively (Fig. 4c), yielding transfer free energies of ΔGb→m,oL = (0.34 ± 0.04) kJ mol−1 and ΔGb→m,oS = −(2.95 ± 0.45) kJ mol−1 (Table S1†). In general, raising the fraction of POPE in the bilayer resulted in decreasing Rb,SATS but increasing Rm,SOLS values (Fig. 5a), indicating that the membrane became saturated at lower SMA(3
1) is characterised by saturating and solubilising polymer/lipid ratios of Rb,SATS = 0.057 ± 0.009 and Rm,SOLS = 0.211 ± 0.008, respectively (Fig. 4c), yielding transfer free energies of ΔGb→m,oL = (0.34 ± 0.04) kJ mol−1 and ΔGb→m,oS = −(2.95 ± 0.45) kJ mol−1 (Table S1†). In general, raising the fraction of POPE in the bilayer resulted in decreasing Rb,SATS but increasing Rm,SOLS values (Fig. 5a), indicating that the membrane became saturated at lower SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) contents but required more polymer for complete solubilisation. As a consequence, the disparate POPE dependencies of the two critical ratios resulted in a broadening of the coexistence range (Fig. 5a). In terms of Gibbs free-energy changes, this means that the transfer from vesicles into SMALPs became increasingly unfavourable for the lipid but even more favourable for the polymer, as reflected in increasing magnitudes of both ΔGb→m,oL and ΔGb→m,oS, respectively (Fig. 5b, Table S1†). Mixed POPC/POPE SMALPs exhibited a z-average diameter of (29 ± 12) nm just beyond Rm,SOLS and further decreased in size, approaching (12 ± 5) nm in the presence of excess SMA(3
1) contents but required more polymer for complete solubilisation. As a consequence, the disparate POPE dependencies of the two critical ratios resulted in a broadening of the coexistence range (Fig. 5a). In terms of Gibbs free-energy changes, this means that the transfer from vesicles into SMALPs became increasingly unfavourable for the lipid but even more favourable for the polymer, as reflected in increasing magnitudes of both ΔGb→m,oL and ΔGb→m,oS, respectively (Fig. 5b, Table S1†). Mixed POPC/POPE SMALPs exhibited a z-average diameter of (29 ± 12) nm just beyond Rm,SOLS and further decreased in size, approaching (12 ± 5) nm in the presence of excess SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S2a–c†). Notwithstanding the differences in the thermodynamics of solubilisation, these particle sizes were essentially identical for all POPC/POPE mixtures tested (Fig. S2c†) and were comparable to those determined for fluid-phase DMPC SMALPs (Fig. 2).
1) (Fig. S2a–c†). Notwithstanding the differences in the thermodynamics of solubilisation, these particle sizes were essentially identical for all POPC/POPE mixtures tested (Fig. S2c†) and were comparable to those determined for fluid-phase DMPC SMALPs (Fig. 2).
 | ||
Fig. 5 Solubilisation thermodynamics of POPC/POPE LUVs by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 30 °C. (a) Saturating and solubilising molar ratios, Rb,SATS and Rm,SOLS, respectively, as functions of the mole fraction of POPE, XPOPE, in the membrane. Data were derived from solubilisation experiments monitored by 31P NMR spectroscopy such as those in Fig. 4. Error bars represent 95% confidence intervals of fits based on eqn (9), (10), and (12).† (b) Vesicle-to-SMALP transfer free energies, ΔGb→m,oL and ΔGb→m,oS, for lipids and SMA(3 1) at 30 °C. (a) Saturating and solubilising molar ratios, Rb,SATS and Rm,SOLS, respectively, as functions of the mole fraction of POPE, XPOPE, in the membrane. Data were derived from solubilisation experiments monitored by 31P NMR spectroscopy such as those in Fig. 4. Error bars represent 95% confidence intervals of fits based on eqn (9), (10), and (12).† (b) Vesicle-to-SMALP transfer free energies, ΔGb→m,oL and ΔGb→m,oS, for lipids and SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively, derived from values in (a) with the aid of eqn (3)–(8).† 1), respectively, derived from values in (a) with the aid of eqn (3)–(8).† | ||
Discussion
The ability to directly solubilise lipids and proteins from membranes into discoidal nanoparticles while retaining a bilayer architecture sets SMA copolymers apart not only from conventional head-and-tail detergents but also from other disc-forming amphiphiles such as bicelle-forming surfactant mixtures27 and protein-bounded nanodiscs.28 In the following, we discuss the most salient conclusions derived from the above experiments, namely, (i) the polymer-mediated solubilisation of gel-phase bilayers, (ii) the influence of acyl-chain packing on solubilisation, (iii) the re-association of lipids from nanodiscs with vesicular bilayers, (iv) the non-selectivity of mixed-membrane solubilisation, (v) the absence of “free” polymer, and (vi) discrepancies between the present thermodynamic observations and previous structural studies6 regarding the polymer/lipid ratio in SMALPs.Solubilisation of gel-phase membranes
Although polymer-induced vesicle dissolution proceeds more slowly below than above Tm,21 we found that, from a thermodynamic viewpoint, solubilisation of gel-phase DMPC (Fig. 3b and c) is more efficient than when the same lipid is present in its liquid–crystalline state (Fig. 1b and c). Moreover, the SMALPs thus formed appear to contain a gel-phase bilayer core near Rm,SOLS but experience acyl-chain melting as the concentration of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is further increased (Fig. 3a and d). Similar observations have previously been made with conventional head-and-tail surfactants29–32 and have been ascribed to the lower tolerance of tightly packed gel-phase membranes against perturbations caused by surfactant insertion into the lipid bilayer.19 Addition of surfactant to gel-phase membranes induces packing defects, and accumulation of high local surfactant concentrations in these defects induces strong positive curvature, which leads to the fracture of the vesicular bilayer at relatively low surfactant contents to give rise to gel-phase, surfactant-depleted lamellar sheets or discs surrounded by a surfactant-rich rim.19,33–35 Further addition of surfactant causes both the overall size of these nanostructures and, accordingly, the size of their gel-phase core to decrease until there is no gel phase left in what can then be regarded as fluid, surfactant-rich micelles.19,35 It seems very plausible that the superior solubilising power of SMA(3
1) is further increased (Fig. 3a and d). Similar observations have previously been made with conventional head-and-tail surfactants29–32 and have been ascribed to the lower tolerance of tightly packed gel-phase membranes against perturbations caused by surfactant insertion into the lipid bilayer.19 Addition of surfactant to gel-phase membranes induces packing defects, and accumulation of high local surfactant concentrations in these defects induces strong positive curvature, which leads to the fracture of the vesicular bilayer at relatively low surfactant contents to give rise to gel-phase, surfactant-depleted lamellar sheets or discs surrounded by a surfactant-rich rim.19,33–35 Further addition of surfactant causes both the overall size of these nanostructures and, accordingly, the size of their gel-phase core to decrease until there is no gel phase left in what can then be regarded as fluid, surfactant-rich micelles.19,35 It seems very plausible that the superior solubilising power of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) below Tm is due to a similar mechanism: the polymer's phenyl groups can self-insert into the gel-phase bilayer only by creating packing defects, leading to the formation of gel-phase nanodiscs at Rb,SATS and throughout the coexistence range. Once solubilisation is complete at Rm,SOLS, titration with more polymer gradually raises the proportion of fluid-phase lipid in the polymer-rich rim at the expense of gel-phase lipid in the nanodisc centre.
1) below Tm is due to a similar mechanism: the polymer's phenyl groups can self-insert into the gel-phase bilayer only by creating packing defects, leading to the formation of gel-phase nanodiscs at Rb,SATS and throughout the coexistence range. Once solubilisation is complete at Rm,SOLS, titration with more polymer gradually raises the proportion of fluid-phase lipid in the polymer-rich rim at the expense of gel-phase lipid in the nanodisc centre.
Influence of acyl-chain packing
POPC is a bilayer-forming lipid naturally occurring in eukaryotic cell and organelle membranes and is widely used for in vitro studies of membranes and membrane-interacting compounds.36 By contrast, POPE does not form bilayers on its own; phospholipids with phosphoethanolamine headgroups are abundant in prokaryotes, accounting, for instance, for up to 70% of the lipids in the inner membrane and the inner leaflet of the outer membrane of Escherichia coli,37 a Gram-negative bacterium extensively used for heterologous protein production.38 Lacking the three N-methyl groups present in phosphocholine, the smaller phosphoethanolamine moiety reduces the lateral pressure in the headgroup regions of POPE-containing mixed lipid bilayers, which is compensated for by increased pressure in the acyl-chain core.39–41 Since these changes in the lateral pressure profile have been shown21 to correlate with slower polymer-induced membrane solubilisation, we tested the influence of increasing POPE contents on the thermodynamics of SMALP formation. Although SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was able to completely solubilise mixed LUVs containing up to 75 mol% POPE (Fig. 4), increasing the lateral pressure in the bilayer core exerted a pronounced influence on the thermodynamics of solubilisation, namely, a decrease in Rb,SATS and a concomitant increase in Rm,SOLS (Fig. 5a) or, equivalently, an increase in the positive value of ΔGb→m,oL and a concomitant decrease in the negative value of ΔGb→m,oS (Fig. 5b). The opposing trends in Rb,SATS and Rm,SOLS can both be explained by considering the effect of POPE on the lateral pressure profile of the membrane. On the one hand, before solubilisation sets in, accommodation of SMA(3
1) was able to completely solubilise mixed LUVs containing up to 75 mol% POPE (Fig. 4), increasing the lateral pressure in the bilayer core exerted a pronounced influence on the thermodynamics of solubilisation, namely, a decrease in Rb,SATS and a concomitant increase in Rm,SOLS (Fig. 5a) or, equivalently, an increase in the positive value of ΔGb→m,oL and a concomitant decrease in the negative value of ΔGb→m,oS (Fig. 5b). The opposing trends in Rb,SATS and Rm,SOLS can both be explained by considering the effect of POPE on the lateral pressure profile of the membrane. On the one hand, before solubilisation sets in, accommodation of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) chains in a membrane having an elevated POPE content may be hampered by high lateral pressure in the bilayer core, so that the membrane becomes saturated with polymer at lower SMA(3
1) chains in a membrane having an elevated POPE content may be hampered by high lateral pressure in the bilayer core, so that the membrane becomes saturated with polymer at lower SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentrations than would be the case with a pure POPC bilayer. On the other hand, upon solubilisation, the SMA(3
1) concentrations than would be the case with a pure POPC bilayer. On the other hand, upon solubilisation, the SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) chains surrounding a bilayer patch rich in POPE will experience increased lateral pressure from the acyl-chain region, which might hamper the insertion of the polymer's phenyl pendant groups and require more polymer molecules per unit length of SMALP edge to hold the nanodisc together.
1) chains surrounding a bilayer patch rich in POPE will experience increased lateral pressure from the acyl-chain region, which might hamper the insertion of the polymer's phenyl pendant groups and require more polymer molecules per unit length of SMALP edge to hold the nanodisc together.
From this and the preceding sections, it is apparent that both the physical state and the chemical composition of the lipid bilayer to be solubilised exert a pronounced influence on the vesicle-to-SMALP transfer free energies and, consequently, on the slopes of the saturation and solubilisation boundaries in the phase diagram. Nevertheless, in all cases investigated so far, the extraction of phospholipids from vesicles to form SMALPs incurs a much lower free-energy penalty than solubilisation by synthetic head-and-tail detergents and even by mild detergents such as bile salts and their derivatives (Fig. 6). Hence, these free-energy values reflect, from a thermodynamic viewpoint of lipid transfer, the native-likeness of nanodiscs formed upon solubilisation of membranes made from various phospholipids by SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which goes hand in hand with their structure- and activity-preserving properties towards membrane proteins.2,4,5,10,15,42
1), which goes hand in hand with their structure- and activity-preserving properties towards membrane proteins.2,4,5,10,15,42
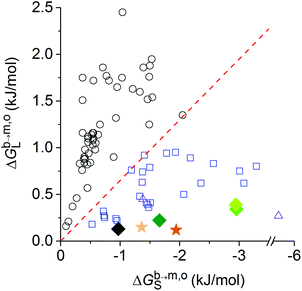 | ||
Fig. 6 Thermodynamic classification of surfactants based on the bilayer-to-micelle transfer free energies of surfactants and lipids, ΔGb→m,oS and ΔGb→m,oL, respectively.7 Synthetic head-and-tail detergents (open black circles) on the one hand and bile salts and their derivatives (open blue squares), the lipopeptides P2A2 and surfactin (open purple triangles), as well as SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (full symbols) on the other hand are separated by a threshold (red dashed line) defined by ΔGb→m,oL = −0.65 × ΔGb→m,oS. Data for SMA(3 1) (full symbols) on the other hand are separated by a threshold (red dashed line) defined by ΔGb→m,oL = −0.65 × ΔGb→m,oS. Data for SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solubilisation refer to DMPC at 10 °C or 30 °C (dark and light orange star, respectively) as well as POPC/POPE (1 1) solubilisation refer to DMPC at 10 °C or 30 °C (dark and light orange star, respectively) as well as POPC/POPE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)–POPC/POPE(1 0)–POPC/POPE(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (dark to light green diamonds); all other data are taken from ref. 7. 3) (dark to light green diamonds); all other data are taken from ref. 7. | ||
Lipid re-association with vesicles
It has been shown5 that SMALPs can deliver individual copies of the homotetrameric K+ channel KcsA into planar bilayers composed of E. coli polar lipid extract for electrophysiological measurements. However, quantitative reconstitution of membrane proteins and lipids from nanodiscs into other membrane systems such as vesicles remains to be demonstrated.43 With the aid of the respective phase diagrams (Fig. 1c and 3c), we accomplished complete re-association of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-solubilised DMPC with vesicles at temperatures both above and below Tm, as evidenced by a loss of the 31P NMR signal arising from small, fast-tumbling particles (Fig. 1d and S1d,† respectively). This observation could formally be explained by polymer-mediated tethering or adsorption of SMALPs to vesicles. In the light of the close agreement of these reconstitution titrations with the phase diagrams obtained from solubilisation experiments (insets in Fig. 1d and S1d†), however, it seems more likely that phospholipid molecules can indeed be reincorporated from SMALPs into vesicular bilayer membranes by addition of excess lipid in order to shift the equilibrium back into the purely vesicular range of the phase diagram. This, in turn, implies that high-yield reconstitution of nanodisc-embedded membrane proteins might also become feasible by simple addition of lipid vesicles to protein-containing SMALPs, without requiring harsher procedures such as polymer precipitation with divalent cations.43 Such a mild yet quantitative reconstitution protocol would facilitate the scrutiny of membrane proteins with vectorial functions, such as channels and transporters, in a membrane-mimetic system with two distinct aqueous compartments.
1)-solubilised DMPC with vesicles at temperatures both above and below Tm, as evidenced by a loss of the 31P NMR signal arising from small, fast-tumbling particles (Fig. 1d and S1d,† respectively). This observation could formally be explained by polymer-mediated tethering or adsorption of SMALPs to vesicles. In the light of the close agreement of these reconstitution titrations with the phase diagrams obtained from solubilisation experiments (insets in Fig. 1d and S1d†), however, it seems more likely that phospholipid molecules can indeed be reincorporated from SMALPs into vesicular bilayer membranes by addition of excess lipid in order to shift the equilibrium back into the purely vesicular range of the phase diagram. This, in turn, implies that high-yield reconstitution of nanodisc-embedded membrane proteins might also become feasible by simple addition of lipid vesicles to protein-containing SMALPs, without requiring harsher procedures such as polymer precipitation with divalent cations.43 Such a mild yet quantitative reconstitution protocol would facilitate the scrutiny of membrane proteins with vectorial functions, such as channels and transporters, in a membrane-mimetic system with two distinct aqueous compartments.
Non-selective lipid solubilisation
From the two distinct 31P NMR signals obtained during the solubilisation of mixed POPC/POPE LUVs (Fig. 4a), we conclude that SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solubilises these two lipids in a non-preferential manner. This is in agreement with a number of studies showing that various SMA copolymers, when present in excess, extract lipids from native3,12,26 and mixed artificial21 membranes in a non-selective fashion. In contrast with these earlier reports, the present results show that non-selectivity applies not only to the final solubilisation product at polymer concentrations much higher than Rm,SOLS but throughout all stages of a solubilisation titration. This might be surprising in view of the stark influence of lipid composition on both the kinetics21 and the thermodynamics (Fig. 5) of solubilisation. It therefore becomes apparent that it is the bulk physical state of the bilayer membrane rather than the properties of individual lipid constituents that governs the solubilisation equilibrium without, however, biasing the lipid composition of the solubilised bilayer.
1) solubilises these two lipids in a non-preferential manner. This is in agreement with a number of studies showing that various SMA copolymers, when present in excess, extract lipids from native3,12,26 and mixed artificial21 membranes in a non-selective fashion. In contrast with these earlier reports, the present results show that non-selectivity applies not only to the final solubilisation product at polymer concentrations much higher than Rm,SOLS but throughout all stages of a solubilisation titration. This might be surprising in view of the stark influence of lipid composition on both the kinetics21 and the thermodynamics (Fig. 5) of solubilisation. It therefore becomes apparent that it is the bulk physical state of the bilayer membrane rather than the properties of individual lipid constituents that governs the solubilisation equilibrium without, however, biasing the lipid composition of the solubilised bilayer.
Absence of free polymer
An important conclusion emerging from all pseudophase diagrams thus far established for various lipid/SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures (ref. 7 as well as Fig. 1c, 3c, and 4c) is that SMA(3
1) mixtures (ref. 7 as well as Fig. 1c, 3c, and 4c) is that SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) does not exist in a “free” form in the presence of lipid vesicles. That is, the polymer's affinity for lipids appears to be so high that, within the vesicular and coexistence ranges, it is always associated with lipids irrespective of the polymer/lipid ratio, albeit in different morphologies, such as vesicular membranes or nanodiscs. Population of a significant pool of free SMA(3
1) does not exist in a “free” form in the presence of lipid vesicles. That is, the polymer's affinity for lipids appears to be so high that, within the vesicular and coexistence ranges, it is always associated with lipids irrespective of the polymer/lipid ratio, albeit in different morphologies, such as vesicular membranes or nanodiscs. Population of a significant pool of free SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the aqueous solution would manifest in a nonzero ordinate intercept in the phase diagrams, as observed for detergents with high critical micellar concentrations.37,44,45 Note, however, that this does not formally rule out the existence of free polymer chains at polymer/lipid ratios higher than Rm,SOLS or in protein-containing samples, as it is conceivable that membrane proteins retain lipid molecules in bilayer patches and, thus, render them unavailable for interaction with the polymer.46 Then, addition of “excess” polymer could, in principle, result in the build-up of a pool of free polymer that coexists with polymer-bounded, protein-containing nanodiscs. Moreover, in view of the bivariate distribution of the length and the styrene/maleic acid ratio of SMA copolymers,43 it is possible that there exists a fraction of relatively short polymer chains rich in maleic acid residues that are too hydrophilic to participate in the solubilisation of lipid membranes and the formation of SMALPs, irrespective of the polymer and lipid concentrations and the resulting aggregate morphologies in the sample. Such a polymer population would neither contribute to any of the phenomena investigated here nor manifest in the present approach based on phase diagrams but might possibly be responsible for some of the adverse effects tentatively ascribed to “free” polymer chains in membrane-protein studies.43
1) in the aqueous solution would manifest in a nonzero ordinate intercept in the phase diagrams, as observed for detergents with high critical micellar concentrations.37,44,45 Note, however, that this does not formally rule out the existence of free polymer chains at polymer/lipid ratios higher than Rm,SOLS or in protein-containing samples, as it is conceivable that membrane proteins retain lipid molecules in bilayer patches and, thus, render them unavailable for interaction with the polymer.46 Then, addition of “excess” polymer could, in principle, result in the build-up of a pool of free polymer that coexists with polymer-bounded, protein-containing nanodiscs. Moreover, in view of the bivariate distribution of the length and the styrene/maleic acid ratio of SMA copolymers,43 it is possible that there exists a fraction of relatively short polymer chains rich in maleic acid residues that are too hydrophilic to participate in the solubilisation of lipid membranes and the formation of SMALPs, irrespective of the polymer and lipid concentrations and the resulting aggregate morphologies in the sample. Such a polymer population would neither contribute to any of the phenomena investigated here nor manifest in the present approach based on phase diagrams but might possibly be responsible for some of the adverse effects tentatively ascribed to “free” polymer chains in membrane-protein studies.43
Polymer/lipid ratio in SMALPs
Provided that there is no free polymer in solution, the polymer/lipid ratio in SMALPs must be identical to the overall ratio in the sample. This means that, with Rm,SOLS = 0.144 in the exemplary case of DMPC at 30 °C (Fig. 1c), the polymer/lipid mass ratio in SMALPs is at least 0.85, corresponding to 46% of the total nanodisc mass being due to SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For a more typical molar ratio of 0.5 (e.g., Fig. 2d), the mass ratio and percentage are as high as 2.9 and 75%, respectively. Using the approximation that the densities of polymer and lipid are similar, we thus arrive at equally high volume fractions of SMA(3
1). For a more typical molar ratio of 0.5 (e.g., Fig. 2d), the mass ratio and percentage are as high as 2.9 and 75%, respectively. Using the approximation that the densities of polymer and lipid are similar, we thus arrive at equally high volume fractions of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in nanodiscs. This appears to be at odds with findings of an earlier study,6 where the dimensions of the lipid core and polymer belt of SMALPs formed from DMPC and the more hydrophilic polymer SMA(2
1) in nanodiscs. This appears to be at odds with findings of an earlier study,6 where the dimensions of the lipid core and polymer belt of SMALPs formed from DMPC and the more hydrophilic polymer SMA(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were characterised by small-angle neutron scattering (SANS). The disc dimensions estimated in that study would result in a volume contribution of the polymer belt of no more than 25%.
1) were characterised by small-angle neutron scattering (SANS). The disc dimensions estimated in that study would result in a volume contribution of the polymer belt of no more than 25%.
In trying to reconcile these seemingly contradictory results, we have to be aware of two major simplifications inherent to the structural model used for analysing and interpreting the SANS data.6 On the one hand, this model assumes SMALPs to consist of a pure lipid core bounded by a strictly separated, pure polymer belt. Obviously, this is, at best, a zeroth-order approximation, since the same6 and other studies2 report spectroscopic evidence indicating intercalation of the polymer's phenyl groups into the acyl-chain region of the lipid bilayer core, thus reducing the “effective” volume of the polymer ascribed to the belt. On the other hand, a considerable amount of the polymer resides in the lipid bilayer or at the lipid/water interface of the SMALP core, as evidenced by the finite Rb,SATS values found here and previously.7 For example, with Rb,SATS = 0.078 for DMPC at 30 °C (Fig. 1c), the polymer/lipid mass ratio in the vesicular membrane at the onset of solubilisation and, thus, in the bilayer core of the SMALPs then formed amounts to 0.46, meaning that SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) accounts for 32% of the total mass in the SMALP core. Conversely, the belt need not consist exclusively of polymer but might accommodate a substantial fraction of lipid. Both of the above factors, that is, the blurry boundary between the core and belt regions of SMALPs as well as the finite mutual solubilities of the lipid and the polymer, are not accounted for in the simple geometric model,6 calling for a more detailed structural scrutiny of SMALPs.
1) accounts for 32% of the total mass in the SMALP core. Conversely, the belt need not consist exclusively of polymer but might accommodate a substantial fraction of lipid. Both of the above factors, that is, the blurry boundary between the core and belt regions of SMALPs as well as the finite mutual solubilities of the lipid and the polymer, are not accounted for in the simple geometric model,6 calling for a more detailed structural scrutiny of SMALPs.
Conclusions
Styrene/maleic acid copolymers exhibit decisive advantages over conventional detergents by combining a strong solubilising capacity with a discoidal nanoparticle morphology harbouring a native-like lipid bilayer. Herein, we have shown that• the kinetics of vesicle dissolution is a poor indicator of the three-stage solubilisation equilibrium, as gel-phase membranes are more susceptible than fluid-phase membranes to SMALP formation;
• the addition of more polymer than minimally required for complete solubilisation results in gradual size reduction of the SMALPs above Tm and seems to gradually abolish the gel phase below Tm;
• the bulk physical properties of the bilayer affect the vesicle-to-SMALP transfer free energies of both the lipid and the polymer and, hence, the saturating and solubilising polymer/lipid ratios defining the degree of detergency or solubilising power;
• polymer-solubilised lipids re-associate with vesicular bilayer membranes upon addition of excess lipid so as to shift the equilibrium back into the vesicular range of the phase diagram;
• polymer-mediated lipid solubilisation is non-preferential throughout the range of polymer/lipid ratios across which SMALP formation proceeds;
• there is no detectable population of free “solubilisation-competent” polymer chains in vesicular suspensions containing phospholipids and styrene/maleic acid copolymers, irrespective of temperature or lipid composition.
Experimental
Materials
DMPC, POPC, and POPE were obtained from Lipoid (Ludwigshafen, Germany). SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) copolymer solution (trade name Xiran SL25010 S25) was kindly provided by Polyscope (Geleen, Netherlands). Chloroform was purchased from Fisher Scientific UK (Leics, UK), D2O from Deutero (Kastellaun, Germany), 85% H3PO4 in D2O from Sigma–Aldrich (Steinheim, Germany), NaCl from VWR (Darmstadt, Germany), and tris(hydroxymethyl)aminomethane (Tris) from Carl Roth (Karlsruhe, Germany). All chemicals were obtained in the highest available purity.
1) copolymer solution (trade name Xiran SL25010 S25) was kindly provided by Polyscope (Geleen, Netherlands). Chloroform was purchased from Fisher Scientific UK (Leics, UK), D2O from Deutero (Kastellaun, Germany), 85% H3PO4 in D2O from Sigma–Aldrich (Steinheim, Germany), NaCl from VWR (Darmstadt, Germany), and tris(hydroxymethyl)aminomethane (Tris) from Carl Roth (Karlsruhe, Germany). All chemicals were obtained in the highest available purity.
Vesicle preparation
LUVs composed of either DMPC or POPC were prepared by suspension of dry lipid powder in Tris buffer (50 mM Tris, 200 mM NaCl, pH 7.4) to a final lipid concentration of 40 mM followed by 35-fold extrusion at 30 °C through two stacked 100 nm polycarbonate filters with a block-heated Mini-Extruder (Avanti, Alabama, USA). Mixed LUVs composed of POPC and POPE were prepared by suspending dry lipid powders in chloroform at 40 mM lipid concentration and mixing at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, or 1
1, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 POPC/POPE molar ratios. Mixed lipid solutions were dried in a rotary evaporator at 60 °C and 20 kPa for 2 h and incubated in a desiccator at room temperature and 5 Pa for 16 h to remove traces of chloroform. Dry lipid films were suspended in Tris buffer at a final concentration of 40 mM total lipid and extruded as described above.
3 POPC/POPE molar ratios. Mixed lipid solutions were dried in a rotary evaporator at 60 °C and 20 kPa for 2 h and incubated in a desiccator at room temperature and 5 Pa for 16 h to remove traces of chloroform. Dry lipid films were suspended in Tris buffer at a final concentration of 40 mM total lipid and extruded as described above.
Preparation of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1) stock solution
1) stock solution
The random copolymer used in this study had a styrene/maleic acid molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a mass-average molar mass of Mw = 10 kg mol−1, and a number-average molar mass of Mn = 4 kg mol−1. To prepare SMA(3
1, a mass-average molar mass of Mw = 10 kg mol−1, and a number-average molar mass of Mn = 4 kg mol−1. To prepare SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) stock solutions, 3 mL of a Xiran SL25010 S25 solution was dialysed against Tris buffer using a 5 mL QuixSep dialyser (Membrane Filtration Products, Seguin, USA) and a Spectra/Por 3 dialysis membrane (Spectrum Laboratories, Rancho Dominguez, USA) with a molar-mass cutoff of 3.5 kg mol−1. Dialysis was performed for 24 h under gentle stirring with membrane and buffer exchange after 16 h. Dialysed SMA(3
1) stock solutions, 3 mL of a Xiran SL25010 S25 solution was dialysed against Tris buffer using a 5 mL QuixSep dialyser (Membrane Filtration Products, Seguin, USA) and a Spectra/Por 3 dialysis membrane (Spectrum Laboratories, Rancho Dominguez, USA) with a molar-mass cutoff of 3.5 kg mol−1. Dialysis was performed for 24 h under gentle stirring with membrane and buffer exchange after 16 h. Dialysed SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was filtered through a 0.22 μm poly(vinylidene fluoride) (PVDF) filter (Carl Roth, Karlsruhe, Germany), and the final SMA(3
1) was filtered through a 0.22 μm poly(vinylidene fluoride) (PVDF) filter (Carl Roth, Karlsruhe, Germany), and the final SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) concentration was determined by refractometry on an Abbemat 500 (Anton Paar, Graz, Austria) using a molar refractive index increment of dn/dc = 1.1178 M−1.7 Note that the same refractive index value was derived using a series of SMA(3
1) concentration was determined by refractometry on an Abbemat 500 (Anton Paar, Graz, Austria) using a molar refractive index increment of dn/dc = 1.1178 M−1.7 Note that the same refractive index value was derived using a series of SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions prepared from polymer powder obtained through hydrolysis of a commercial styrene/maleic anhydride product from the same supplier (Xiran SZ25010). Molar concentrations were calculated from mass concentrations on the basis of the above number-average molar mass. Samples were aliquoted, stored at −20 °C, and thawed prior to use.
1) solutions prepared from polymer powder obtained through hydrolysis of a commercial styrene/maleic anhydride product from the same supplier (Xiran SZ25010). Molar concentrations were calculated from mass concentrations on the basis of the above number-average molar mass. Samples were aliquoted, stored at −20 °C, and thawed prior to use.
31P NMR spectroscopy
For solubilisation experiments, 40 mM lipid in the form of LUVs and 17.5 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in Tris buffer were used to prepare samples containing 2.5, 5.0, 7.5, or 10.0 mM lipid and 0–7 mM SMA(3
1) in Tris buffer were used to prepare samples containing 2.5, 5.0, 7.5, or 10.0 mM lipid and 0–7 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For reconstitution experiments, a mixture containing 2.5 mM DMPC and 0.28–0.40 mM SMA(3
1). For reconstitution experiments, a mixture containing 2.5 mM DMPC and 0.28–0.40 mM SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the form of SMALPs was incubated with 1.9–7.5 mM DMPC in the form of LUVs. All samples contained 10% D2O for locking and were incubated for at least 16 h at 10 °C or 30 °C before measurements were performed at 10 °C or 30 °C on an Avance 400 spectrometer (Bruker Biospin, Rheinstetten, Germany) operating at a 31P resonance frequency of 162 MHz. We used a 5 mm broadband inverse probe to acquire 256 scans with an inverse-gated 1H decoupling sequence, an acquisition time of 1.6 s, a sweep width of 9746 Hz, and a relaxation delay of 6 s. Data were multiplied by an exponential function with a line-broadening factor of 1.0 Hz before Fourier transformation. Chemical shifts were referenced to 85% H3PO4 in D2O as external standard at 0 ppm. Total peak areas were calculated by numerical integration in TopSpin 3.2, while peak positions and individual peak areas in lipid mixtures were obtained by fitting the corresponding data to a Lorentzian-shaped curve (eqn (13)†) by nonlinear least-squares analysis.47 Dependencies of 31P NMR peak areas on SMA(3
1) in the form of SMALPs was incubated with 1.9–7.5 mM DMPC in the form of LUVs. All samples contained 10% D2O for locking and were incubated for at least 16 h at 10 °C or 30 °C before measurements were performed at 10 °C or 30 °C on an Avance 400 spectrometer (Bruker Biospin, Rheinstetten, Germany) operating at a 31P resonance frequency of 162 MHz. We used a 5 mm broadband inverse probe to acquire 256 scans with an inverse-gated 1H decoupling sequence, an acquisition time of 1.6 s, a sweep width of 9746 Hz, and a relaxation delay of 6 s. Data were multiplied by an exponential function with a line-broadening factor of 1.0 Hz before Fourier transformation. Chemical shifts were referenced to 85% H3PO4 in D2O as external standard at 0 ppm. Total peak areas were calculated by numerical integration in TopSpin 3.2, while peak positions and individual peak areas in lipid mixtures were obtained by fitting the corresponding data to a Lorentzian-shaped curve (eqn (13)†) by nonlinear least-squares analysis.47 Dependencies of 31P NMR peak areas on SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/lipid ratios were analysed in terms of a three-stage model (eqn (9)–(12)†) by nonlinear least-squares analysis.47
1)/lipid ratios were analysed in terms of a three-stage model (eqn (9)–(12)†) by nonlinear least-squares analysis.47
Dynamic light scattering
DLS measurements were performed on a Zetasizer Nano S90 (Malvern Instruments, Worcestershire, UK) equipped with a 633 nm He–Ne laser and a photodetector mounted at an angle of 90°. Samples containing lipid and SMA(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were incubated for 16 h at 30 °C or 10 °C for DMPC experiments above or below Tm, respectively, or at 30 °C for POPC/POPE mixtures. Measurements were carried out in a 45 μL quartz glass cuvette with a 3 mm × 3 mm cross-section (Hellma Analytics, Müllheim, Germany) at 30 °C or 10 °C. Each sample was measured twice, once with the attenuator position automatically optimised for determination of size distributions and a second time with maximum open attenuator position to ensure comparability of total scattering intensities. Data analysis was performed by fitting the experimentally determined autocorrelation function with a non-negatively constrained least-squares function48 to obtain the intensity-weighted particle size distribution and by cumulant analysis49 to yield the z-average particle size and the width of the size distribution as derived from the associated polydispersity index (PDI). Assuming Gaussian distributions of both the decay rate and the particle size, the standard deviation of the z-average particle size is
1) were incubated for 16 h at 30 °C or 10 °C for DMPC experiments above or below Tm, respectively, or at 30 °C for POPC/POPE mixtures. Measurements were carried out in a 45 μL quartz glass cuvette with a 3 mm × 3 mm cross-section (Hellma Analytics, Müllheim, Germany) at 30 °C or 10 °C. Each sample was measured twice, once with the attenuator position automatically optimised for determination of size distributions and a second time with maximum open attenuator position to ensure comparability of total scattering intensities. Data analysis was performed by fitting the experimentally determined autocorrelation function with a non-negatively constrained least-squares function48 to obtain the intensity-weighted particle size distribution and by cumulant analysis49 to yield the z-average particle size and the width of the size distribution as derived from the associated polydispersity index (PDI). Assuming Gaussian distributions of both the decay rate and the particle size, the standard deviation of the z-average particle size is  .50
.50
Author contribution
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Acknowledgements
Dr. Harald Kelm (University of Kaiserslautern) is gratefully acknowledged for invaluable help with 31P NMR experiments. We thank Dr. Jana Broecker (University of Toronto), Jonas Dörr (Utrecht University, Netherlands), Abraham Olusegun Oluwole, Anne Grethen, Erik Frotscher, and Bartholomäus Danielczak (all University of Kaiserslautern) for helpful discussions. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through International Research Training Group (IRTG) 1830, by the Carl Zeiss Foundation with a scholarship to R. C. A., and by the Deutsche Akademische Austauschdienst (DAAD) with grant no. 56041134 to S. K.References
- T. J. Knowles, R. Finka, C. Smith, Y. Lin, T. Dafforn and M. Overduin, J. Am. Chem. Soc., 2009, 131, 7484 CrossRef CAS PubMed.
- M. C. Orwick, P. J. Judge, J. Procek, L. Lindholm, A. Graziadei, A. Engel, G. Gröbner and A. Watts, Angew. Chem., Int. Ed., 2012, 51, 4653 CrossRef CAS PubMed.
- A. R. Long, C. C. O'Brien, K. Malhotra, C. T. Schwall, A. D. Albert, A. Watts and N. N. Alder, BMC Biotechnol., 2013, 13, 41 CrossRef CAS PubMed.
- M. Jamshad, J. Charlton, Y. Lin, S. J. Routledge, Z. Bawa, T. J. Knowles, M. Overduin, N. Dekker, T. R. Dafforn, R. M. Bill, D. R. Poyner and M. Wheatley, Biosci. Rep., 2015, 35, 1 CrossRef CAS PubMed.
- J. M. Dörr, M. C. Koorengevel, M. Schäfer, A. V. Prokofyev, S. Scheidelaar, W. van der Cruijsen, T. R. Dafforn, M. Baldus and J. A. Killian, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 18607 CrossRef PubMed.
- M. Jamshad, V. Grimard, I. Idini, T. J. Knowles, M. R. Dowle, N. Schofield, P. Sridhar, Y. Lin, R. Finka, M. Wheatley, O. R. T. Thomas, R. E. Palmer, M. Overduin, C. Govaerts, J. Ruysschaert, K. J. Edler and T. R. Dafforn, Nano Res., 2015, 8, 774 CrossRef CAS.
- C. Vargas, R. Cuevas Arenas, E. Frotscher and S. Keller, Nanoscale, 2015, 7, 20685 RSC.
- R. Zhang, I. D. Sahu, L. Liu, A. Osatuke, R. G. Comer, C. Dabney-Smith and G. A. Lorigan, Biochim. Biophys. Acta, 2014, 1848, 329 CrossRef PubMed.
- C. Logez, M. Damian, C. Legros, C. Dupré, M. Guéry, S. Mary, R. Wagner, C. M'Kadmi, O. Nosjean, B. Fould, J. Marie, J. Fehrentz, J. Martinez, G. Ferry, J. A. Boutin and J. L. Banères, Biochemistry, 2016, 55, 38 CrossRef CAS PubMed.
- S. Gulati, M. Jamshad, T. J. Knowles, K. A. Morrison, R. Downing, N. Cant, R. Collins, J. B. Koendrick, R. C. Ford, M. Overduin, I. Kerr, T. R. Dafforn and A. J. Rothnie, Biochem. J., 2014, 461, 269 CrossRef CAS PubMed.
- M. Orwick-Rydmark, J. E. Lovett, A. Graziadei, L. Lindholm, M. R. Hicks and A. Watts, Nano Lett., 2012, 12, 4687 CrossRef CAS PubMed.
- D. J. K. Swainsbury, S. Scheidelaar, R. van Grondelle, J. A. Killian and M. R. Jones, Angew. Chem., Int. Ed., 2014, 53, 11803 CrossRef CAS PubMed.
- D. Li, J. Li, Y. Zhuang, L. Zhang, Y. Xiong, P. Shi and C. Tian, Protein Cell, 2015, 6, 229 CrossRef PubMed.
- S. J. Routledge, L. Mikaliunaite, A. Patel, M. Clare, S. P. Cartwright, Z. Bawa, M. D. B. Wilks, F. Low, D. Hardy, A. J. Rothnie and R. M. Bill, Methods, 2016, 95, 26 CrossRef CAS PubMed.
- S. Paulin, M. Jamshad, T. R. Dafforn, J. Garcia-Lara, S. J. Foster, N. F. Galley, D. I. Roper, H. Rosado and P. W. Taylor, Nanotechnology, 2014, 25, 285101 CrossRef PubMed.
- V. Postis, S. Rawson, J. K. Mitchell, S. C. Lee, R. A. Parslow, T. R. Dafforn, S. A. Baldwin and S. P. Muench, Biochim. Biophys. Acta, 2015, 1848, 496 CrossRef CAS PubMed.
- A. Periasamy, N. Shadiac, A. Amalraj, S. Garajová, Y. Nagarajan, S. Waters, H. D. T. Mertens and M. Hrmova, Biochim. Biophys. Acta, 2013, 1828, 743 CrossRef CAS PubMed.
- D. Lichtenberg, Biochim. Biophys. Acta, 1985, 821, 470 CrossRef CAS.
- H. Heerklotz, Q. Rev. Biophys., 2008, 41, 205 CrossRef CAS PubMed.
- D. Lichtenberg, H. Ahyayauch and F. M. Goñi, Biophys. J., 2013, 105, 289 CrossRef CAS PubMed.
- S. Scheidelaar, M. C. Koorengevel, J. D. Pardo, J. D. Meeldijk, E. Breukink and J. A. Killian, Biophys. J., 2015, 108, 279 CrossRef CAS PubMed.
- M. Roux and P. Champeil, FEBS Lett., 1984, 171, 169 CrossRef CAS PubMed.
- M. T. Paternostre, M. Roux and J. L. Rigaud, Biochemistry, 1988, 27, 2668 CrossRef CAS PubMed.
- D. Levy, A. Gulik, M. Seigneuret and J. L. Rigaud, Biochemistry, 1990, 29, 9480 CrossRef CAS PubMed.
- J. Schiller, M. Müller, B. Fuchs, K. Arnold and D. Huster, Curr. Anal. Chem., 2007, 3, 283 CrossRef CAS.
- I. Prabudiansyah, I. Kusters, A. Caforio and A. J. M. Driessen, Biochim. Biophys. Acta, 2015, 1848, 2050 CrossRef CAS PubMed.
- U. H. N. Dürr, R. Soong and A. Ramamoorthy, Prog. Nucl. Magn. Reson. Spectrosc., 2013, 69, 1 CrossRef PubMed.
- A. Nath, W. M. Atkins and S. G. Sligar, Biochemistry, 2007, 46, 2059 CrossRef CAS PubMed.
- S. K. Patra, A. Alonso and F. M. Goñi, Biochim. Biophys. Acta, 1998, 1373, 112 CrossRef CAS.
- S. K. Patra, A. Alonso, J. L. R. Arrondo and F. M. Goñi, J. Liposome Res., 1999, 9, 247 CrossRef CAS.
- T. Nyholm and J. P. Slotte, Langmuir, 2001, 17, 4724 CrossRef CAS.
- B. Carion-Taravella, S. Lesieur, J. Chopineau, P. Lesieur and M. Ollivon, Langmuir, 2002, 18, 325 CrossRef CAS.
- S. Funari, B. Nuscher, G. Rapp and K. Beyer, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8938 CrossRef CAS PubMed.
- F. Ollila and J. P. Slotte, Biochim. Biophys. Acta, 2002, 1564, 281 CrossRef CAS.
- H. Heerklotz, Biophys. J., 2002, 83, 2693 CrossRef CAS PubMed.
- D. E. Warschawski, A. A. Arnold, M. Beaugrand, A. Gravel, É. Chartrand and I. Marcotte, Biochim. Biophys. Acta, 2011, 1808, 1957 CrossRef CAS PubMed.
- H. Goldfine, J. Lipid Res., 1984, 25, 1501 CAS.
- K. Duquesne and J. N. Sturgis, Methods Mol. Biol., 2010, 601, 205 CAS.
- R. S. Cantor, J. Phys. Chem. B, 1997, 101, 1723 CrossRef CAS.
- R. S. Cantor, Biochemistry, 1997, 36, 2339 CrossRef CAS PubMed.
- E. Strandberg and A. S. Ulrich, Biochim. Biophys. Acta, 2015, 1848, 1944 CrossRef CAS PubMed.
- A. J. Bell, L. K. Frankel and T. M. Bricker, J. Biol. Chem., 2015, 290, 18429 CrossRef CAS PubMed.
- J. M. Dörr, S. Scheidelaar, M. C. Koorengevel, J. J. Dominguez, M. Schäfer, C. van Walree and J. A. Killian, Eur. Biophys. J., 2016, 45, 3 CrossRef PubMed.
- O. O. Krylova, N. Jahnke and S. Keller, Biophys. Chem., 2010, 150, 105 CrossRef CAS PubMed.
- S. Keller, H. Heerklotz, N. Jahnke and A. Blume, Biophys. J., 2006, 90, 4509 CrossRef CAS PubMed.
- N. Jahnke, O. O. Krylova, T. Hoomann, C. Vargas, S. Fiedler, P. Pohl and S. Keller, Anal. Chem., 2014, 86, 920 CrossRef CAS PubMed.
- G. Kemmer and S. Keller, Nat. Protocols, 2010, 5, 267 CrossRef CAS PubMed.
- P. Hassan, S. Rana and G. Verma, Langmuir, 2015, 31, 3 CrossRef CAS PubMed.
- D. E. Koppel, J. Chem. Phys., 1972, 57, 4814 CrossRef CAS.
- M. Holzer, S. Barnert, J. Momm and R. Schubert, J. Chromatogr. A, 2009, 1216, 5838 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr02089e |
| This journal is © The Royal Society of Chemistry 2016 |
