Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly energetic compositions based on functionalized carbon nanomaterials
Qi-Long
Yan
a,
Michael
Gozin
*a,
Feng-Qi
Zhao
b,
Adva
Cohen
a and
Si-Ping
Pang
c
aCenter for Nanoscience and Nanotechnology, Faculty of Exact Science, Tel Aviv University, Tel Aviv 69978, Israel. E-mail: cogozin@gmail.com; qilonyan@post.tau.ac.il; Tel: +972-3-640-5878
bScience and Technology on Combustion and Explosion Laboratory, Xi'an Modern Chemistry Research Institute, Xi'an 710065, China
cSchool of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China
First published on 28th January 2016
Abstract
In recent years, research in the field of carbon nanomaterials (CNMs), such as fullerenes, expanded graphite (EG), carbon nanotubes (CNTs), graphene, and graphene oxide (GO), has been widely used in energy storage, electronics, catalysts, and biomaterials, as well as medical applications. Regarding energy storage, one of the most important research directions is the development of CNMs as carriers of energetic components by coating or encapsulation, thus forming safer advanced nanostructures with better performances. Moreover, some CNMs can also be functionalized to become energetic additives. This review article covers updated preparation methods for the aforementioned CNMs, with a more specific orientation towards the use of these nanomaterials in energetic compositions. The effects of these functionalized CNMs on thermal decomposition, ignition, combustion and the reactivity properties of energetic compositions are significant and are discussed in detail. It has been shown that the use of functionalized CNMs in energetic compositions greatly improves their combustion performances, thermal stability and sensitivity. In particular, functionalized fullerenes, CNTs and GO are the most appropriate candidate components in nanothermites, solid propellants and gas generators, due to their superior catalytic properties as well as facile preparation methods.
Introduction
Energetic materials (EMs) are a class of materials with a large amount of stored chemical energy, which can be rapidly released; typical subclasses of EMs include explosives, pyrotechnics and propellants. Gunpowder (GP), which was invented in ancient China, is considered as the first EM in human history. It is a mixture of sulfur, charcoal, and potassium nitrate (saltpeter). Charcoal as a form of carbon together with sulfur is a fuel, while the saltpeter is an oxidizer.1,2 Due to its ability to rapidly generate heat and gas, GP has been widely used as a firearm propellant and in pyrotechnics. As a primitive energetic composition, GP is no longer widely in use and has been replaced by more advanced energetic systems, both in civil and military applications, which are still undergoing fast development. During the past few decades, researchers have been seeking safer energetic materials that are highly energetic, insensitive, nontoxic, and environmentally friendly.3 In newly developed propellants and explosives, carbon-rich materials are gradually being replaced by high-nitrogen materials (HNMs) with a higher energy content and a better oxygen balance.4 However, these HNMs are in most cases not safe enough for practical applications. Recently, a variety of carbon nanomaterials (CNMs) have been developed as carriers of EMs, combining to form advanced structures with higher performances. Some of them, as coating or encapsulating materials, are also very effective in improving the safety of energetic systems.5It is well known that solid carbon materials (SCMs) can be found in different forms of bulk phases (e.g. diamonds) as well as molecular forms (e.g. fullerenes).6 In fact, CNMs have a unique place in nanoscience owing to their exceptional electrical, thermal, chemical and mechanical properties. They have found application in such diverse areas as composite materials, energy storage and conversion, sensors, drug delivery, field emission devices and nanoscale electronic components, due to their strong adsorption capacity, chemical stability, and high mechanical strength as well as easy modification.7 Currently, several types of SCMs are used in the field of EMs, which can be divided into two groups. One group is nano-powdered carbon, such as carbon black (CB) and nanodiamond, where the latter can be used as a reducing agent in energetic compositions.8 The other is CNMs with a rich pore structure and a large surface area, including fullerenes, expanded graphite (EG), carbon nanotubes (CNTs), graphene, and graphene oxide (GO). The application of these materials in the field of energetic compositions has been recently summarized.9 To further expand the scope of their applications in EMs, nano-sized carbon materials with a large surface area can be functionalized with energetic groups or coupled with binders, energetic compounds, metal fuels or combustion catalysts to form novel advanced functional nanostructures with better properties that can be used in propellants, explosives and pyrotechnics.10 In recent years, extensive research has been carried out on the preparation and application of carbon nanomaterials in EMs,11 especially graphene, GO, and CNTs and their composites, as combustion catalysts or energetic components.12,13 In addition, activated carbon fiber and graphite have also been investigated with respect to propellant manufacture and waste treatment. Relevant research topics include design, synthesis, characterization and properties of carbon-based nanomaterials; potential usage of nano-sized carbon materials in rocket propulsion; catalytic effect and mechanisms of carbon-based nano-sized catalysts on decomposition and combustion of energetic materials; and application and characterization of carbon nanomaterials in the purification of waste water in the explosive industry.14
In the past few decades, a large number of papers have been published regarding the preparation and applications of CNMs, based on which several review papers are available, mainly in Chinese literature.8,12,15–18 Experimental and theoretical data on new carbon nanostructures using fullerenes and nanotubes as well as monolithic diamond-like nanoparticles, nanofibers and various nanocomposites as starting materials are summarized in a Russian journal.15 Their atomic and electronic structures and the nature of their chemical bonds and their physicochemical properties are compared. The applications of fullerene materials in catalytic reactions, including hydrogen transfer and hydrosilylation, pyrolysis of alkanes, H2–D2 exchange, coupling and alkyl transfer reactions are reviewed.16 Recent advances in the application of graphene-based catalysts in energetic materials are also introduced, revealing that they have a significant catalytic effect on the combustion of solid propellants.17,18 Graphene-based EMs usually have larger energy release rates; moreover, the burning rate and mechanical properties, as well as the sensitivity of propellants can be improved using graphene or GO.19 However, these review papers are not accessible for most Western researchers due to language barriers. Besides, many other papers reporting CNTs, EG and CB materials and their applications in the field of EMs have still not been reviewed and summarized. To make such a large amount of data on CNMs, especially that published in Chinese journals, available to all readers, this review aims to summarize preparation methods of CNM-based EMs as well as their effects on the performance and sensitivity of the corresponding energetic compositions.
Classification of carbon nanomaterials
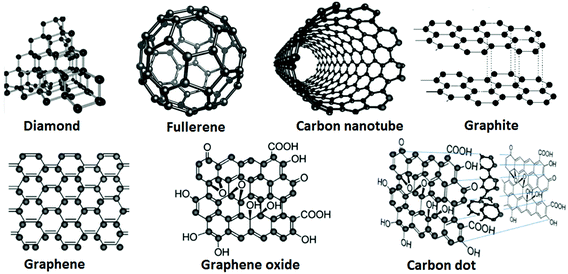
Carbon is one of the most important natural elements and it has sp3, sp2, and sp hybridized orbitals. The anisotropic sp2 in molecular carbon atoms may lead to anisotropy in some crystals of these materials (Table 1). Therefore, carbon materials have widely extended properties. In fact, more and more new carbon materials continue to be discovered and artificially prepared. There is no other element like carbon that can form such diverse and completely different substances as a single element, in terms of structures and properties, such as 3-D diamond crystal and graphite, 2-D graphite sheets, 1-D CNTs and 0-D fullerene molecules. It has been discovered that carbon materials are mostly superior in terms of their hardness, optical properties, heat resistance, radiation characteristics, chemical resistance, electrical insulation, electrical conductivity, and surface and interface properties to many other materials. Carbon materials can probably cover the characteristics of all the substances on the planet, such as “the hardest to the softest”, “insulator to semiconductor to super conductor”, “thermal insulator to thermal conductor” and “fully light-absorbing to completely transparent”, so these materials have a wide range of applications, as listed in Table 2. In general, CNMs have attracted more and more attention in recent years due to the possibility of specific preparation of a wide range of variously-shaped nano-carbon crystals in the form of needles, tubes, spheres and many other morphologies.20 For example, the three key conjugated CNMs are graphene, CNTs and fullerenes. These materials have very different morphologies with unique chemical properties but they still have much in common, especially in terms of basic building units (Fig. 1).| Bond types | Hybridization type | |||
|---|---|---|---|---|
| sp | sp2 | sp3 | Mixed | |
| Coordination | 2 | 3 | 4 | Variable |
| Average C–C length (nm) | 0.121 | 0.133 | 0.154 | — |
| 0.142 | ||||
| Binding energy | 46 kJ mol−1 | 35 kJ mol−1 | 20 kJ mol−1 | — |
| Typical examples | Acetylene | Ethylene | Adamantane, cyclododecane (CF)n, SiC, B4C | — |
| Benzene | ||||
| Identified carbon phases | Carbene (hexagonal facets bulk crystal C60) | Graphite (in-plane) (a cubic, hexagonal) | Diamonds | C60 |
| Undetermined carbon phases | C2 to C20 | 1-Graphite 3d-sp2 bct-4 polystyrene | 6H-Diamonds-BC-8 | Carbophene |
| Carbon molecules | Graphyenes | |||
| Fullerenes | CNTs | Graphite | Diamond | Amorphous C | |
|---|---|---|---|---|---|
| 0-Dimensional | 1-Dimensional | 2-Dimensional | 3-Dimensional | Amorphous | |
| DoH, decomposition of hydrogen; MF, molecular fiber; DC, directional crystallization; FM, ferromagnetic; SC, superconductive; TC, thermal conductivity; HR, heat resistance; AR, abrasion resistance; CR, corrosion resistance; ULM, ultra-lubricating materials; OM, optical material; SLM, super lightweight material; USM, ultra-high strength material; PVD, Physical vapor deposition; CVD, chemical vapor deposition; ERM, energy raw materials; EEM, electricity–electronic materials; CC, catalyst carrier. | |||||
| Preparation methods | CVD, ablation, discharge | Discharge, DoH, fluoride, CVD and ablation | CVD, evaporation, ablation | High-pressure synthesis, CVD | CVD, PVD, sputtering, plasma |
| Morphology | Single crystal, amorphous | Fibers | FM, crystalline, DC | Crystalline | Amorphous, fiber |
| Characteristics | Semi-conductive, catalytic, FM, SC | Conductive, high strength, catalytic, high TC | Conductive, catalytic, intercalating | High hardness, high TC, HR, AR | High hardness, conductive, CR |
| Applications | ULM, nonlinear OM | SLM, USM, ERM, CC | Electronic, X-ray OM, CC | ULM, EEM, coating, CC | ULM, electronic, coating, CC |
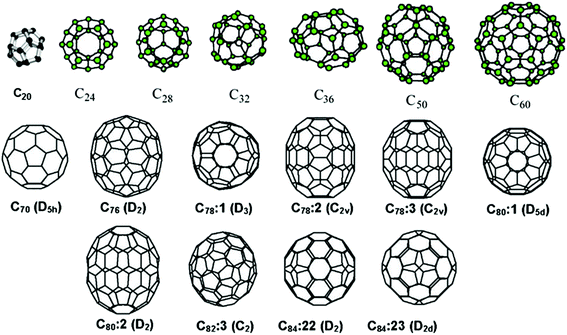
These CNMs can transform to each other under certain experimental conditions; hence, initial study on CNTs has origins in fullerene research. There are many types of fullerene derivatives (e.g. C20, C32, C60, C70, and C84), where buckminsterfullerene (C60) is a typical and well-known example.21 Each fullerene molecule possesses the characteristic of being a pure carbon cage, each atom bonded to three others, as in graphite (Fig. 2).
Table 2 shows examples of various typical carbon materials. As mentioned in the Introduction, current CNMs, including fullerene, graphene, CNTs and other carbon materials, have many excellent physical and chemical properties, and some of them are widely used in the field of EMs. Unlike graphene, every fullerene has exactly 12 pentagonal faces with a different number of hexagonal faces (e.g., C60 has 20). CNTs can be viewed as graphene sheets rolled into hollow tubes, which can be classified into single-walled, double-walled and multi-walled nanotubes. As a special cylindrical form of fullerene, CNTs were discovered more than 30 years before fullerenes but this discovery was not fully appreciated at that time. Recently, the production of CNTs exceeded several thousand tons per year, and these materials are used for applications in energy storage, automotive parts, boat hulls, sporting goods, filters, electronics, coatings, carriers of catalysts and electromagnetic shields.22 CNT-related publications were more than triple during the past decade, while the rate of patent applications also dramatically increased. Although most of the CNM production output was of unorganized structures, organized CNT architectures such as “forests”, yarns and regular sheets were manufactured in smaller volumes.23 In addition to fullerenes and CNTs, other CNMs, including graphene and GO, are currently under intensive investigation.24–28 In the following sections, newly developed preparation methods for several mentioned CNMs, together with functionalized EMs and energetic compositions based on these materials, are summarized in more detail.
CNM-based energetic nanomaterials
Energetic composites containing carbon black
CB can be produced by thermal decomposition or partial combustion of hydrocarbons, such as oil and natural gas.33 The characteristics of a particular type of CB vary depending on the manufacturing process. The material produced by the furnace process, currently the most widely used method, is called “furnace black”, distinguishing it from CB manufactured by other processes. The latter is usually used in energetic compositions. Furnace black is obtained by blowing an aerosol of petroleum or coal into a high-temperature chamber. This method is efficient for mass production of CB due to high yields as well as better particle size and structure control. Other preparation methods include the channel process (partially combusting fuel), the acetylene black process (thermally decomposing acetylene gas) and the lampblack process (collecting soot from fumes generated by burning oils or pine wood). Recently, a new method has been developed to produce CB from the hydrolysis, carbonization and pyrolysis of rice husk.34 The specific surface area (SSA) of CB obtained from rice husk was increased from 389 (that of the starting materials) to 1034 cm2 g−1 and the pore volume was increased from 0.258 to 0.487 cm3 g−1. This method is also applicable to the preparation of CB from other types of biomass. For better application of CB in different industrial products, it needs to be dispersed in different solvents. For example, stable high-load dispersions of CB in PMA (propyleneglycol methylether acetate) can be prepared by a ball-milling process. Another type of CB is graphitized carbon black (GCB), which is a non-porous form of amorphous carbon. The GCB powder is made up of highly agglomerated nanoparticles with a spherical morphology, and it has been observed from high-resolution TEM images that it has a core–shell structure. A 5 nm graphitic shell covers an amorphous carbon core (Fig. 3).
 | ||
| Fig. 3 TEM images of GCB material (crystallized in a 3H graphitic structure with a d002 interlayer space of 0.346 nm, which is slightly larger than that of an ideal graphitic structure, 0.335 nm). Reprinted from ref. 37 published in J. AOAC Int. (2009) with permission of Publishing Technology. | ||
Highly graphitized CB has been used to evaluate the isosteric heat of adsorption to determine the differences in the adsorption mechanisms of strong polar and non-polar fluids,35–38 allowing its use in chemical residue analysis.39 GCB materials are hydrophobic and are frequently used to effectively trap organic compounds from water or water vapour.
As a super adsorbent, CB can be easily combined with nano-sized EMs,36 forming new types of energetic mixtures with better performances, which are described in the following sections.
The reinforcing potential of CB is closely related to the number of high energy sites. Therefore, it can be used as an active filler in the rubber industry, including in rubber-based propellants to improve their physical and dynamic properties. The surface energy difference between rubbers and CB is large, which has a negative influence on the stability of a CB dispersion in a rubber matrix and on its distribution in blends of different rubbers. It was suggested that plasma polymerization could be used to modify the surface of CB by depositing a thin film of polymer over it.47 Fullerene soot, as a by-product of fullerene production, can be used in this plasma modification process.48 In this case, both the CB and the polymer matrix had a comparable surface energy, where the polymer matrix could be an elastomer, such as styrene-butadiene rubber (SBR), butadiene rubber (BR) or ethylene–propylene diene rubber (EPDR), which are widely used in polymer bonded explosives (PBXs) and propellants.49 There are alternative ways to improve the mechanical strength of CB-containing polymer blends, such as ball-mill mixing, annealing and quenching heat treatment.50 The first process can break down highly structured CB into finer particles, while the latter two induce CB to form a coagulation structure in the polymers.
The shock wave generated from the detonation of EMs may in turn cause phase transition in CB, due to extreme pressure and high temperature. Such phase transitions provide a route for the preparation of nanodiamond from CBs, where a cubic crystal diamond structure is obtained. This method is significantly different from the preparation of diamonds using graphite as a carbon source.51 In the past, CB was commonly used in solid propellants but recently it was also used in primary explosives to improve their ignition delays as well as to reduce sensitivity. For example, a primary explosive, bis-(5-nitrotetrazole) tetraammine-cobalt(III) perchlorate (BNCP) has been combined with CB.52 It was proved that CB can decrease the laser initiation threshold of BNCP, the detailed mechanism of which will be discussed in a later section. CB can also be combined with potassium nitrate (KN) to obtain a new type of propellant (CBKN) for special application in blasting valves.53 Such blasting valves are used in nuclear power stations in pressurized water reactors. Comparing black powder (HY6) and sulfur-free black powder (WHY6) to CBKN propellant, it was shown that the latter has a higher auto-ignition temperature (over 321 °C) and a lower burn rate, indicating that CBKN has better thermal stability. In addition to the above-mentioned application, CB may have further applications in EMs. For instance, they may be used in the development of nanothermites, for which the requirements for their mechanical and electrostatic sensitivities are high. Such sensitivities may be modulated using pristine or chemically modified CB as additives, as has already shown for the performance improvement of WO3/Al nanothermite.54
Energetic mixtures based on nanodiamonds (nD)
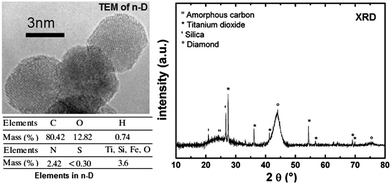 | ||
| Fig. 4 TEM picture and XRD pattern showing the chemical composition of nDs used to form nD/PC compositions. Reprinted from ref. 79 published in Propellants, Explos., Pyrotech. (2009) with permission of John Wiley & Sons, Inc. | ||
It can be seen that the diameter of the nDs is less than 5 nm, with a carbon content of about 80% and an O content of 12.8%. It seems that the Sa content in Sa/KC mixtures has little effect on their impact sensitivity (IS), while the IS of nDs/KC compositions strongly depends on the nD content. However, nDs/KC compositions with a larger content of nDs are very sensitive to friction, due to the hardness of the diamond. The nDs/KC compositions were found to be deflagrating primary explosives with a maximum propagation rate of 3960 m s−1 at 12.3 MPa when the content of nDs is 22 wt%. Such compositions are considered as “green” primers. The nDs/KC reactive formulations usually decompose in two possible combustion modes, i.e. (i) continuous or (ii) intermittent, depending on the proportion and pressing level of the nDs used to shape the pellets.80
In addition to direct use of nDs as an ingredient in energetic compositions, they can also be used to coat particles of high EMs. It was reported that well-dispersed and uniformly shaped DnDs were produced and used to coat of micron-sized RDX particles.81 SEM images of these materials are shown in Fig. 5. It has been found that the DnDs could reduce the energy barrier of thermal decomposition of RDX and improve its reactivity. However, excessive coating of over 1/3 of nDs hindered decomposition and gas diffusion, acting as an inert shell.
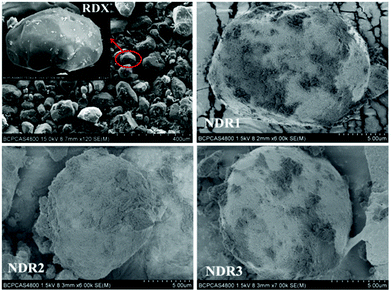 | ||
Fig. 5 SEM images of RDX and nDs, where the ratio of nDs to RDX is kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (NDR1), 1 8 (NDR1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (NDR2), and 1 6 (NDR2), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (NDR3), which corresponds to increasing the mass proportions of the nD coating to 1/9, 1/7, and 1/5, respectively. Reproduced from ref. 81 with permission from the PCCP Owner Societies. 4 (NDR3), which corresponds to increasing the mass proportions of the nD coating to 1/9, 1/7, and 1/5, respectively. Reproduced from ref. 81 with permission from the PCCP Owner Societies. | ||
Similar to the other CNMs, in addition to physical combination with energetic components, nDs could be functionalized with energetic or other functional groups. However, this topic is unexplored and no data has been published in open literature on this issue to date. The most important reason for chemical functionalization of nDs is that detailed and unambiguous characterization of the surface structure of nD particles remains an extremely challenging task. In fact, it was shown that treating nD particles in a reduction reaction resulted in enrichment of hydroxyl and hydroxymethyl functional groups, paving the way for a new generation of nD-based EMs.
Energetic composites based on expanded graphite
Recently, a new preparation procedure was developed, where natural graphite was initially dispersed in H2O2 and then introduced into an autoclave to proceed with a hydrothermal process.84 In all the cases, under the influence of heat, the graphite layers are separated as in an accordion, expanding the graphite flakes (Fig. 6).85 High-quality EbG has a large proportion of intercalated layers, where sulphur- or nitrogen-containing compounds are used as intercalating agents. Depending on the nature of the graphite and the intercalating agent, expansion can commence at temperatures as low as 180 °C and can occur suddenly and rapidly. In the case of free expansion, the final volume between the layers can be several hundred times greater than the initial volume. It is well known that electrochemical insertion of Na+ into graphite is greatly hindered by the insufficient interlayer spacing, while EG could be a good option to replace the graphite used in a Na+ battery anode (Fig. 7).86 EG can usually be prepared from fine expandable graphite flakes (160 μm in size), using acetic anhydride as an inserting agent and potassium dichromate as an oxidant.87 The best known conditions for this process are as follows: mole fractions of graphite, acetic anhydride, concentrated sulfuric acid and potassium dichromate should be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1
3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, with a reaction time of 50 min and a reaction temperature of 45 °C. Similarly, micron-sized EG is prepared by a chemical oxidation method, using sulfuric and glacial acetic acids as inserting reagents and potassium dichromate as an oxidizing agent.88 The optimum mass ratio of raw materials EbG
0.6, with a reaction time of 50 min and a reaction temperature of 45 °C. Similarly, micron-sized EG is prepared by a chemical oxidation method, using sulfuric and glacial acetic acids as inserting reagents and potassium dichromate as an oxidizing agent.88 The optimum mass ratio of raw materials EbG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4
H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3COOH
CH3COOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2CrO7 for the latter process is 1
K2CrO7 for the latter process is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, while the reaction time is 60 min at room temperature. The micron-sized EG has a larger infrared extinction coefficient than the raw graphite, due to inclusion of some open or semi-open small holes that can absorb infrared radiation. The expansion usually occurs at the very beginning of the reaction and extension of the reaction time has little effect on the microstructure of the final EG.89 After its preparation, the EG is ready for application in various fields, including preparation of advanced EMs.
0.5, while the reaction time is 60 min at room temperature. The micron-sized EG has a larger infrared extinction coefficient than the raw graphite, due to inclusion of some open or semi-open small holes that can absorb infrared radiation. The expansion usually occurs at the very beginning of the reaction and extension of the reaction time has little effect on the microstructure of the final EG.89 After its preparation, the EG is ready for application in various fields, including preparation of advanced EMs.
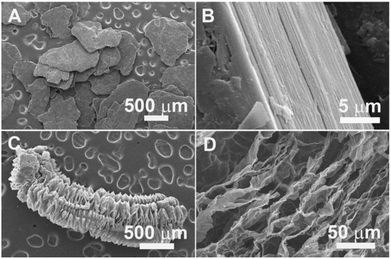 | ||
| Fig. 6 SEM images of the characteristic structures of flake graphite (A, B) and expanded graphite (C, D). Reproduced from ref. 85 with permission of Copyright 2013, The Electrochemical Society. | ||
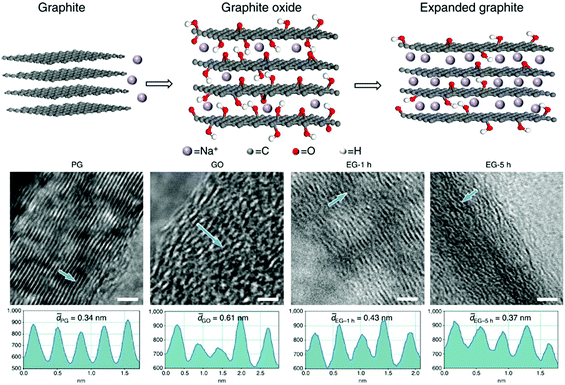 | ||
| Fig. 7 Above is a schematic diagram: (left) Na+ cannot be electrochemically intercalated into graphite because of the small interlayer spacing; (middle) electrochemical intercalation of Na+ into GO is enabled by the enlarged interlayer distance resulting from oxidation; however, the intercalation is limited by steric hindering from large amounts of oxygen-containing groups; (right) a significant amount of Na+ can be electrochemically intercalated into EG owing to a suitable interlayer distance and reduced oxygen-containing groups in the interlayers. Below are high-resolution TEM images showing cross-sectional layered structures for PG, GO, EG-1 h and EG-5 h. Reprinted from ref. 79 with permission. Copyright © 2014, Nature Publishing Group. | ||
| Materials | Preparation methods | Application fields | Contributors |
|---|---|---|---|
| EG | 1. Using natural graphite flakes as raw material, acetic anhydride as inserting agent and potassium dichromate as oxidant | Antistatic and anti-electromagnetic materials, Electronic devices, flame retardants, energetic materials | Ji-Hui et al. (2006)87 |
| 2. By dispersing natural graphite in H2O2 with subsequent hydrothermal treatment in autoclave | Kuan et al. (2012)84 | ||
| 3. Micron-sized EG can be prepared by chemical oxidation, using sulfuric acid and glacial acetic acid as inserting reagents and potassium dichromate as oxidizing agent. | Ba et al. (2011)88 | ||
| Porous carbon | Prepared from energetic carbon precursors, alkali propiolates, via ultrasonic spray pyrolysis | Purification, catalysts | Xu et al. (2012)332 |
| EG/PP | EG/polypropylene (PP) nanocomposites are prepared by solid-state ball-milling followed by low-temperature melt mixing. | Engineering materials, binders | Mohammad et al. (2013)90 |
| EG/Mg(OH)2 | By mixing Mg(OH)2 powder and EG. | Chemical heat pumps | Zamengo et al. (2014)91 |
| γ-Fe2O3/EG | Nano γ-Fe2O3/EG is prepared by a sol–gel and low temperature self-combustion technique. | Magnetic materials | Zhang et al. (2011)93 |
| CaCl2/CH3NH2/EG | Mixed using a cylindrical solid–gas reactor. | Chemical heat pumps | Balat et al. (1993)90 |
EMs based on functionalized C60 fullerene
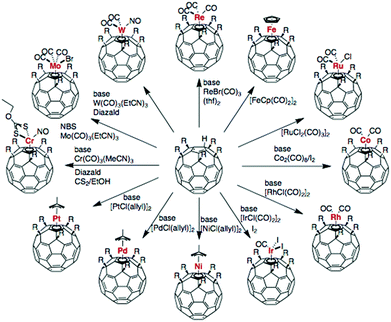 | ||
| Fig. 8 Synthesis paths for exohedral metal fullerene complexes. Reprinted from ref. 106 with permission from Copyright © 2011, Royal Society of Chemistry. | ||
Exohedral metallofullerenes may open a new way to prepare a variety of novel energetic materials and combustion catalysts. Some functionalized fullerenes can be heated to their ignition temperature with low-intensity, continuous-wave (c.w.) laser irradiation.107 Laser-induced ignition was demonstrated with various types of laser radiation at intensities as low as 100 W cm−2. As shown in Fig. 9, process (a) is the laser-induced cleavage of functional groups, which induces acoustic shock-waves or otherwise imparts kinetic energy to the fullerene cages; (b) energetic fullerene cages collide, leading to coalescence or disintegration; (c) coalescence of SWCNTs into larger SWCNTs, MWCNTs and carbon onions is promoted by the heat generated by the incident laser, cleavage of the functional groups and the exothermic coalescence processes, together with the catalytic action of cleaved functional groups and defects in the nanostructures; (d) alternatively, disintegration of fullerene cages supplies carbon for the simultaneous formation of SWCNTs, MWCNTs and carbon onions, with intact fullerene cages acting as nucleation sites. The simultaneous formation of various carbon nanostructures can be catalysed by cleaved functional groups and the defects present in intact fullerene cages. The energetic fullerene cages collide, leading to coalescence or disintegration.
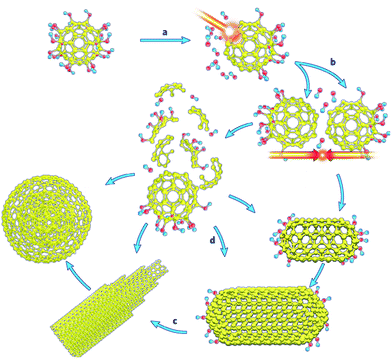 | ||
| Fig. 9 Proposed mechanism for laser-induced transformation of functionalized fullerenes. Reprinted from ref. 107 with permission. Copyright © 2008, American Chemical Society. | ||
 | ||
| Scheme 1 Preparation procedure of MDFP.108. | ||
The optimum molar ratio of C60, 2,4-dinitrobenzaldehyde and N-methylglycine reactants was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, while the reaction was conducted at 95 °C for 40 h.109 After the latter publication, the preparation of energetic fullerene derivatives became a popular topic and many new compounds were synthesized. Typical representatives of this family of compounds are nitro-fulleropyrrolidine derivatives, which are synthesized by 1,3-dipolar cycloaddition reactions of C60 and the nucleophilic substitution reaction of N-unsubstituted fulleropyrrolidine110 (Scheme 2). In particular, Cp-1 to Cp-4 compounds are prepared by 1,3-dipolar cycloaddition, while Cp-6 and Cp-7 are prepared from Cp-2 via nucleophilic substitution.
6, while the reaction was conducted at 95 °C for 40 h.109 After the latter publication, the preparation of energetic fullerene derivatives became a popular topic and many new compounds were synthesized. Typical representatives of this family of compounds are nitro-fulleropyrrolidine derivatives, which are synthesized by 1,3-dipolar cycloaddition reactions of C60 and the nucleophilic substitution reaction of N-unsubstituted fulleropyrrolidine110 (Scheme 2). In particular, Cp-1 to Cp-4 compounds are prepared by 1,3-dipolar cycloaddition, while Cp-6 and Cp-7 are prepared from Cp-2 via nucleophilic substitution.
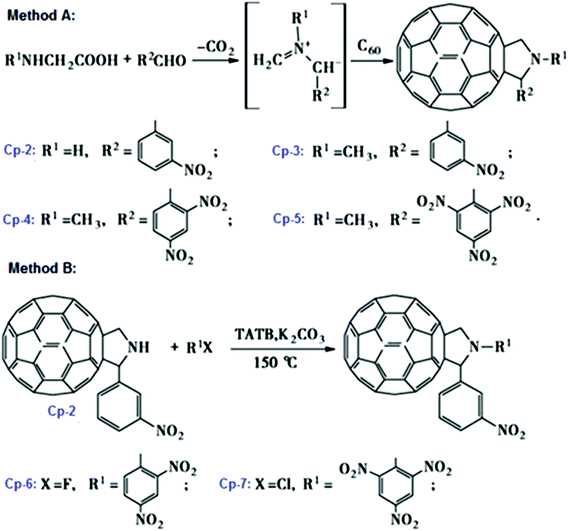 | ||
| Scheme 2 Preparation methods of N-unsubstituted fulleropyrrolidine. Reprinted from ref. 110 published in Chin. J. Energet. Mater. (2009) with permission. | ||
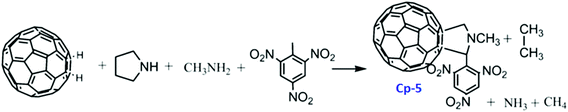
It was found that the mechanical sensitivity of HMX was greatly reduced when it was mixed with 1% of N-methyl-2-(3-nitrophenyl) fulleropyrrolidine (Cp-3, MNPFP). Moreover, N-methyl-2-(N-ethylcarbazole)-fulleropyrrolidine (Cp-8, MECFP) and N-methyl-2-(4′-N,N-diphenylaminophenyl)-fulleropyrrolidine (Cp-9, MDPAFP) were synthesized via microwave irradiation (MR; Scheme 3).111 A photoinduced intramolecular electron transfer process from a C60 moiety to a carbazole moiety has been studied by nanosecond laser flash photolysis. The performance of N-methyl-3-(2′,4′,6′-trinitrobenzene)-fulleropyrrolidine (Cp-5, MTNBFP) has been estimated using a self-consistent field calculation method based on the following overall isodesmic reaction (Scheme 4).
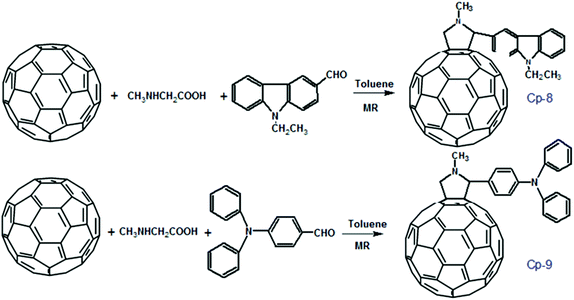 | ||
| Scheme 3 Preparation method of N-methyl-2-(4′-N,N-diphenylaminophenyl)-fulleropyrrolidine under microwave irradiation. | ||
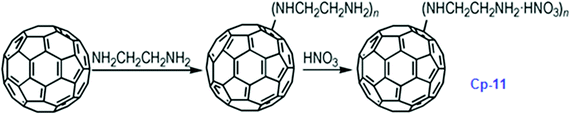
It has been calculated that the enthalpy of formation of Cp-5 is 2782.2 kJ mol−1, while its detonation velocity and pressure are 3282 km s−1 and 4.443 GPa, respectively.112 Upon complete combustion, the total heat of combustion for Cp-5 is 2.17 × 105 kJ mol−1 due to its high carbon content. It seems that this compound may not be useful as the main ingredient of an explosive or a propellant but it might be a good additive for heat-generating energetic compositions. To improve the energetic content of fullerene derivatives, more nitro groups can be introduced on the fullerene skeleton. Such a strategy has been recently attempted, where a high-nitrogen-content derivative, C60(NO2)14 (FP, CP-10), was prepared and characterized,113 using a prolonged treatment of C60 in benzene with very high concentrations of N2O4. However, Cp-10 is not so thermally stable and it deflagrates when heated above 170 °C in nitrogen or air, releasing a considerable amount of heat. This compound may be used as a powerful explosive, depending on its sensitivity, which has not been reported. In addition to the nitro-derivatives of fullerene, fullerene nitrates have also been reported that can be used in propellant compositions as energetic burn rate modifiers. As a typical example, fullerene ethylenediamine nitrate (Cp-11, FEDN) was synthesized by reacting fullerene and ethylenediamine in diluted nitric acid (Scheme 5).114
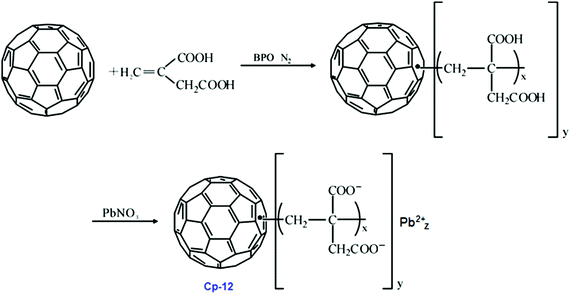
Cp-11 can undergo a three-step decomposition process starting at about 100 °C. The details of its decomposition mechanism will be described in a later section. Energetic fullerene catalysts improve the specific impulse of solid propellants, while decrease their pressure exponents. There is another type of polymeric catalyst based on fullerene, the so-called fullerene itaconic acid copolymer lead salt (FIAL, Cp-12), which may have a better effect on combustion.115 This was prepared via a two-step reaction using C60, itaconic acid and lead nitrate as starting materials (Scheme 6).
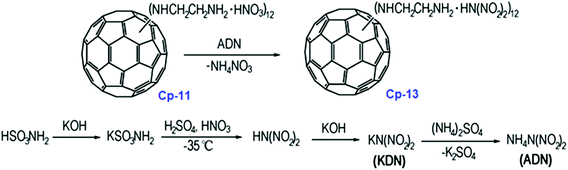
As shown in Scheme 6, the reaction time and temperature had little effect on the final content of lead. However, the optimum reaction conditions for the preparation of Cp-12 were found to be 25 °C with a pH of 6.9. The resulting catalyst, Cp-12, is quite thermally stable, with a decomposition temperature peak at 304 °C. Based on the aforementioned fullerene nitrate (FN, Cp-11), a more energetic derivate, fullerene ethylenediamine dinitramide (FED, Cp-13) could be obtained via an ion exchange reaction with ammonium dinitramide in 84% yield (ADN; Scheme 7).116
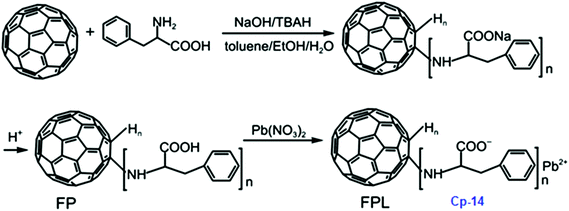
The Cp-13 product has the appearance of a yellowish solid and its structure was analyzed by UV-vis, FT-IR, elemental analysis and XPS techniques. Based on these analyses, Cp-13 was found to have a formula of H12C60(HNCH2CH2NH2·HN(NO2)2)12 and it was proposed for use as an oxidizer in solid propellants like ADN. To combine the advantages of fullerene and lead salts as propellant catalysts, another lead salt based on fullerene phenylalanine (FPL, Cp-14) was prepared. Analogously to Cp-12, Cp-14 can be prepared using the same strategy, where the Pb cation comes from Pb(NO3)2 (Scheme 8).117,118
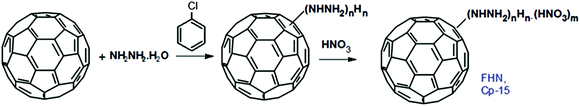
FPL was found to have a significant catalytic effect on the decomposition of 1,3,5-trinitroperhydro-1,3,5-triazine (RDX) and it may be used as an efficient combustion catalyst in solid propellants. On the basis of the above-mentioned synthetic method, a new potential energetic combustion catalyst, fullerene hydrazine nitrate (FHN, Cp-15) was designed and prepared in a two-step process. Fullerene hydrazine (FH) was first prepared in 84% yield from fullerene and hydrazine hydrate, and then the obtained FH was reacted with concentrated nitric acid to form the FHN salt (Scheme 9).119
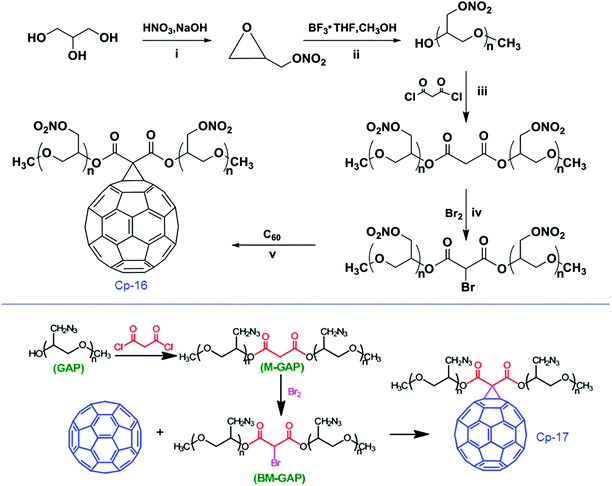
Elemental and other chemical analyses of FH showed a composition of C, 69.38%, N, 26.86% and H, 3.76%, strongly indicating that the formula of FH, obtained under these reaction conditions, is C60(NHNH2)10H10. The corresponding formula of the FHN salt was found to be C60(NHNH2)10H10·4HNO3, with an oxygen content of 14.9%. In general, C60 can either be combined with energetic moieties, as shown above, or be functionalized with energetic polymers. Two novel fullerene-based energetic polymers have been prepared recently, including C60-poly(glycidyl nitrate) (C60-PGN, Cp-16)120 and C60-glycidyl azide polymer (C60-GAP, Cp-17).121 The former polymer was synthesized through a modified Bingel reaction of C60 with bromomalonic acid PGN ester, in the presence of amino acid in dimethyl sulfoxide, while the latter was prepared using the same reaction between C60 and bromomalonic acid glycidyl azide polymer ester (Scheme 10). Both Cp-16 and Cp-17 have excellent thermal stability over 200 °C and can be used as energetic binders for solid propellants.
For better comparisons, the preparation methods and applications of the above-mentioned energetic fullerene derivatives are summarized in Table 4.
| Materials | Preparation method | Application field | Contributors |
|---|---|---|---|
| FEN, fullerene ethylenediamine nitrate; FED, fullerene ethylenediamine dinitramide H12C60(HNCH2 CH2NH2·HN(NO2)2)12; mNPF, N-methyl-2-(3-nitrophenyl) pyrrolidino-[3′,4′:1,2] fullerene; NFD, nitro fulleropyrrolidine derivatives; PF, polynitro[60]fullerene, C60(NO2)14; MTNBFP, N-methyl-3-(2′,4′,6′-trinitrobenzene)-fulleropyrrolidine; FPGN, [60]Fullerene-poly(glycidyl nitrate); MDFP, N-methyl-2-(1,3-dinitrophenyl) fulleropyrrolidine; FIA-Pb, fullerene itaconic acid copolymer lead salt; FFGAP, functionalized [60]fullerene-glycidyl azide polymer; FPL, fullerene phenylalanine lead salt; FHN, fullerene hydrazine nitrate; NPF, trinitrophenyl C60 derivative. | |||
| FPGN | Through a modified Bingel reaction of C60 and bromomalonic acid PGN ester in the presence of amino acid and dimethyl sulfoxide | Energetic binder | Gong et al. (2015)120 |
| FFGAP | Using a modified Bingel reaction of [60]fullerene (C60) and bromomalonic acid glycidyl azide polymer ester (BM-GAP) | Energetic binder | Huang et al. (2015)121 |
| FPL | Fullerene phenylalanine (FP) was dissolved in 10 mL water, then the pH of the FP water solution was adjusted to 6.96 using 1 mol L−1 HNO3 by adding Pb(NO3)2 (0.01 mol) and the mixture was stirred at 30 °C for 3 h | Propellant catalyst | Guan et al. (2014)117 |
| FHN | Fullerene hydrazine is synthesized from fullerene and hydrazine hydrate and then it reacts with concentrated nitric acid to form fullerene hydrazine nitrate | Energetic combustion catalyst | Guan et al. (2014)119 |
| FEN | Using fullerene, ethylenediamine and dilute nitric acid as raw materials | Propellant catalysts | Chen et al. (2014)116 |
| FED | Via ion exchange reaction of fullerene ethylenediamine nitrate and ammonium dinitramide (ADN) | Energetic components | Chen et al. (2014)114 |
| mPF | Glycyl-porphyrin (20 mg) and C60 (42 mg) are dissolved in 45 ml of anhydrous toluene under stirring, then three-fold excess of benzaldehyde was added | Catalyst for explosives | Jin et al. (2014)114 |
| FIA-Pb | Prepared via a two-step reaction using C60, itaconic acid and lead nitrate as raw materials | Propellant catalyst | Liu et al. (2013)115 |
| PF | Prolonged treatment of C60 in benzene with very high concentrations of N2O4 | Magnetic material | Cataldo et al. (2013)113 |
| MTNBFP | Not available in the literature | Primary explosive | Tan et al. (2010)112 |
| NFD | Synthesized by 1,3-dipolar cycloaddition reactions of C60 and a nucleophilic substitution reaction of N-unsubstituted fulleropyrrolidine | Propellant catalyst | Jin et al. (2009)110 |
| FDFP | Prepared by the Prato reaction with a reactant molar ratio of C60, 2,4-dinitrobenzaldehyde and N-methylglycine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
Energetic catalyst | Jin et al. (2006)109 |
| NPF | Prepared via C60 reaction with trinitrochlorobenzene and sodium azide | Energetic components | Wang et al. (1996)108 |
As shown in Table 4, it is clear that most of the energetic fullerene derivatives were obtained directly by reacting C60 with the corresponding energetic precursors. Almost all the studies have been reported during the past 5 years, strongly indicating that this research field is in its infancy. These energetic fullerene derivatives could either be used as combustion catalysts or as energetic binders. However, the compatibility and sensitivity have not yet been systematically investigated for all these materials; this is very important with respect to their practical applications.
EMs based on graphene, GO or reduced GO
GO is another form of graphene that is also very attractive for preparation of graphene-based materials.129–131 In fact, thin films of GO could be reduced in solution under various reducing conditions and the reduction converts GO into a reduced-GO (rGO) material, which exhibits better electrical conductivity.131,132 In addition to its use in making rGO for electronic devices, GO has been used in catalytic oxidation,133–135 biotechnology136–139 and as a surfactant.140
Recently, a facile and scalable liquid-phase preparation method for aqueous solutions of isolated graphene sheets from low-temperature EG, was reported.141 It has been shown that graphene sheets exfoliated from EG could be restored to an extended conjugated sp2 network. Later on, novel single- and multi-layer graphene quantum dots (GQDs) were prepared from CX-72 CB by chemical oxidation (Fig. 10).142 Single-layer GQDs were demonstrated to be excellent probes for cellular imaging, while multi-layer GQDs may offer great applications in optoelectronic devices.
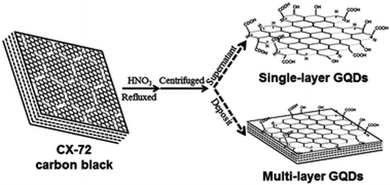 | ||
| Fig. 10 The preparation procedures for single- and multi-layer GQDs. Reprinted with permission from ref. 141 with permission of © 2009 IOP Publishing Ltd. | ||
Preparation of GO was first reported more than a century ago and it could be achieved by several approaches. In general, the conversion of graphite to GO is conducted in two steps: (1) the oxidation of graphite to GO; (2) the removal of impurities (e.g. acids and manganese salts). Brodie's method, reported in 1859, was the pioneering one, where he used fuming HNO3 and KClO3 as the intercalatant and oxidant.143 Nevertheless, this method has several flaws, including a long reaction time and the generation of toxic gases during the reaction. In 1958, Hummers and Offeman developed another method, which is currently the most widely employed way for the synthesis of GO.144 They used H2SO4 to intercalate graphite in the presence of NaNO3, while using KMnO4 as an oxidizer. Recently, Tour et al.145 improved the Hummers method by excluding NaNO3, while increasing the amount of KMnO4 and performing the reaction in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2SO4/H3PO4. However, there are still several problems with this method. To solve these problems, an improved Hummers method has recently been developed.146 In the latter method, GO is prepared using a simple purification process in a high yield, using small graphite flakes as a raw material. In fact, GO in the solid state gets gradually “reduced” and becomes darker in colour upon prolonged storage in air at room temperature. Such disproportionation reactions are highly exothermic.147 In the past, thermal reduction/exfoliation of GO was a typical method for rGO production, which, however, has the drawback of requiring specialized equipment for rapid thermal shock absorption. Currently, a new alternative method is available, based on vacuum and microwave-assisted exfoliation, which has solved the problem of poor reduction of GO with a low C/O ratio.148 Microwave irradiation of GO under vacuum leads to outgassing from GO and the creation of plasma, which aids temperature distribution and hydrogenation. As mentioned earlier, graphene, GO and rGO derivatives are well-known in the field of electronic technology and biotechnology. Although many of these functionalized carbonaceous nanomaterials have been used in various energetic compositions, the number of publications in this field is limited.149 For instance, GO itself is a highly energetic material, is thermally unstable and can readily undergo exothermic disproportionation reactions to produce chemically modified graphene under mild heating conditions.
1 mixture of H2SO4/H3PO4. However, there are still several problems with this method. To solve these problems, an improved Hummers method has recently been developed.146 In the latter method, GO is prepared using a simple purification process in a high yield, using small graphite flakes as a raw material. In fact, GO in the solid state gets gradually “reduced” and becomes darker in colour upon prolonged storage in air at room temperature. Such disproportionation reactions are highly exothermic.147 In the past, thermal reduction/exfoliation of GO was a typical method for rGO production, which, however, has the drawback of requiring specialized equipment for rapid thermal shock absorption. Currently, a new alternative method is available, based on vacuum and microwave-assisted exfoliation, which has solved the problem of poor reduction of GO with a low C/O ratio.148 Microwave irradiation of GO under vacuum leads to outgassing from GO and the creation of plasma, which aids temperature distribution and hydrogenation. As mentioned earlier, graphene, GO and rGO derivatives are well-known in the field of electronic technology and biotechnology. Although many of these functionalized carbonaceous nanomaterials have been used in various energetic compositions, the number of publications in this field is limited.149 For instance, GO itself is a highly energetic material, is thermally unstable and can readily undergo exothermic disproportionation reactions to produce chemically modified graphene under mild heating conditions.
 | ||
| Fig. 11 Structure of sandwich complex of TATB/Graphene layer formed due to strong π–π attraction. Reprinted from ref. 153 with permission. Copyright © 2010, American Chemical Society. | ||
The calculated detonation enthalpy of the TATB/graphene complex is 1.61 kJ g−1 with a density of 2.1 g cm−3, while the detonation pressure and detonation velocity are 10.5 GPa and 2.40 km s−1, respectively. The lubricating properties of graphene are better than those of graphite, and a series of complexes with adjustable properties could be obtained by adjusting the proportion and the order of overlaying layers. However, this type of material is still in a design stage. In common practice, EMs should be safe enough to be handled even in the case of primary explosives. To improve the electrostatic sensitivity of lead styphnate (LS), graphene nanoplatelet-lead styphnate composites (GLS) were prepared either by adding graphene nanoplatelets (GNP) to the reaction solution or by coating normal LS with GNPs. The composites showed an excellent anti-electrostatic performance with depressed electrostatic spark sensitivity and static electricity accumulation.154
Although potassium picrate (KPA) is a less powerful explosive than LS, it has many important applications. KPA is somewhat shock-sensitive and even more sensitive than the parent picric acid. If ignited in a confined space, KPA will detonate. Ultrafine KPA was prepared under the action of a crystalline controlling agent and its thermal sensitivity can be improved by graphene-doping. KPA doped with graphene nanoparticles (having a larger surface area) may partially hinder effective collisions of KPA particles and accelerate the heat dispersion,155 and hence the mechanical sensitivity of KPA may be decreased. In addition to primary explosives, high-energy solid propellants also need to be safer to allow their use in low vulnerability (LOVA) munitions.
In particular, ammonium perchlorate (AP) is the main ingredient of high-energy composite propellants. However, its surface properties need to be improved for better safety. A nano-composite based on AP and graphene aerogels (AP/GA, Fig. 12) was prepared by a sol–gel method and characterized by scanning electron microscopy (SEM), elemental analysis and X-ray diffraction (XRD) techniques.156 It can be seen in Fig. 12a and b that (similarly to GA) there are still many voids inside the AP/GA nano-composite material with an SSA of 49.18 m2 g−1. However, a large number of AP particles, with an average particle size of 69.4 nm, are attached to the skeleton of graphene. Elemental analysis of this material shows that the content of AP can reach to 94%, which is very promising as the main energetic component.157 Based on AP/GA nanocomposites, a novel GA/Fe2O3/AP nanostructured energetic material was prepared by a sol–gel method, which was followed by a supercritical CO2 drying technique (Fig. 13).158 It has been demonstrated that Fe2O3 and AP are well dispersed in GA layers on a nanometer scale, where Fe2O3 exhibits a catalytic effect on the thermal decomposition of AP.
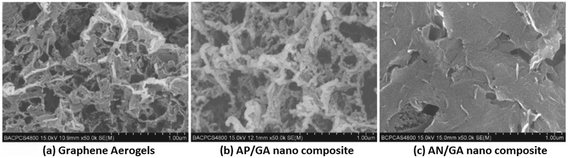 | ||
| Fig. 12 SEM images of GA (a) and AP/GA (b) and AN/GA (c) nano composites. Reprinted from ref. 156 with permission. Copyright © 2012, Chin. J. Explos. Propellants. | ||
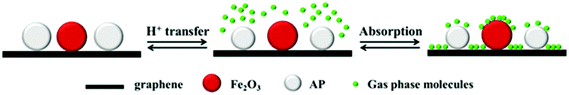 | ||
| Fig. 13 The principle of GA/Fe2O3/AP nanostructured energetic composite formation, where Fe2O3 and AP nanoparticles are added and trapped in the porous three-dimensional networks of GA. Reprinted from ref. 158 with permission. Copyright © 2014, Springer Science. | ||
Fig. 14 shows the SEM images of GA and a GA/Fe2O3/AP nano-structured energetic composite. It is apparent that the morphology of GA is uniform on a large scale and exhibits a three-dimensional network of randomly oriented sheet-like structures with a wrinkled texture as well as rich hierarchical pores. It is very similar to AP/GA; a large amount of AP crystallizes on the networks of GA and covers the surface. N2 adsorption–desorption isotherms of GA and the corresponding nanocomposites are shown in Fig. 14c and d.
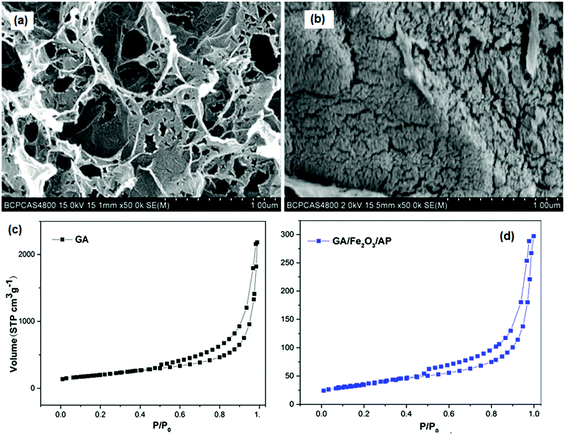 | ||
| Fig. 14 SEM images of GA (a) and GA/Fe2O3/AP nanostructured energetic composite (b); Nitrogen adsorption–desorption isotherms for GA (c) and GA/Fe2O3/AP nanostructured energetic composite (d). (reproduced from Lan, J. Sol-Gel. Sci. Technol., 2015, 74, 161–167.) Reprinted from ref. 158 with permission. Copyright © 2014, Springer Science. | ||
For GA/Fe2O3/AP nano-structured energetic composites and GA, the adsorption–desorption curves indicate an open wedge-shaped meso-porous structure for this material, resulting from the presence of the graphene sheets.159 The tested SSAs of GA and the nano-composite are 717 and 123 m2 g−1, respectively, while the total pore volumes (Vtot), determined by the Barrett–Joyner–Halenda (BJH) method for GA and the nano-composite, are 3.37 and 0.46 cm3 g−1, respectively. After filling GA with AP, the nano-composite shows significant decreases in its SSA and Vtot, indicating that AP decreases the SSA of the product.
In addition to AP, GA can also be used to prepare nanocomposites of ammonium nitrate (AN), another common oxidant used in propellants and explosives. Similarly, this can be prepared by the sol–gel method followed by a supercritical CO2 drying process.160 According to SEM and elemental analysis, nano-sized AN uniformly disperses in the GA, with an average particle size of 71 nm and a mass fraction of 92.7% (Fig. 12c). All the above-mentioned materials are metastable intermolecular composites (MICs), which are among the most attractive EMs in terms of their performance. MICs are generally composed of a fuel and an oxidizer with particle sizes in the nanometer range. The heat and mass transfer are greatly enhanced due to the nanoscale contact of oxidizer and fuel particles. The most promising application for MICs is nanothermites. On the basis of graphene functionalization, long-range electrostatic and short-range covalent interactions could be harnessed to produce multifunctional EMs through hierarchical self-assembly of a nanoscale oxidizer and a fuel into highly reactive macrostructures.
A self-assembly method of Al and Bi2O3 nanoparticles on functionalized graphene sheets (FGS) was recently reported.161 The self-assembly process and the chemical bonds formed are shown in Fig. 15. Such a nanocomposite structure was formed in a colloidal suspension phase that ultimately condenses into ultra-dense macrostructures, which contain 5 wt% of GO. The TEM and SEM photos of GO/Al/Bi2O3 composites, as well as Al and Bi2O3 nanoparticles are shown in Fig. 15. In this case, the oxygen atom from the OH group on Al is protonated by the hydrogen of the COOH group on GO, which creates a carboxylate anion (GO–COO−) group on the GO (Fig. 15).
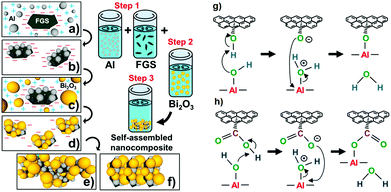 | ||
Fig. 15 Schematic of the self-assembly process: (a) electrostatic attraction of Al to GO, (b) covalent bonding of GO/Al existing as a stable GO/Al dispersion, (c) electrostatic attraction of Bi2O3 to GO/Al nanostructures, and (d) noncovalent assembly of Bi2O3 on GO/Al; the instability of the GO/Al/Bi2O3 dispersion continues the self-assembly process to form ultradense macrostructures ((e) and (f)); chemical interactions between (g) hydroxyl groups of GO and surface hydroxyl groups of Al nanoparticles leading to C–O–Al covalent bonding and (h) carboxylic groups of GO and hydroxyl groups of Al nanoparticles leading to O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Al covalent bonding. Reprinted from ref. 161 with permission. Copyright © 2014, American Chemical Society. C–O–Al covalent bonding. Reprinted from ref. 161 with permission. Copyright © 2014, American Chemical Society. | ||
The covalent bonding between Al and GO and the absence of covalent bonding between Bi2O3 and GO, were shown by various spectroscopic methods. Fig. 16 shows the orientation of the GO/Al/Bi2O3 nanostructures within the larger macrostructures. It is suggested that the formation of this structure is driven by two-particle-size modes, where the smaller sized and less planar GO/Al/Bi2O3 forms randomly oriented macrostructures, while the more planar one tends to condense into layered macrostructures for larger-sized assemblies. The layered assembly probably arises from the preference of the larger 2-D GO/Al/Bi2O3 and they geometrically align with one another due to van der Waals interactions. Regardless of their structure, the GO/Al/Bi2O3 nanocomposites have excellent fuel/oxidizer contact in comparison to randomly mixed Al/Bi2O3, resulting in enhanced reactivity.
 | ||
| Fig. 16 TEM (a–d) and SEM (e, f) images of GO(5%)/Al/Bi2O3 composites self-assembled from nano to macro length scales: (a) a few layered GO sheets, (b) Al nanoparticles with an average diameter of 80 nm, (c) Bi2O3 nanoparticles with a size range of 90–210 nm and (d) layered nanoscale building block of a GO densely decorated with Al first and then with Bi2O3; The inset in (d) shows a ultradense assembly of Al and Bi2O3 on GO; ultradense macro structures self-assembled from GO/Al/Bi2O3 nano-composites in layered (e) and random (f) orientations. Reprinted from ref. 161 with permission. Copyright © 2014, American Chemical Society. | ||
A remarkable enhancement in measured energy release from 739 to 1421 J g−1 was found for this novel composite when it was compared to a normal Al/Bi2O3 mechanical mixture. In addition to the aforementioned method, a pyrocarbon (PyC) coating can also be introduced to the Al2O3–SiO2 system, using in situ oxidation of PyC by thermolysis of AN.162 The formation of nitrogen oxides during the decomposition of AN enabled the oxidation of PyC at relatively low temperatures. In this way, a PyC coating can be easily introduced to the three-dimensional all-oxide fiber-reinforced composite (Fig. 17). For the composites without PyC interphases (Fig. 17a), the fracture surface was very homogeneous and almost devoid of pull-out fibers. As shown in Fig. 17b, the fibers were tightly surrounded by the matrix and no fiber/matrix (F/M) debonding occurred, suggesting strong interfacial bonding. A large number of micro-pores are distributed on the fiber surface due to chemical corrosion of the matrix. Obvious fiber pull-out behaviour is shown for the composites with PyC interphases (Fig. 17c) due to weak interfacial bonding, which has been confirmed by SEM imaging (Fig. 17d). This indicates that the porous coating is favourable to F/M debonding, thereby improving the performance. As mentioned earlier in this section, insensitive energetic materials are greatly desirable for LOVA ammunition compositions. The capability to control compositions and properties for target EMs is essential to obtain an optimal performance, controlled energy release, and low sensitivity. With these goals in mind, a new study on relatively insensitive explosive 1,1-diamino-2,2-dinitroethylene (FOX-7) has been carried out.163 The FOX-7 nanocrystals were prepared under confinement of mesoporous carbon FDU-15 via a self-assembly process. It has been indicated that complete impregnation can be achieved in N-methyl-2-pyrrolidone at 100–110 °C, and the maximum amount of FOX-7 in the FOX-7/FDU-15 composites was 43.8 wt%. There are many other functionalized graphenes that are only used in energy storage such as batteries and fuel cells. One should refer to a comprehensive review on graphene derivatives in energy storage applications for further details.164
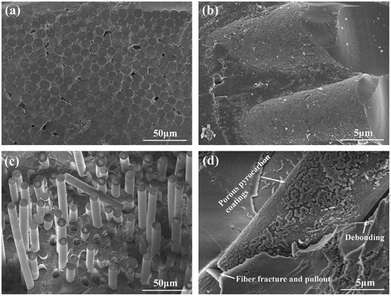 | ||
| Fig. 17 Fracture surfaces of the N440/AS composites without (a, b) and with (c, d) PyC interphases. Reprinted from ref. 162 with permission. Copyright © 2015, Elsevier Ltd. | ||
EMs based on GO and rGO
Compared with graphene, GO seems to be more suitable and more easily functionalized into energetic compounds. GO itself is considered to be a potential EM. Upon heating, GO can readily undergo violent exothermic decomposition due to the extensive oxygenic functional groups on the basal plane (phenol, hydroxyl, and epoxide) and at the edges (carboxylic).165 This is also the case for rGO, which is shown to retain some oxygenic groups as a result of imperfect reduction. Therefore, rGO is also energetic, yet not as energetic as GO. DSC results suggest that GO takes up to 0.8 kJ g−1 of heat to trigger its deoxygenating reaction. Because the heavy addition of inert graphite impaired the energy output of the composite explosives, GO and rGO were promising candidates to replace graphite in insensitive high-energy PBX compositions.On the one hand, GO and rGO act as space-qualified lubricants, which strongly improve the low-temperature performance of hypergolic ionic liquids by reduction of their viscosity.166 However, matching the type of graphene to the specific ionic-liquid functionality is very important in achieving the optimum performance. On the other hand, GO and rGO enhance the ignition and burning properties of hypergolic ionic liquids. For example, it has been found that the laser ignition and burn rates of nitrocellulose (NC) microfilms can be enhanced upon doping with GO,167 where a Nd:YAG (1064 nm, 20 ns) laser was used to ignite GO-doped NC films at low temperatures. The researchers studied several types of NC microfilms doped with different concentrations of GO, (e.g. 0.1 wt%, 0.5 wt%, 1 wt% and 2 wt%). Some of the morphologies of these films are shown in Fig. 18. The pure NC films are continuous, smooth, and homogeneous (Fig. 18a). After introducing GO into NC films, the morphology of the latter changes significantly and its surface becomes rough.
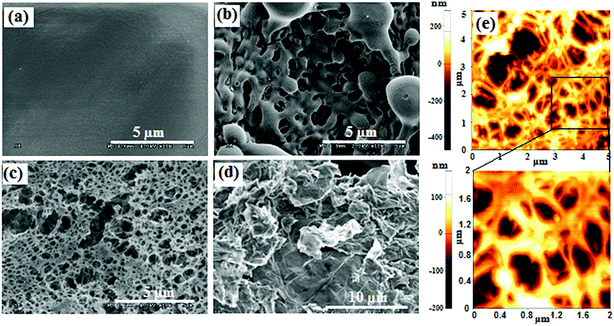 | ||
| Fig. 18 SEM and AFM images of pure and GO-doped NC films. SEM image of (a) pure NC film, (b) 0.5%, and (c) 2% GO-doped NC film; (d) as-produced GO; and (e) AFM images of 1% GO-doped NC film. (Reproduced from Zhang et al., Appl. Phys. Lett., 2013, 102, 141905.) Reprinted from ref. 165 with permission. Copyright © 2013, AIP Publishing LLC. | ||
Moreover, an increase in the concentration of GO led to the formation of highly porous structures (Fig. 18b and c). AFM imaging and comparison of GO-doped NC containing 0.5 wt% of GO with that containing 1 wt% shows that the latter has larger pores with diameters from 0.5 μm to 3 μm (Fig. 18e).
In addition to burn rate enhancement, GO can also be used to decrease the sensitivity of energetic crystals. For instance, to improve the safety of 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), GO was introduced by a solvent-antisolvent method (Fig. 19).168 In a typical procedure, HMX was dissolved in DMF at 40 °C and then GO (about 2 wt%) was added. After GO was completely dispersed in HMX solution in DMF by sonication, the mixture was injected into CH2Cl2 (in which GO is much less soluble). After filtration, washing, and dying at 40 °C, the HMX/GO composite was obtained. SEM images of this material are shown in Fig. 20. It can be seen that GO is not capable of changing the shape of HMX crystals. The morphology difference between HMX and HMX/GO was found to be considerable. The surfaces of unmodified HMX crystals are smooth and clean, while some wrinkles are observed in composite HMX/GO, which may significantly improve the safety of HMX. It was also shown that GO sheets exhibited an even better desensitizing effect than C60 and CNTs.
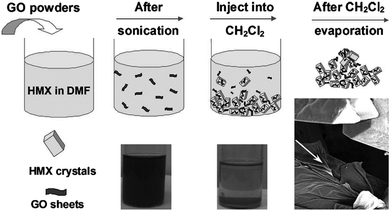 | ||
| Fig. 19 Procedures for the formation of the HMX/GO composites. Reprinted from ref. 168; published with permission. Copyright © 2013, John Wiley & Sons, Inc. | ||
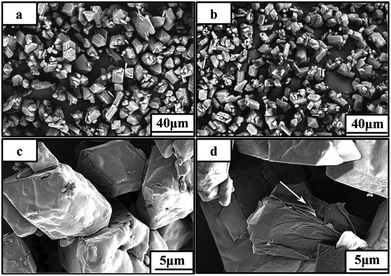 | ||
| Fig. 20 SEM images of HMX and HMX/GO-2 at low-magnification (a, b) and high-magnification (c, d). Reprinted from ref. 168 with permission. Copyright © 2013, John Wiley & Sons, Inc. | ||
As well as simple coating of energetic crystals, GO can also be functionalized with energetic moieties. It has been reported that tetrazine derivatives can be covalently grafted to GO through nucleophilic substitution, forming a new type of material (FGs-Tz).169 The general synthetic route, leading to the grafting of substituted chlorotetrazines to FGS2, which is GO with a nominal C/O of 2, is shown in Scheme 11. The reactions of FGS2 with chlorotetrazines take a long time (48 h) requiring a temperature of 120 °C. The reaction between Tz1 and FGS2 was found to be unsuccessful and undesirable side reactions took place between the Tz1 and the other components of the suspension. It has been shown by DSC that the unmodified FGS2 has a strong exothermic peak at ∼230 °C due to rapid thermal reduction releasing H2O vapour and CO2. This exotherm was not present in thermally reduced FGS and in the FGS-Tz compounds, indicating that the FGS2 was reduced during the reaction between chlorotetrazines and FGS2. There might be an exotherm above 300 °C for FGS-Tz3 and FGS-Tz4, because the decomposition was not complete below that temperature.
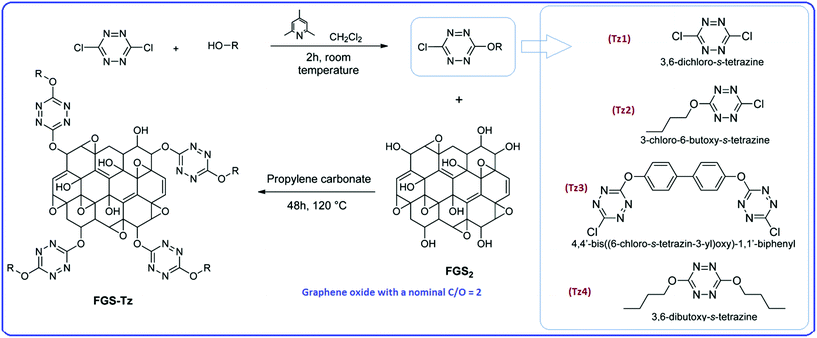 | ||
| Scheme 11 General synthetic route leading to the grafting of substituted chlorotetrazines to FGS2.169 | ||
It is, therefore, not clear whether these functionalized FGS-Tz can be considered as energetic materials. In contrast to the GO grafted with tetrazines, the pure tetrazines are less stable, indicating that GO has a stabilization effect on these tetrazine molecules. All the graphene, GO and rGO-based EMs and their preparation methods, together with their potential applications, are summarized in Table 5.
| Materials | Preparation method | Applications | Contributors |
|---|---|---|---|
| NdG, N-doped graphene; GA, graphene aerogel; AP, ammonium perchlorate; GKPA, graphene-doped ultrafine potassium picrate; FOX-7, 1,1-diamino-2,2-dinitroethylene; GL, 551 glue; FGS-Tz, tetrazine derivative functionalized GO with a nominal C/O of 2. | |||
| GA/Fe2O3/AP | By a facile sol–gel method and supercritical carbon dioxide drying technique | Propellants | Lan et al. (2015)158 |
| GA/AN | By a sol–gel and supercritical CO2 drying method | Propellants | Lan et al. (2015)160 |
| GO-Tzs | Tetrazine derivatives are covalently grafted to GO through nucleophilic substitution, at 120 °C for 48 h in propylene carbonate | Not clear | Li Y. et al. (2015)169 |
| CL-20/GL/rGO | Graded CL-20 particles are coated with 551 glue by an emulsion polymerization method and then mixed with reduced GO | Energetic compositions | Yu L. et al. (2014)265 |
| Al/Bi2O3/FGS | Formed in a colloidal suspension phase that ultimately condenses into ultra-dense macrostructures | Energetic devices | Thiruvengadathan et al. (2014)161 |
| AP/GA | The nanostructured energetic composite is prepared by the sol–gel method | Solid propellants | Wang et al. (2014, 2012)157,158 |
| GKPA | Prepared under the action of a crystalline controlling agent and modified by graphene | Energetics | Liu et al. (2014)81 |
| HMX/GO | A two-dimensional GO was introduced to HMX by the solvent–nonsolvent method | Propellants and explosives | Li R. et al. (2013)168 |
| GLS | Prepared either by adding graphene nanoplatelets (GNP) to the reaction solution or by coating normal lead styphnate (LS) with GNP | Primary explosives | Li Z. M. et al. (2013)154 |
| GO/NC | Pure and GO-doped NC films are obtained through mixing pure NC–acetone solution and various weight ratios of GO water solution | Propellants | Zhang X. et al. (2013)165 |
| GO/FOX-7 | FOX-7 nanocrystals are synthesized in mesoporous carbon FDU-15 through self-assembly impregnation in N-methyl-2-pyrrolidone at 100–110 °C | Explosives and propellants | Cai H. et al. (2013)163 |
According to Table 5, graphene or its aerogels can be combined with either catalysts or oxidizers. The sol–gel method is commonly used for preparation of these materials. Pure GO and rGO are energetic but there are not many energetic nanomaterials or composites based on these yet, except for composites with HMX, NC and FOX-7. These energetic components were either deposited on the skeleton of GO or coated by thin layers of GO or rGO. All the above-mentioned composite materials are novel and were reported very recently. All these materials can be considered as promising candidate components for energetic compositions, as combustion catalysts or main ingredients.
EMs based on functionalized CNTs
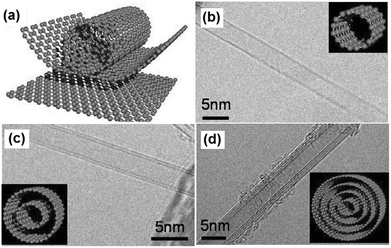 | ||
| Fig. 21 (a) Schematic diagram of an individual layer of honeycomb-like carbon called graphene and how this can be rolled to form a CNT; (b)–(d) HR-TEM images of single-, double- and multi-walled carbon nanotubes (insets are their corresponding images). Reprinted from ref. 170 with permission. Copyright © 2006, International Union of Pure and Applied Chemistry. | ||
CNTs can be endcapped by Cu and Fe nanoparticles for energetic and catalyst applications (Fig. 22). This is essential for large-scale production of high-purity SWCNTs,182 double-walled CNTs (DWCNTs)183 and MWCNTs.184 Preparation of DWCNTs, with lengths of up to 2.2 mm, were achieved with a selectivity of 85% by precisely controlling the thickness of the iron catalyst.183 A variety of aligned CNT structures have been prepared predominately on non-conducting substrates, posing limitations on applications where conductive substrates/contacts are required.172,173,177 To circumvent this problem, aligned MWCNTs can be directly grown on Inconel 600 metallic alloy, using vapor-phase catalyst delivery.185 Controlled synthesis has endowed SWCNTs with narrow distributions of tube diameter and a large fraction of a predetermined tube type.186,187
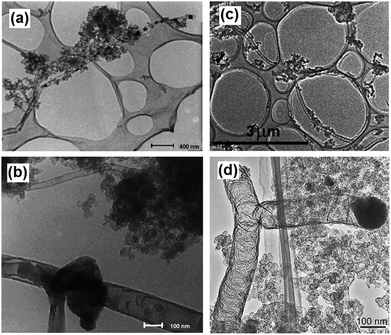 | ||
| Fig. 22 TEM image of an end-capped CNT with Cu-particles (a), a tube-within-a-tube CNT (b), a segmented CNT with a length of a dozen microns (c) and a CNT–T-junction with a crystalline Fe-particle at its end (d). Reprinted from ref. 188 with permission. Copyright © 2004 Elsevier Ltd. | ||
It is possible that CNTs formed in the detonation processes of the high-nitrogen compound, cyanuric triazide (TAT), contain a small number of nitrogen atoms. Elemental analysis showed that less than 3 wt% nitrogen was present when using a C/N-containing material as the precursor.188,189 It was reported that the nitrogen atoms in CNx nanotubes can be inhomogeneously distributed with enrichment of carbon on the external surface.190 All tubes prepared using nickel, copper or steel cartridges were of a multi-walled type but the morphology and the yield depended on the metal. For Ti-based catalysts, no CNT formation was detected, indicating that this metal has no catalytic effect on nanotube formation. In addition to TAT, polyazidopyrimidines and metal/fluorocarbon pyrolants can also be used to prepare CNTs.191,192 2,4,6-Triazidopyrimidine (TAP) was found to be a good precursor for CNTs in the presence of Ni(ClO4)2. SEM and TEM images of CNTs generated by catalytic detonation of TAP are presented in Fig. 23. It can be seen that hollow-channel CNT structures, with lengths of 3–20 μm and diameters of 60–80 nm are obtained. The yield of CNTs is estimated to be greater than 90%. They have a bamboo-like morphology and these “compartmentalized nanotubes” are segmented with a relatively uniform length of about 90 nm. As well as the detonation synthesis method, SWCNTs and “carbon nano-carpet rolls” (CNCR) were found in the combustion residue of a pyrolant based on poly(carbon-monofluoride) (PMF, (CFx)n) and were rich in Mg particles.192 More investigations should be done to clarify the mechanism of CNT formation during combustion of EMs. In addition to organized CNT structures, a variety of tailored structures with enhanced properties have been synthesized for special applications, such as: 1-D yarns, fibers, ropes and brushes, 2D sheets and buckypapers and 3D foams and sponges. 1-D nitrogen-containing CNTs (1-D NCNSs) have emerged in the past two decades as exceptionally promising nanomaterials due to their unique physical and chemical properties, which enable a broad range of applications in various fields of modern technology.193 All these CNTs or NCNS materials can be used in the field of EMs in the following ways: (1) functionalized with polymeric binders, (2) coupled with pyrotechnics, (3) used as carriers of combustion catalysts, (4) combined with metal fuels and (5) functionalized with energetic moieties/groups.
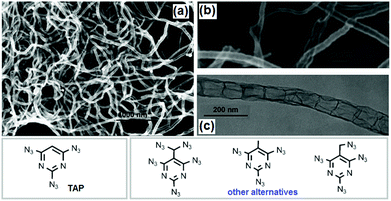 | ||
| Fig. 23 SEM (a, b) and TEM (c) images of CNTs generated by catalytic detonation of TAP (other polyazidopyrimidines can be used for this purpose) (reproduced from Ye et al., Angew. Chem., Int. Ed., 2006, 45, 7262–7265). Reprinted from ref. 191 with permission. Copyright © 2006, WILEY-VCH Verlag GmbH & Co. KGaA. | ||
The thermal stability was also increased, while its glass transition temperature (Tg) was decreased to below −40 °C. Later on, another similar energetic binder – 3,3′-bisazidomethyloxetane-3-azidomethyl-3′-methyloxetane (BAMO-AMMO), covalently modified with CNTs was synthesized by the same research group.195 Their preparation schemes as well as SEM images of the obtained BAMO-AMMO films are shown in Fig. 24. During preparation, BAMO-AMMO random copolymer (or TME) was added to a reactor under a N2 atmosphere, followed by mixing with TDI and dibutyltin dilaurate (DBD). The reaction mixture was then blended uniformly and cured at 60 °C for 7 days. Similarly to the GAP–CNT composite, upon using 1 wt% of CNT-OH with an R value of 1.4, the elongation ratio of the CNTs/BAMO-AMMO binder was increased to 380% and the tensile strength to 10.4 MPa, which was 82.5% higher than that of the traditional trimethylolethane (TME)/BAMO-AMMO energetic binder. However, the Tg of the CNTs/BAMO-AMMO binder (−34 °C) is slightly higher than that of GAP–CNTs.
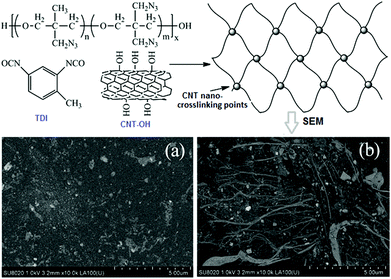 | ||
| Fig. 24 Synthetic scheme for CNT/BAMO-AMMO thermoset polyurethane elastomer and its SEM images with different CNT mass fractions: (a) 0.5 wt% and (b) 1.0 wt%; (reproduced from Zhang et al., J. Energet. Mater., 2015, 33, 305–314.) Reprinted from ref. 195 with permission. Copyright © 2015 Taylor & Francis. | ||
In addition to energetic polymers, CNTs can also be used to enhance inert polymers such as fluoropolymer, which is a very important binder for PBXs.196,197 The mechanical strength of PBXs needs to be improved for application in penetration warheads and nuclear bombs. MWCNT/fluoropolymer (F2314) composites were prepared via a melt blending process and the effect of the MWCNT content on the viscoelastic properties of MWCNT/F2314 were explored using a dynamic mechanical analysis (DMA) method.198 It has been shown that at 80 °C and 0.1 MPa, when the MWCNT content was increased from 2 wt% to 20 wt%, the constant creep strain rate of MWCNT/F2314 composites was decreased by 84.7%.
In order to achieve the goal of molecular-level mixing, a new wet chemical method was proposed to embed KNO3 in CNTs, forming uniform novel KNO3/CNTs nanoenergetic materials.200,201 The latter material can be integrated into a copper thin-film microbridge (CTFM) positioned on a glass substrate. It has been shown that the hollow cavities of the CNTs were filled with crystalline KNO3 and that the entire surface of the micro-initiator was well distributed without significant agglomeration. Compared with single-layer CTFMs, the micro-initiator exhibited a more violent electrical explosion process than the micro-initiator without CNTs. Also, the CNT-containing micro-initiator had a longer duration electrical explosion and a higher peak temperature, due to enhancement of heat generation by CNTs. It has been shown that the decomposition heat of CNT-based micro-initiators is about 876.1 J g−1 (peak temperature: 386.8 °C). High-speed photography of the ignition process for these micro-initiators showed that fast chemical reaction of CNTs was involved in the electric explosion process, resulting in more heat release. Fig. 25 shows TEM images of KN/CNTs nEMs as well as SEM images of the sectional view and surface morphology of the micro-initiator, after electrophoretic deposition (EPD).
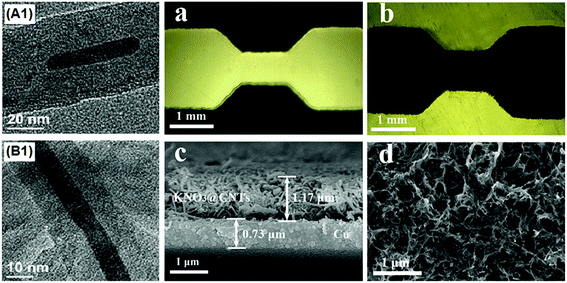 | ||
| Fig. 25 TEM images of the KN/CNTs nano energetic materials (nEMs) achieved by wet chemical method (A1 and B1), showing the legible lattice stripe (B1); micrographs of the Cu thin-film microbridge (a), the micro-initiator after EPD (b); SEM images of the sectional view (c) and the surface morphology of the micro-initiator after EPD (d). Reprinted from ref. 200 with permission. Copyright © 2012 Elsevier B.V. | ||
It can be seen in Fig. 25 that the KN nanocrystals homogeneously fill the hollow cavities of CNTs, without impurities attaching on the exterior wall. The average proportion of CNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KN is estimated as about 10
KN is estimated as about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from TEM images. The bulk density of KN is 2.109 g cm−3 and the density of MWCNTs is typically observed to be around the same as KN. It is clear that the KNO3/CNTs nEMs tightly covered the CTFM. The whole surface was well distributed without significant agglomeration. The CNT arrays showed excellent electron field emission properties.202 When KNO3/CNTs nEMs were integrated with a CTFM deposited onto a ceramic substrate, the electro-explosion performance was greatly improved. This means that, due to the superior electric and thermal conductivities of CNTs, the enhanced chemical reaction of the KNO3/CNTs nEMs is beneficial for the miniaturization of electro-pyrotechnics. In fact, there are several reported techniques for integrating EMs on an electronics or microelectromechanical system (MEMS) chip. These include modifications to the bulk substrate material, deposition of thin films and growth of nanotubes on the top of the substrate.203
1 from TEM images. The bulk density of KN is 2.109 g cm−3 and the density of MWCNTs is typically observed to be around the same as KN. It is clear that the KNO3/CNTs nEMs tightly covered the CTFM. The whole surface was well distributed without significant agglomeration. The CNT arrays showed excellent electron field emission properties.202 When KNO3/CNTs nEMs were integrated with a CTFM deposited onto a ceramic substrate, the electro-explosion performance was greatly improved. This means that, due to the superior electric and thermal conductivities of CNTs, the enhanced chemical reaction of the KNO3/CNTs nEMs is beneficial for the miniaturization of electro-pyrotechnics. In fact, there are several reported techniques for integrating EMs on an electronics or microelectromechanical system (MEMS) chip. These include modifications to the bulk substrate material, deposition of thin films and growth of nanotubes on the top of the substrate.203
The EMs systems involved are nanoporous energetic silicon, energetic CNTs and solution-deposited nitramines (e.g. RDX). As well as micro-ignitor applications, CNTs can also be used to stabilize the extremely sensitive new “green” primary explosives. Those that are currently used, such as lead azide (LA) and lead styphnate (LS), are very toxic and harmful to the people who work with them. Nowadays, more and more novel environmentally friendly EMs have been developed to replace LA and LS but many of the materials are simply too sensitive to handle. Copper azide (CA) is one of these cases and it detonates easily from electrostatic charges during handling. It has been found that the sensitivity of CA can be suppressed when it is encapsulated in conducting containers, such as anodic aluminium oxide (AAO)-templated CNTs.204 The preparation procedure of CA/AAO–CNT composites and the morphologies of the involved intermediates are shown in Fig. 26.
 | ||
| Fig. 26 Schematics of the chronological steps of the synthesis process: (a) commercial alumina membrane; (b) carbon-coated alumina membrane; (c) pores of the carbon-coated membrane filled with colloidal Cu–O NP solution; (d) Cu–O NP-filled CNTs after dissolution of the alumina membrane; (e) Cu NP-filled CNTs after H2 reduction; (f) CA NPs inside the CNTs. (g) TEM image of Cu–O NP-filled CNTs, (h) an enlarged view showing rod-like NPs inside the CNT framed in (g); TEM micrograph of (i) CuN3/Cu(N3)2-filled CNT and (j) SAED pattern of the CuN3-filled CNTs. Reprinted from ref. 204 with permission. Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
It can be seen in Fig. 26 that the colloidal copper oxide nanoparticles (nano Cu–O) of ∼5 nm are synthesized and fill CNTs with a diameter of ∼200 nm, produced by template synthesis. The Cu–O inside the CNTs is reduced by hydrogen to elemental copper and reacts with hydronic acid gas to produce copper azide. It was found that upon ignition, the 60 μm long, straight, open-ended CNTs guide decomposition gases along the tube channels, without fracturing the nanotube walls. These novel materials are potentially useful as nano-detonators and “green” primary explosives. This is also the case for BNCP, which can either be coupled with CB or encapsulated by CNTs (Fig. 27).52
 | ||
| Fig. 27 SEM images of CNTs and BNCP/CNTs. Reprinted from ref. 52 published in Chin. J. Energet. Mater. (2013) with permission. | ||
The encapsulating material should be electrically conducting in order to dissipate electric charges and prevent detonation due to static electricity. If the CNTs are straight and open-ended, nanotube channels can potentially drive the shock waves along their lengths, increasing the efficiency of initiation of the secondary explosives, thus retaining the initiated particles within the nanotubes.205 In addition to primary explosives, the properties of pyrotechnic compositions used in flashbang or solid state laser pump sources can also be modified by CNTs. To improve the pump efficiency of the Zr/KClO4 pyrotechnic reagent, high strength CNTs with strong adsorption capacity were introduced.206 It was shown that the inclusion of CNTs had a significant effect on the thermal behavior and light radiation energy. When the content of CNTs is 0.5 wt%, the total radiation energy of the pyrotechnics can reach 1830 J g−1.
Regarding combustion catalysts for propellants, on one hand, they should maintain their activity during production of propellants, which means that the catalyst should be chemically compatible with various ingredients of the propellants.208,209 On the other hand, the particle size of the catalysts should be as small as possible with a surface structure that can be uniformly dispersed in the propellant matrix. Nano-sized catalysts or additives are highly promising for improving the combustion performance of propellants but they are very active and need to be coated with inert materials. A combination of these nano-sized catalysts with CNTs was a promising solution. For instance, a nano Cu/CNT composite catalyst (see Fig. 28) was prepared by a liquid reductive deposition method, using CNTs as the carrier.210 The researchers used CuCl2·2H2O as the source of Cu ions and KBH4 as a reducing agent. The reaction was conducted at 50 °C with addition of disodium EDTA and PVP as surfactant and complexing agents. It has been shown that Cu/CNTs can remarkably decrease the peak decomposition temperature (Tp) of AP/HTPB propellant.
 | ||
| Fig. 28 SEM and TEM images of CNTs, Cu/CNT and CuO/CNT combustion catalysts. Reconstructed from several figures that appeared in ref. 210, 211, 215 and 216 with permission. | ||
Similarly, a CuO catalyst can also be carried by CNTs, forming a novel CuO/CNT composite (black powder, Fig. 28).211 Taking cupric acetate and CNTs as the reactants, CuO/CNTs can also be prepared by a solvent-infusion method at normal pressure and at a temperature of 100 °C. The catalytic effect of this material on N-guanylurea-dinitramide (FOX-12 or GUDN) was found to be significant and the resulting composite material had excellent thermal stability and low sensitivity. In addition to the above-mentioned composites, metal fuels or catalysts can also be encapsulated inside CNTs. SWCNTs were proposed to be the best protective coating for energetic metal nanoparticles and as a base for propellant catalysts.211 An advanced technology has been developed to prepare metalized CNTs using a CO2 laser for ablation of the composite graphite target212 or electroless deposition on top of MWCNTs.213 SEM and TEM images of the resulting product, as well as its XRD spectra are shown in Fig. 29.
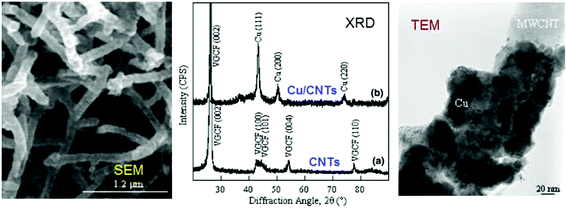 | ||
| Fig. 29 SEM and TEM images and XRD spectra of CNTs and Cu/CNTs prepared by an electroless deposition method. Reprinted from several figures published in ref. 213 with permission. Copyright © 2004 Elsevier B.V. | ||
It can be seen in Fig. 29 that copper layers with an average thickness of 40 nm have been deposited on the surfaces of MWCNTs. This provides an effective means to fabricate powder metal-coated CNT composites. Based on this principle, Fe2O3/MWCNT composite particles were also produced by a mild physical absorption method using molten Fe(NO3)3·9H2O as a precursor.214 It has been shown that a large amount of hematite-phase Fe2O3 uniformly fills the MWCNTs, where the fraction of Fe2O3 is about 25.8 wt%. In fact, this concept is also applicable to binary catalysts. For instance, an SnO2–Cu2O/CNT composite catalyst was prepared by an integrative infusion method based on liquid-phase deposition, using CuCl2·2H2O, SnCl4·2H2O and CNTs as reactants.215 It was shown that SnO2 and Cu2O nanoparticles were uniformly attached on CNTs with oval morphologies and a layer thickness of 5–10 nm (Fig. 28). This has a positive catalytic effect on the decomposition of FOX-12, which can also be catalyzed by the aforementioned CuO/CNTs. The catalytic effects of these materials will be further discussed in a separate section, in conjunction with the thermal behavior of several other related materials.
Another binary catalyst that is widely used in solid propellants is CuO·PbO. The catalyst can also be modified by addition of CNTs, forming a CuO·PbO/CNT composite material by a microemulsion method.216 The obtained catalyst has particle sizes below 50 nm (Fig. 28). In addition to copper and lead oxides, cobalt and aluminum oxides can also be deposited on CNTs for better catalytic activity. It was reported that a Co–Al mixed metal oxides/CNTs (CoAl-MMO/CNT) nanocomposite could be synthesized from a Co–Al layered double hydroxide/CNT composite precursor.217 It has been shown that that in this nanocomposite, the cobalt oxide nanoparticles and Co-containing spinel-type complex metal oxides are well-dispersed on the surface of CNTs, forming a heterostructure of CoAl-MMO and CNTs, which has an excellent catalytic effect on energetic oxidizers such as AP.
 | ||
| Fig. 30 SEM images (A and B) and EDS spectra (A′ and B′) of Ag/CNTs prepared by two different methods: (A) silver mirror reaction and (B) hydrothermal method. Reprinted from several figures published in ref. 221 with permission of Acta Phys.-Chim. Sinica. | ||
As the most widely used metal fuel, Al has been intensively investigated regarding its oxidation and combustion behavior, which has a significant effect on the final performance of Al-containing propellants and explosives. Controlling the Al oxidation rate and temperature, as well as reaction enthalpy is important for its practical use. CNTs were introduced to Al initially for enhancing its mechanical strength, due to the large tensile strength of CNTs (150 GPa).223–225 However, the thermal behavior could also be tailored by CNTs according to a recent report.222 CNTs have several functions, which can be used to control the sizes and thermal properties of Al particles by changing the CNT content. Morphological modifications of Al/CNT mixtures during different stages of ball-milling of Al particles with CNTs are shown in Fig. 31. This figure shows the morphological changes that Al/CNT mixtures undergo with the progress of milling time. The morphology can be categorized into three types based on milling time: in Stage I, Al particles were coated by CNTs and spherical Al particles were distorted into platelets. In Stage II, Al particles disintegrated into smaller sizes and CNTs were partially embedded inside Al particles, giving them the appearance of sea-urchins, and in Stage III, Al/CNT particles aggregated into larger sizes with CNTs homogeneously dispersed inside the Al matrix.
 | ||
| Fig. 31 Morphological modifications of Al/CNT mixtures with ball-milling time: (a) Stage I (30 min); CNTs coated on the surface of Al particles, (b) stage II (120 min); CNTs embedded inside Al particles while a portion of the CNTs remain on the Al surface, and (c) Stage III (180 min); Aggregation of Al particles with embedded CNTs inside. Reprinted from ref. 222 with permission of Crown copyright © 2013, Published by Elsevier Ltd. | ||
In addition to NO2 groups, many other energetic groups can be introduced to CNTs. In fact, SWCNTs modified with tetrazolyl groups (SWCNTs-CN4) were prepared by a diazo-reaction, where covalent bonds were formed to maintain the structure of the five-membered ring.228 It was confirmed by X-ray photoelectron spectroscopy (XPS) that the nitrogen content in this SWCNTs-CN4 material was about 14.8%, while the ratio of tetrazolyl groups to carbon atoms was about 4.9%. Similarly, on the basis of diazonium chemistry, SWCNTs can be decorated with nitrobenzenes, via microwave irradiation of the reaction mixture.229 It has been found that even covalent functionalization introduces defects that could reduce conductivity and that thermopower waves rapidly propagate along fuel-coated decorated nanotubes, producing electrical power. A schematic representation of reaction propagation on a SWCNT decorated with trinitrobenzenes and the reaction conditions required to produce different nitrobenzene-functionalized SWCNTs, as well as their Raman spectra and DSC thermograms are shown in Fig. 32. It is clear that the energy from a chemical reaction at one end propagates through the 1-D conductor, initiating reactions in the attached molecules at the wave-front. The energetically decorated SWCNTs appear to generate considerable heat when decomposing at high temperatures. It can be seen from Fig. 32d that MNP-, DNP- and TNP-SWCNTs can undergo exothermic reactions between 300 and 330 °C during linear heating, except for the BrP-SWCNTs (functionalized with a non-energetic 4-bromophenyl group).
 | ||
| Fig. 32 (a) Schematic representation of reaction propagation on a single-walled carbon nanotube decorated with trinitrobenzenes; (b) synthesis of nitrophenyl-SWCNTs via diazonium reactions, where “i” represents NOBF4, acetonitrile, 0 °C, 4 h (for MNP- and DNP-SWNT) and “ii” denotes NOHSO4 (NaNO2 + H2SO4), H2O, room temperature, 3 h (for TNP-SWCNT); (c) Raman spectra of energetically decorated SWCNTs with modes at 1200 cm−1 increased in the Raman spectra after covalent functionalization (excited at 785 nm); (d) DSC curves at 10 °C min−1 under nitrogen where all the nitrophenyl-SWCNTs decompose with slow heat release, whereas the BrP-SWCNTs do not release heat at all. Recombination of several figures from ref. 229 reprinted with permission. Copyright © 2011, American Chemical Society. | ||
In addition to covalently functionalized CNTs with energetic groups, energetic compounds can non-covalently functionalize CNTs via doping or adsorption. For instance, BNCP can be doped with CB or CNTs,52 whereas TATB can be adsorbed by CNTs.230 Theoretical calculations show that TATB deforms remarkably when it is non-covalently attached to the surface of CNTs, especially on their inner wall. The diameter of the CNT determines the distortion degree of the TATB molecule inside but it has little effect on the deformation of a TATB molecule bound to the outside of the CNT. Non-covalent combination of TATB with CNTs is exothermic due to the negative adsorption energy. TATB adsorption on the inner wall of CNTs was energetically more favourable than that of adsorption on the outer wall. In both cases, more stable adsorption occurs as the diameter of the nanotube increases. As a comparison, these researchers also theoretically investigated the structure, adsorption energy and initial dissociation reaction of the energetic 3,3′-diamino-4,4′-azofurazan (DAAzF), assembled inside or outside SWCNTs.231 They found that most of the adsorption processes of SWCNTs with larger diameters are exothermic and thermodynamically favourable, indicating that DAAzF spontaneously assembles inside the CNTs. Based on these theoretical findings, CNTs were successfully used as a host for some high-nitrogen content compounds. For instance, CNTs (10,10) were selected as a nanoscale matrix to host N-oxides of 3,3′-azo-bis(6-amino-1,2,4,5-tetrazine) (DAATO) molecules, using a CVD (chemical vapor deposition) method for obtaining so-called nitrogen-doped CNTs (N-CNTs).232 Their SEM and TEM images, as well as XPS spectra are shown in Fig. 31. It has been stated that the DAATO contains an average of 3.2–3.6 oxygen atoms per molecule,233 resulting in five possibilities for positioning and quantifying oxygen atoms per molecule (Fig. 33c).
 | ||
| Fig. 33 SEM and TEM images for the morphology (a), N 1s XPS spectra (b) of N-CNTs synthesized at 980 °C and the structural geometry of DAATO/CNT (10, 10) (c). Recombination of several figures from ref. 232 reprinted with permission. Copyright © 2009 Elsevier Ltd. | ||
The diameter and growth rate of these novel hybrid materials are obviously temperature-dependent. It has been found that a single N-CNT has a diameter of 60 nm when its preparation temperature is 980 °C (Fig. 33), while the diameter is only around 30 nm at 800 °C, because as temperature decreases, agglomeration of the catalyst reduces, resulting in thinner N-CNTs. It has also been shown that a lower synthesis temperature usually results in curled and disordered graphitic layers but a higher temperature favours the formation of a better-organized layered graphitic structure.234 N-CNTs have a nitrogen content of 10.4% when the temperature is 800 °C. As well as tetrazine, triazole moieties can also be used to functionalize MWCNTs using ‘click’ chemistry.235 The resulting product was successfully incorporated into a polyurethane (PU) matrix by in situ polymerization, where interactions with the PU polymer were enhanced. As a result, the heat of decomposition of the PU/MWCNTs nano-composites was significantly increased due to inclusion of triazole moieties.
In addition to the above-mentioned CNT-based energetic materials, more and more novel EMs are under development. As a comparison, the preparation and applications of existing CNT-based EMs are summarized in Table 6. It can be seen that most functionalized CNTs are used for catalysts or energetic additives of propellants and explosives due to their relatively lower energy content and promising catalytic activity. Several of them can be used as ingredients of pyrotechnics, such as thermites and ignitors. The preparation procedures for these materials are typically facile, including microemulsion, hydrothermal, reductive deposition, ultrasonic and CVD, as well as straightforward chemical reaction methods. Combination of CNTs with sensitive EMs provides a way to improve the sensitivity and performance of the parent EMs. It has been reported that N-doping of CNTs can create well-localized and highly active sites on the CNT surface.236 When CNTs are filled with highly EMs, e.g. a polymeric nitrogen chain, an effective electric field is created between the CNT surface and the nitrogen chain with a minimal C–N distance. This inclusion process stabilizes the highly energetic polynitrogen chain, which is unstable under ambient conditions.237 This approach helps in the utilization of highly unstable EMs.
| CNT based EMs | Preparation methods | Applications | Contributors |
|---|---|---|---|
| CNT-OH, hydroxylated CNTs; BAMO-AMMO, 3,3′-bisazido methyloxetane-3-azidomethyl-3′-methyloxetane; TDI, toluene diisocyanate; TNB, trinitrobenzene; STP, stannic tetrachloride pentahydrate; AAO, anodic aluminum oxide; BNCP, bis(5-nitrotetrazolato) cobalt(III) perchlorate; TOC, thionyl chloride; NA, 4-nitroaniline. | |||
| SWCNTs-CN4 | Prepared by a diazo-reaction on the wall of SWCNTs | Energetic materials | Ji et al. (2015)228 |
| MWCNT/F2314 | Obtained from the melt blending of fluoropolymer and MWCNT mixture | PBXs, pyrotechnics | Lin et al. (2015)198 |
| KNO3/CNTs | By mechanically mixing and grinding to nanosize | Energetic initiator | Guo et al. (2012200 and 2014202) |
| Zr/KClO4/CNTs | By grinding a mixture of Zr and KCIO4 for 20 min | Pyrotechnic reagent | Liu et al. (2015)206 |
| CNT/BAMO-AMMO | Using CNT-OH as a crosslinking agent, covalently modified BAMO-AMMO | Energetic binder | Zhang et al. (2013194 and 2015195) |
| Fe2O3/MWCNTs | By mild and superior physical absorption method using molten Fe(NO3)3·9H2O as the precursor | Thermites, pyrophoric substrates | Assovskiy et al. (2009)212 and Wang et al. (2014)214 |
| GAP/CNTs binder films | Using hydroxylation CNTs as crosslinking agent, GAP as prepolymer, TDI as curing agent | Propellants, PBXs | Zhang et al. (2013)194 |
| Al/CNT composites | Al/CNT mixture was engineered by mechanical pulverization | Thermites, propellants | Jeong et al. (2013)222 |
| Nano BNCP/CNT | Prepared from tetraamine BNCP doped with CNTs | Detonators, pyrotechnics | Chen et al. (2013)52 |
| CoAl–MMO/CNT | Synthesized from Co–Al layered double hydroxide/CNTs precursor | Propellant catalyst | Fan et al. (2013)217 |
| HMX/CNTs | Prepared with an ultrasonic compositing method | Energetic compositions | Zeng et al. (2012)248 |
| Ag/CNTs | Using silver mirror reaction and hydrothermal methods | Explosive catalyst | An et al. (2012)221 |
| Al/Teflon/CNTs | By blending of Al with Teflon and CNTs | Energetic binder | Kappagantula et al. (2012)277,278 |
| Nitro-SWNTs | Prepared by chemical transformation of the carboxylic acid groups introduced on the wall of CNTs | Energetic materials | Forohar et al. (2012)227 |
| HMX-P/MCNTs | HMX-polymeric binder system doped with a small mass fraction (1 wt%) multiwalled CNTs or graphite | Microwave materials | Zeng et al. (2012)248 |
| NB-SWNTs | Single-walled CNTs decorated with mono-, di- and tri-NB via diazonium chemistry | Thermopower wave generators | Abrahamson et al. (2011)229 |
| SnO2–Cu2O/CNTs | Prepared by integrate infusioning with liquid phase deposition under normal temperature and pressure using CuCl2·H2O, STP, and CNTs as the reactants | Explosive catalyst | Zhang et al. (2011)215 |
| AAO/CNTs | AAO is simply mixed with templated CNTs | Nano-detonators | Pelletier et al. (2010)204 |
| Nd-CNTs | Synthesis from nitrogen-doped CNTs using chemical vapor deposition method | Energetic components | Zhong et al. (2010)232 |
| PbO·CuO/CNTs | Prepared via a microemulsion process | Catalyst for propellants | Ren et al. (2010)216 |
| MSWCNT | Using a CO2 laser for ablation of the composite graphite target | Catalyst for propellants | Esawi et al. (2010)225 |
| CuO/CNTs | Using sol-infusion method under normal pressure and low temperature (at 100 °C) | Catalyst for propellants | Liu et al. (2008)210 |
| Nano Cu/CNTs | By liquid reductive deposition method and using the carbon nanotubes as the carrier | Catalyst for propellants | Liu et al. (2008)211 |
| NA/SWNTs | Functionalization is achieved on CVD-produced SWNTs by acidification to –COOH followed by reaction with TC, and then NA | Energetic component | Wang et al. (2003)226 |
Effects of CNMs on performances of EMs
Catalytic decomposition, ignition and combustion
It was found from the decomposition of HMX/CNT composites that the CNTs reduced the decomposition Ea and the peak temperature (Tp) of HMX.248 About 1 wt% of CNTs in this mixture reduced the Ea of HMX by more than 70 kJ mol−1, resulting in a higher decomposition rate. However, the heat of decomposition and Tp of HMX decreased as the CNT content increased. Similar research has been carried out for hexanitrohexaazaiso wurtzitane (CL-20) and aminonitrobenzodifuroxan (CL-18) using DSC and TG techniques.249,250 It was shown that CNTs also bring down the initial thermolysis temperature of these explosives. It was suggested that the reason for this phenomenon is that CNTs promote homolytic scission of the N–NO bond, thereby promoting formation of NO˙ free radicals. This is also the case for AP-based compositions, where the decomposition rate of AP has been raised above its typical onset temperature.251
In fact, the porous structure of CNT bundles, having a very large SSA, can be considered as a combustion catalyst nano-carrier, forming many types of highly-promising composite catalysts. The synergistic effect of the CNTs with the catalysts greatly improves catalytic activity and the agglomeration problem of nano-sized catalysts can also be resolved. For instance, the catalyst Ag/CNTs can change the thermal decomposition mechanism of RDX, where the dominant liquid phase reaction changes to a faster gas-phase reaction, resulting in a lower Tp.221 The effect is also similar for a PbO–CuO/CNT composite catalyst,230 which has a greater influence on the thermal decomposition of RDX compared to the parent PbO–CuO. Another two nano-catalysts, CuO/CNTs231 and SnO2–Cu2O/CNTs,239 were also shown to decrease the Tp of FOX-12 decomposition, meanwhile the heat release and Ea were decreased accordingly. When CNTs are used to encapsulate energetic molecules, such as nitromethane (NM), they form a NM/CNT composite material, which has very different thermal behavior in comparison with the parent NM.252 It has been shown that a transition state (TS) exists for the thermal decomposition of NM/CNT (5, 5), while there is no TS for dissociation of a C–N bond in the gas phase of a “stand-alone” NM molecule.253 In fact, SWCNTs can change the molecular structure and electronic charge of the NO2 and CH3 groups of NM during its decomposition. The energy barrier of the TS (in the CNT-bound NM) was calculated to be about 198 kJ mol−1, which is 21 kJ mol−1 lower than that of the aforementioned C–N dissociation of free NM. Meanwhile, the energy barriers for the nitromethane–methyl nitrite rearrangement and H-migration, as well as C–N homolytic dissociation are significantly decreased.254,255 This is also the case for nitramide encapsulated in SWCNTs.256 Regarding some other ingredients of composite propellants, CNTs have little effect on the thermal decomposition of phase-stabilised ammonium nitrate (PSAN) and GAP, while they were shown to stabilize HTPB binder.257
The thermal behavior of ADN is greatly affected by CNT-based catalysts. For instance, Fe2O3/CNTs and Fe·Cu/CNTs decrease the decomposition Tp of ADN by 12.1 °C and 11.6 °C, respectively, when just 1 wt% of the catalyst is used.258 These results were observed when a large amount of hematite phase Fe2O3 uniformly filled the MWCNTs and the mass fraction of Fe2O3 in such a composite was about 25.8 wt%.204 In the presence of 10 wt% Fe2O3/MWCNT composites, the second Tp of AP decreased by 116 °C and the first exothermic peak disappeared with a 200 kJ mol−1 decomposition heat increase.
Graphene, a two-dimensional carbon crystal with monoatomic thickness, is considered to be a basic structural unit of CNTs and graphite. It also has a large SSA and can form many complexes with energetic molecules. Ni/graphene nano-composites were prepared via a one-step mixing method and then their catalytic effect on the thermal decomposition of AP was investigated.259 It was shown that when the content of Ni/graphene composites is about 1% of the mixture, the catalytic effect is the most significant. In this case, the second decomposition peak temperature decreased by 97.3 °C, while the first peak disappeared. Later, these researchers used the same methodology to prepare a complex of Mn3O4 nanoparticles with graphene, which also exhibited a significant catalytic effect on AP decomposition.260 Similarly, two decomposition peaks in the latter AP formulation merged into one peak. Meanwhile, the Tp was reduced by 141.9 °C, as a result of the synergy between Mn3O4 and graphene. In contrast to graphene, GO has functional groups on its surface, It is easier to combine with other substances, extending the range of application of GO-based materials. It was reported that CuO/GO nano-composites prepared from a water/isopropanol solvent are very effective in catalyzing AP decomposition and combustion.261 In addition, when some other groups were introduced to GO, the catalytic activity was significantly improved. For instance, nitrated GO decreased the Tp of AP decomposition by 106 °C,262 while the heat of decomposition increased from 875 to 3236 J g−1.
The thermal properties of AN oxidizer, frequently used in propellants and emulsion explosives, can also be modified by integration with CNMs.263,264 When AN was mixed with activated carbon (AC) or CNTs, violent reactions took place upon its melting. However, mixtures of AN with graphite or fullerene showed only mild exothermic peaks (decomposition), similar to those of pure AN. It was also found that the formation of carbon dioxide was more distinct for AN/AC and AN/CNT mixtures than for other mixtures. When BNCP was doped with CB, its Tp decreased from 289.9 to 276.7 °C, while after doping BNCP with CNTs, the Tp was significantly further reduced. There was an obvious endothermic peak and as the nanotube diameter decreased, the decomposition peak for BNCP became narrower and sharper, accompanied by a disappearing shoulder.52 In order to compare the thermal behavior and catalytic effects of different CNM-based EMs, the thermal decomposition data, as well as the kinetic parameters of all above-mentioned materials are summarized in Table 7.
| Samples | Exothermic peaks (10 K min−1) | Kinetic parameters (Kissinger) | ||||
|---|---|---|---|---|---|---|
| T o/°C | T p/°C | ΔH | E a | Ln (A) | Contributors | |
T
o, onset temperature of the peaks; Tp, peak temperature of thermal events; ΔH, heat release in J g−1; Ea, activation energy; A, pre-exponential factor; na, not available; aCNTs with diameter of 10–20 nm, while bCNTs with diameter of 60–100 nm; Ag-CNT-1 and -2 means the mass ratio of Ag/CNTs is 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 9 1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Nomenclature: CL-20, 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane; FEN, fullerene ethylenediamine nitrate; FED, fullerene ethylenediamine dinitramide, H12C60(HNCH2 CH2NH2·HN(NO2)2)12; mNPF, N-methyl-2-(3-nitrophenyl)pyrrolidino- [3′,4′:1,2] fullerene; PF, polynitro[60]fullerene, C60(NO2)14; FPGN, [60]fullerene-poly(glycidyl nitrate); FPL, fullerene phenyl-alanine lead salt; FFGAP, functionalized [60]fullerene-glycidyl azide polymer; FPL, fullerene phenylalanine lead salt; FHN, fullerene hydrazine nitrate; FDN, derivative with molar ratio of C60, 2,4-dinitrobenzaldehyde and N-methylglycine 1 1, respectively. Nomenclature: CL-20, 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane; FEN, fullerene ethylenediamine nitrate; FED, fullerene ethylenediamine dinitramide, H12C60(HNCH2 CH2NH2·HN(NO2)2)12; mNPF, N-methyl-2-(3-nitrophenyl)pyrrolidino- [3′,4′:1,2] fullerene; PF, polynitro[60]fullerene, C60(NO2)14; FPGN, [60]fullerene-poly(glycidyl nitrate); FPL, fullerene phenyl-alanine lead salt; FFGAP, functionalized [60]fullerene-glycidyl azide polymer; FPL, fullerene phenylalanine lead salt; FHN, fullerene hydrazine nitrate; FDN, derivative with molar ratio of C60, 2,4-dinitrobenzaldehyde and N-methylglycine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; NPF, trinitrophenyl C60 derivative; SCCNT, SnO2–Cu2O/CNT composites; PCC, PbO·CuO/CNTs; CCNT, CuO-CNTs (8.8 nm, 5%); GA, graphene aerogel; nD-1, nD/RDX = 1 6; NPF, trinitrophenyl C60 derivative; SCCNT, SnO2–Cu2O/CNT composites; PCC, PbO·CuO/CNTs; CCNT, CuO-CNTs (8.8 nm, 5%); GA, graphene aerogel; nD-1, nD/RDX = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, while it is 1 8, while it is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for nD-2. 4 for nD-2. |
||||||
| ε-CL-20/glue | 231.2 | 250.5 | 1495 | Na | na | Yu et al. (2014)265 |
| ε-CL-20/glue/G | 233.0 | 247.7 | 1027 | Na | na | |
| ε-CL-20/glue/GO | 217.2 | 234.3 | 1678 | Na | na | |
| ε-CL-20/glue/rGO | 234.3 | 250.6 | 1613 | Na | na | |
| RDX | 216.7 | 222.6 | na | 171.7 | 40.84 | Guan et al. (2014)114 |
| GAP/FHN | 112.5 | 154.9/242.0 | na | Na | na | |
| RDX/FHN | 212.6 | 218.7 | na | 154.8 | 36.94 | |
| HMX | 277.3 | 283.8 | na | 358.2 | 76.95 | |
| HMX/FHN | 279.2 | 281.6 | na | 334.7 | 72.11 | |
| RDX | 208.7 | 240.4 | 499.5 | 174.0 | 36.38 | Jin et al. (2014)114 |
| RDX/mNPF | 206.4 | 234.0 | 891.8 | 166.8 | 35.00 | |
| HMX | 276.3 | 278.1 | — | 470.6 | — | Zeng et al. (2012)248 |
| HMX/CNTs | 273.2 | 275.9 | — | 397.6 | — | |
| PF | 160.2 | 189.7/247.0 | 2017.7 | Na | na | Cataldo et al. (2013)113 |
| FED | 150.3 | 202.9 | 1037.7 | Na | na | Chen et al. (2014)114 |
| FEN | 100.0 | 172.3/360.5 | na | Na | na | |
| FPL | 151.3 | 243.6/473.2 | na | Na | na | Guan et al. (2014)114 |
| FPL/RDX | 189.3 | 206.1 | na | 140.8 | 34.95 | |
| FGAP | 100.3 | 154.9/242.0 | na | Na | na | Huang et al. (2015)121 |
| FPGN | 435.2 | 473.3/615.3 | 1280 | 170.9 | 36.43 | Gong et al. (2015)120 |
| BNCP | 269.6 | 289.9 | na | Na | na | Chen et al. (2013)52 |
| BNCP/CB | 260.7 | 276.8 | na | na | ||
| BNCP/CNTs | 263.1 | 280.1a/277.8b | na | Na | na | |
| BNCP/SWCNTs | 262.2 | 278.0 | na | Na | na | |
| FOX-12 | 206.3 | 218.5 | 1540 | 258.3 | 59.30 | Liu et al. (2008)210,211 |
| FOX-12/CCNT | 189.5 | 198.3 | 1872 | 178.6 | 42.24 | |
| FOX-12/SCCNT | 192.4 | 204.1 | 1150 | 181.2 | 45.62 | Zhang et al. (2011)215 |
| RDX/PCC | 208.2 | 226.9 | na | 100.7 | 26.29 | Ren et al. (2010)216 |
| RDX | 205.2 | 240.2/253.3 | 2546 | 174.0 | 36.38 | An et al. (2012)221 |
| RDX/Ag-CNT-1 | 203.7 | 241.0/252.1 | 2451 | Na | na | |
| RDX/Ag-CNT-2 | 203.2 | 249.8 | 2120 | Na | na | |
| AP | 263.2 | 297.0/406.2 | 621 | Na | na | Wang et al. (2012)231 |
| AP/GA | 265.1 | 322.5 | 2110 | Na | na | |
| AP/GA/Fe2O3 | 257.8 | 312/348 | 1985 | Na | na | Lan et al. (2015)158 |
| AP/HTPB | 274.4 | 335.9/405.8 | 1940 | Na | na | Liu et al. (2008)210 |
| AP/HTPB/CNTs | 266.9 | 328.5/380.9 | 2170 | Na | na | |
| AP/HTPB/Cu-CNTs | 271.2 | 353.4 | 2780 | Na | na | |
| RDX/nD-1 | 217.9 | 244.6 | 894.6 | 127.5 | 11.30 | Tong et al. (2009)81 |
| RDX/nD-2 | 215.1 | 237.3 | 625.6 | 199.9 | 19.10 | |
As shown in Table 7, addition of flake graphite to ε-CL-20 induces slightly earlier decomposition of this explosive with much lower heat release, while the addition of GO or rGO to ε-CL-20 enhanced the decomposition of an ε-CL-20/glue formulation with remarkable improvement in the heat release.265 However, only rGO additive could improve the thermal stability of ε-CL-20. Catalysts based on fullerene (CBF) reduced the decomposition Tp and apparent Ea and raised the exothermic heat of FOX-12. When the mass ratio of CBF/FOX-12 is 1-to-5, the decomposition Tp of FOX-12 is reduced by 20.3 °C, while the heat release is increased by 332 J g−1. The corresponding values are 18.08 °C and 148 J g−1 for pure CBF.215 Similarly, the catalytic effect of the Ag/CNT nanocomposite on the thermal decomposition of RDX was investigated by DSC. It was indicated that the irregularly globose nano-Ag particles, evenly attached to the surface of the CNTs, form a promising catalyst for RDX, greatly accelerating the decomposition rate of RDX, with a reduced Ea. This is also the case for a PbO·CuO/CNTs catalyst, which can accelerate the decomposition of RDX and reduce the Tp by 14.1 °C.216 The nDs can also slightly reduce the Tp of RDX.81 In contrast, when a fullerene-based catalyst (mNPF) is added to RDX, its Tp drops from 240.4 °C to 234.0 °C and the corresponding exothermic heat increases from 499.5 to 891.8 J g−1. There is another fullerene-based catalyst, FED, whose catalytic effect has not yet been studied. It was shown that FED started to decompose at 150 °C with a Tp of 203 °C and an enthalpy of 1037.7 J g−1. The total mass loss of FED in the temperature range 100–800 °C is 49.7%, with intense decomposition of –N(NO2)2 and partial decomposition of the branched chain.114 It was further found that FHN can also accelerate the decomposition of RDX and HMX. The peak temperatures of the exothermal decomposition of these nitramines were reduced117 and the corresponding Ea of RDX was decreased by 17 kJ mol−1, while that of HMX was reduced by more than 20 kJ mol−1. In addition to stabilizing common explosives, CNTs also have a significant influence on the stability of energetic binders. For example, the onset temperature of the CNTs/BAMO-AMMO composition was measured to be 236 °C, which is 7 °C higher than that of the corresponding TME/BAMO-AMMO energetic binders.195
It was recently reported that energetic derivatives of C60-fullerene have very unique thermal properties. For instance, MTNBFP has a very high standard molar enthalpy of formation and could be a highly-promising new candidate for primary explosives.113 The most probable initial dissociation path for MTNBFP is the release of NO2 according to dynamic dissociation energies, which are 58.5, 172.5 and 239.2 kJ mol−1. In terms of energetic polymers based on fullerene, it has been shown that C60-PGN is thermally stable up to 200 °C. The decomposition Ea of C60-PGN was measured to be 170.9 3 kJ mol−1 (by the Kissinger method) and 168.3 kJ mol−1 (by the Ozawa–Doyle method).120 C60-PGN is more stable than any other known polynitrofullerene. C60-GAP decomposition shows a three-step thermal process,121 where the first step is due to the reaction of the azide group and fullerene at approximately 150 °C. The second mass loss step is due to the decomposition of the main chain of the remaining GAP and N-heterocyclic at approximately 240 °C. The final decomposition step could be attributed to the burning of amorphous carbon or the carbon fullerene cage at around 600 °C.
Highly energetic polynitro[C60]fullerene (PF) starts to decompose below 160 °C, with two peaks at 189.7 and 247.0 °C, with a combined heat release of 2017.7 J g−1. The energy content is comparable to those of HMX and CL-20. Its total mass loss of 47.3% corresponds to the theoretical value of 47.2%, corresponding to the calculated formula of C60(NO2)14. During decomposition, PF releases a mixture of nitrogen oxides: NO2 and NO with minor amounts of N2O. A residue of oxidized carbon was obtained after the main decomposition step. By further release of CO2 and CO above 700 °C, the residue is reduced to a carbonaceous matter free of oxygenated groups, also showing the presence of a small amount (<10%) of C60 fullerene.114 Theoretical investigation shows that explosion of PF starts with NO2 group isomerization into C–O–N–O, followed by emission of NO molecules and formation of CO groups on the buckyball surface.266 Then, NO oxidizes into NO2 and the C60 structure falls apart, liberating CO2. As a comparison, FEN decomposes just in two steps: the first-stage with 40.8% mass loss (100–250 °C), due to intense scission of NO3- and partial decomposition of the branched chain, releasing H2O, CO2, CO, N2O and NO2. The second-stage has 59.2% of the mass loss (250–580 °C), due to the decomposition of residual branched chains on a carbon cage, releasing CO2.115 For comparison of the decomposition processes of different energetic fullerene derivatives, their TG/DTG curves under a heating rate of 10 °C min−1 are shown in Fig. 34.
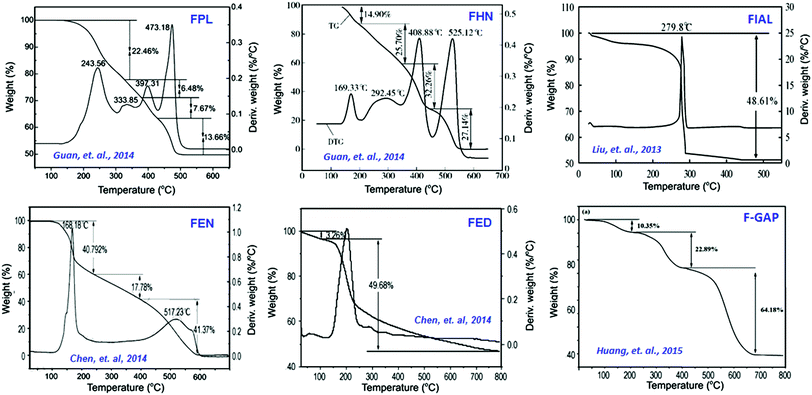 | ||
| Fig. 34 TG-DTG curves of several energetic fullerene derivatives: FPL (fullerene phenylalanine lead salt), FHN, FIAL (fullerene itaconic acid copolymer lead salt), FEN, FED and F-GAP (C60-GAP). Several figures that appeared in the listed ref. 93, 95, 97 and 101 are recombined with permission. | ||
According to Fig. 34, at least two steps are involved in the decomposition of these fullerene derivatives, which started between 100 and 150 °C. In particular, FPL undergoes a four-step exothermic process starting at 135 °C,117 which can reduce the decomposition Tp of RDX by more than 20 °C and decrease Ea by 30 kJ mol−1. FHN undergoes at least four decomposition steps, under a N2 atmosphere, starting at around 150 °C, while FEN decomposes in only two steps, as described above. Moreover, FHN decomposed completely in air with a trace amount of residue. FED initially decomposes in one slow step, with a mass loss of 3.3%, followed by a main decomposition step, with a mass loss of around 50%. It can also be seen that F-GAP decomposes in three steps under an air atmosphere. The first stage of thermal degradation appears at 150 °C, with around 10.4% mass loss. The other two onsets of thermolysis appear at 300 °C and 600 °C, with approximately 22.9% and 64.2% total mass losses, respectively. In contrast, GAP decomposes at approximately 200 °C, with the main gaseous products being CO, HCN, HCHO and NH3.267–269 It is indicated that the initial thermal degradation of F-GAP is probably due to the scission of the –N3 group via the intramolecular or intermolecular cycloaddition of –N3 to the fullerene carbon cage, as well as subsequent nitrogen release. The second step that takes place between 200 and 400 °C is due to decomposition of GAP's main chain and N-heterocyclic decomposition, while the final mass loss step is caused by decomposition of the fullerene cage (Scheme 12).
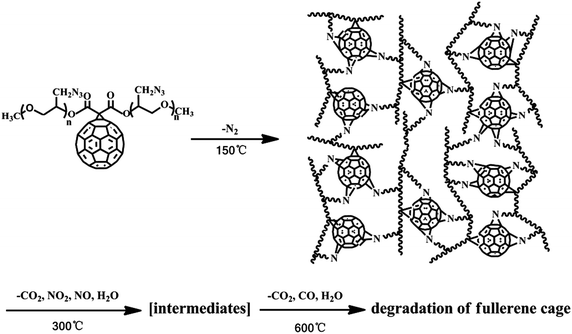 | ||
| Scheme 12 Thermal decomposition mechanism of C60-GAP under air atmosphere (adapted from Huang et al., Polymers, 2015, 7, 896–908). Reprinted from ref. 229 with permission. Copyright © 2011, American Chemical Society. | ||
As shown in Table 7, the decomposition of AP can be greatly affected by CNMs. For instance, the presence of GA, on the one hand, has a catalytic effect on the decomposition of AP. On the other hand, the oxidants produced from AP may react with GA, promoting heat release (Scheme 13).156 There was only one exothermic peak observed in DSC for AP/GA nanocomposites (in contrast to two peaks observed for pure AP); this is 83.7 °C lower than the second peak of AP, with a total heat release of 2110 J g−1. It has been shown that as-prepared AP/GA nanostructured energetic composites have numerous nanoscale pores and their SSA is about 49.2 m2 g−1. These properties result in their accelerated decomposition and greater total heat release.157 Graphene also changes the decomposition mechanism of AP, as shown in Scheme 13. In contrast, the Cu/CNTs catalyst remarkably decreased the second peak of AP, with a higher decomposition heat.210 The GA/Fe2O3 nanostructured catalyst dispersed in AP showed a significant catalytic effect in promoting the thermolysis of AP. In the latter case, only a single decomposition step was observed, with a decreased peak temperature and a large increase in the total heat release. Thus, the as-prepared GA/Fe2O3/AP nanostructured energetic composite could be a promising candidate as a component for solid propellants.158 GA also has a significant catalytic effect on the decomposition of AN,159 whose Tp was decreased by 33.7 °C and total heat release was increased by 532.8 J g−1. In comparison to GA and GA/Fe2O3, the addition of CoAl-MMO and CoAl-MMO/CNT to AP resulted in decomposition occurring via a single exothermic process. For example, in the case of the CoAl-MMO additive, AP shows a maximum peak temperature of 297 °C. Surprisingly, when using CoAl-MMO/CNT, the second peak temperature of AP is significantly decreased to 271 °C, strongly suggesting that this nanocomposite is the most efficient catalyst for promoting the decomposition of AP.217
Based on the above-mentioned results, one can see that CNTs and their functionalized analogs have a strong catalytic effect on energetic compounds such as RDX, FOX-12 and AP. In fact, CNTs also have a significant effect on the oxidation of Al, which is a very important ingredient in many propellants and explosives. It was shown that the CNTs can be fully embedded into Al aggregates, forming sea urchin-type Al/CNT particles. This material has the largest exothermic enthalpy at a lower oxidation temperature for both γ-Al2O3 and α-Al2O3 phases.222 This was attributed to fast heat transfer into Al particles via partially embedded CNTs. The TG curves of Al/CNTs at a heating rate of 5 °C min−1 are shown in Fig. 35. The results presented in Fig. 35 show that the exothermic enthalpy of Al oxidation is strongly dependent on the content of CNTs and increases to −188 kJ g−1 at 20 wt% of CNTs.222 Moreover, the oxidation process of pure Al particles takes place in the range 580 to 970 °C (TGA measurements). The first oxidation peak of pure Al at 580 °C is known as γ-Al2O3 phase formation, while the second peak, near 970 °C is known as α-Al2O3 phase formation. This is in agreement with the typical route of alumina phase transformations: amorphous–Al2O3 → γ → (δ) → (θ) → α-Al2O3.270 Typically, amorphous–Al2O3 transforms to more thermodynamically stable crystalline polymorphs, as the layer thickness reaches a critical value.271 Once the formation of the γ-Al2O3 phase is completed, the oxide layer thickness reaches a critical thickness to form an α-Al2O3 phase near 970 °C. However, the oxidation behavior of the Al/CNT (10 wt%) mixture was found to be very different from pure Al. The weight of the Al/CNT mixture was reduced during the initial stage of oxidation (400–600 °C; Fig. 35b). This phenomenon was explained by the burning of CNTs, which are located on the surface of Al particles.
 | ||
| Fig. 35 (a) TGA profile under air, at a ramping rate of 5 °C min−1 for different stages: Stage I (60 min), Stage II (120 min), and Stage III (180 min). The inset image shows the schematics of Al/CNTs mixture morphologies and (b) the corresponding DTG; the inset shows SEM images of Al/CNTs mixtures obtained after TGA terminated at 700 and 1000 °C. Note that the scales of the y-axes in (b) are different from each other, to allow better visualization of each peak. Reprinted from ref. 222 with permission of Crown copyright © 2013 Published by Elsevier Ltd. | ||
The weight increase at near 600 °C was due to the formation of a γ-Al2O3 layer, which was almost nullified by the weight decrease from burning of the CNTs. The formation temperature of the α-Al2O3 phase shifted from 970 to 907 °C, while the weight increase reached 145% (much larger than that of pure Al) due to enhanced oxidation in the presence of CNTs. As shown above, although the structure and shape of graphene and CNTs are different, both these nanomaterials are capable of facilitating electron transfer and promoting chemical reactions, as well as catalytic effects. These effects could be further enhanced by addition of other catalysts. GO, CNTs and fullerene CNM-based catalysts have large SSAs and thus high reactivity, especially when these nanomaterials are derivatized with additional functional groups.
It was further shown that the addition of both CNTs and graphene can significantly enhance the thermal transport properties of energetic composites made of Al and Teflon.277 However, composites containing CNTs are much more sensitive to impact. In contrast, although graphene cannot significantly sensitize energetic composites to ignition, it does induce greater overall reactivity. Increasing the concentration of CNM additives may decrease the overall thermal diffusivity and increase the sites for probable hot spot formation during impact.278 TEM images of the above-mentioned energetic composites containing CNT, graphene and nano-C are shown in Fig. 36. In fact, the heat conductivity of polymeric materials can be improved by incorporating CNTs279 and this is also the case for pyrotechnic compositions. According to the aforementioned results, CNTs are more effective than other CNMs in improving the ignition performance of pyrotechnic compositions. In particular, SWCNTs are the best choice for this purpose and it has been demonstrated that the burn rate and pressurization rate of SWCNTs/Al/CuO nanocomposites achieve maximum values of about 240 ms−1 and 5.9 ± 0.8 psi μs−1, respectively, when the content of SWCNT is just 1 wt%.280 The preparation method of SWCNTs/Al/CuO nanocomposites, their morphology and flash irradiation ignition process are shown in Fig. 37. The mixing ratio of fuel and oxidizer was fixed at Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuO = 3
CuO = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (wt%). Then, the SWCNTs were added to the nEMs precursor solution in mixing ratios of 1, 2, 5 and 10 wt%. The nano-Al particles were firmly attached to the surface of CuO NPs, while the SWCNTs appeared to be homogeneously distributed in an Al and CuO NP-based matrix. As the content of SWCNTs increased to more than 2 wt%, both the burn rate and the pressurization rate of the as-prepared SWCNT-nEM composites decreased significantly. The researchers believe that this was due to the large amount of SWCNTs in the nEM matrix, which may increase the heat dissipation, resulting in a very limited temperature increase for the SWCNT-nEM composite, thus hindering the oxidation and subsequent ignition of the nEMs. In the latter case, the remote optical ignition and controlled-explosion reactivity of nEMs was achieved by incorporating less than 2 wt% of SWCNTs, as optical ignition agent.
7 (wt%). Then, the SWCNTs were added to the nEMs precursor solution in mixing ratios of 1, 2, 5 and 10 wt%. The nano-Al particles were firmly attached to the surface of CuO NPs, while the SWCNTs appeared to be homogeneously distributed in an Al and CuO NP-based matrix. As the content of SWCNTs increased to more than 2 wt%, both the burn rate and the pressurization rate of the as-prepared SWCNT-nEM composites decreased significantly. The researchers believe that this was due to the large amount of SWCNTs in the nEM matrix, which may increase the heat dissipation, resulting in a very limited temperature increase for the SWCNT-nEM composite, thus hindering the oxidation and subsequent ignition of the nEMs. In the latter case, the remote optical ignition and controlled-explosion reactivity of nEMs was achieved by incorporating less than 2 wt% of SWCNTs, as optical ignition agent.
 | ||
| Fig. 36 TEM images of Al/Teflon energetic composites combined with CNTs, graphene flakes and nano carbon. Reprinted from ref. 277 with permission. Copyright © 2012, managed by AIP Publishing LLC. | ||
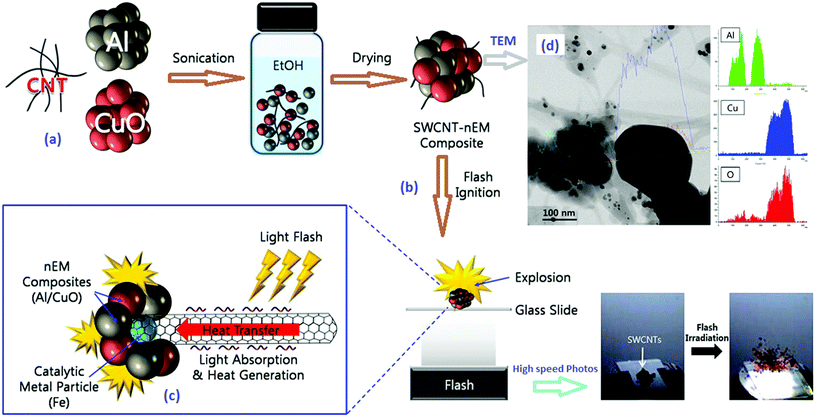 | ||
| Fig. 37 Schematics of (a) the fabrication of SWCNT-nEM composites, (b) the flash ignition of an SWCNT-nEM composite, (c) the optical ignition mechanism of an SWCNT-nEM composite, and (d) scanning TEM image and line scan analysis of SWCNT/Al/CuO nanocomposites. Recombination of several figures from ref. 280 reprinted with permission. Copyright © 2012 The Combustion Institute. Published by Elsevier Inc. | ||
In addition to ignition by irradiation, the ESD ignition properties of pyrotechnics could also be tailored by highly electro-conductive nano-fillers, including CNTs, graphene nano platelets (GNP) and their combinations. It has been found that the ignition and the electrical conductivity of a binary composite of Al and polytetrafluoroethylene (PTFE) can be changed significantly.281 Instead of GNP, a lower volume fraction of CNTs is adequate to create an electrical conductivity percolation threshold. However, a CNT/GNP composite could create a better percolating network. In this way, compositions that are insensitive to ESD ignition become sensitive due to improvement in electrical conductivity. Based on this strategy, flash-ignitable nEMs composed of Al/CuO nanoparticles for underwater explosion were invented using “sea urchin-like” CNTs (SUCNTs, Fig. 38).282 The underwater ignition of nEMs is a big challenge because water perturbs the reactants prior to ignition and quenches the subsequent combustion. The SUCNTs can absorb the irradiated flash energy and rapidly convert it into thermal energy. Similar to the above-mentioned Al/CuO system, the maximum burn rate was achieved by adding 1 wt% of SUCNTs into the nEM matrix. However, the resulting maximum underwater burn rate was found to be only about 34 m s−1, which is much lower than the burning rate of this composition in air (240 m s−1).280
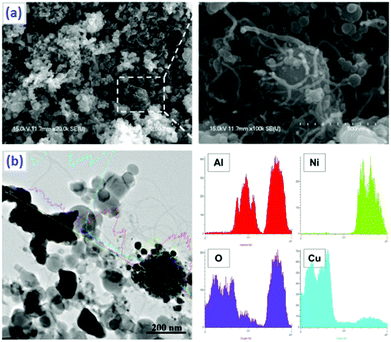 | ||
| Fig. 38 (a) SEM and (b) scanning TEM images of SUCNT/nEM. Reprinted from ref. 282 with permission. Copyright © 2014 The Combustion Institute and published by Elsevier Inc. | ||
In addition to CNTs, graphene could also enhance the combustion properties of propellants. A comparative study showed287 that the ignition temperature was lowered and burning rates were increased for colloidal suspensions of nitromethane with graphene, compared to liquid nitromethane monopropellant alone. It has been further reported288 that the addition of raw fullerene soot (FS), extracted FS (EFS), pure C60 and CB additives to nitroglycerine (NG) markedly accelerated its liquid phase decomposition. The effect of these nanomaterials on the combustion properties of double-base propellants (DB) has been also evaluated. As indicated earlier, CNTs have a strong catalytic effect on the decomposition of AP and they are also a promising combustion catalyst for AP. It was proven that as the CNT content increased, the burning rate of an AP composite also increased with a lower pressure exponent (n).251Table 8 lists the burn rate and n of different propellants when using CNMs as burn rate modifiers. Regarding applications of CNM additives as catalysts, in the early 1960s, the USA began using AC in solid propellants, where Bice289 and Ives290 found that AC increased the burning rate of AN-based propellants. These researchers also reported that a certain amount of carborane/AC composites increased the burning rate of the same propellant from 5.5 to 9.2 mm s−1. Moreover, they showed that the burn rate of aluminized composite propellants may be increased to 25 mm s−1 at 7 MPa by using AC as a modifier.291
| Propellants | μ/(mm·s−1) at different pressures | n (3–11 MPa) | Ref. | ||||
|---|---|---|---|---|---|---|---|
| 3 MPa | 5 MPa | 7 MPa | 9 MPa | 11 MPa | |||
| RDX used was a mixture of 80 μm and 50 μm particle sizes, with a weight ratio of 1/1; AP particle sizes were in the range of 120–200 μm; the N content in NC was 12.6%; CMDB, RDX/AP/NC/NG/additives/catalyst with a mass ratio of 20/10/29/32.5/5.0/3.5; FS, was composed of 10% fullerenes and 90% CB; EFS, containing about 80% C60 and 20% C70; DBP, double-base propellants containing (NC + NG)/DEP/C2 with a mass ratio of 89/8.5/2.0, where DEP is diethyl phthalate and C2 is a stabilizer; the contents of Bi2O3 and Bi2O3-CNTs are 2.5 wt%. | |||||||
| AP | 4.70 | 6.33 | 7.39 | 8.93 | 10.14 | 0.586 | 251 |
| AP/CNT(1%) | 7.84 | 9.46 | 11.11 | 12.53 | 13.26 | 0.427 | |
| AP/CNT(2%) | 9.47 | 11.51 | 13.78 | 14.54 | 16.28 | 0.406 | |
| AP/CNT(3%) | 10.64 | 13.63 | 14.83 | 16.39 | 17.86 | 0.386 | |
| AP/CNT(4%) | 13.24 | 15.02 | 16.12 | 17.49 | 18.30 | 0.247 | |
| 5 MPa | 7 MPa | 9 MPa | 10 MPa | 11 MPa | (5–11 MPa) | 206 | |
| AP/HTPB | 5.75 | 7.03 | 7.37 | 8.58 | 9.12 | 0.572 | |
| AP/HTPB/CNT | 6.68 | 7.84 | 8.90 | 9.76 | 10.08 | 0.538 | |
| AP/HTPB/Cu-CNT | 7.93 | 8.92 | 10.00 | 10.48 | 10.85 | 0.408 | |
| 2 MPa | 4 MPa | 6 MPa | 8 MPa | 12 MPa | (2–8 MPa) | ||
| CMDB | 3.71 | 5.88 | 7.87 | 10.23 | 14.30 | 0.721 | 288 |
| CMDB/CB | 3.96 | 6.10 | 7.82 | 9.74 | 13.57 | 0.642 | |
| CMDB/FS | 3.76 | 5.88 | 7.82 | 9.90 | 13.63 | 0.692 | |
| CMDB/EFS | 3.50 | 5.76 | 7.55 | 9.36 | 13.52 | 0.706 | |
| CMDB/C60 | 3.57 | 5.72 | 7.49 | 9.49 | 13.46 | 0.697 | |
| DB | 1.22 | 2.36 | 4.55 | 5.93 | 8.53 | 1.171 | 299 |
| DB/PbO–GO | 4.68 | 8.15 | 10.09 | 10.99 | 10.38 | 0.630 | 300 |
| DB/Cu2O–PbO/GO | 8.17 | 11.36 | 12.96 | 14.33 | 16.74 | 0.405 | 301 |
| DB/Bi2O3/GO | 3.01 | 6.98 | 10.21 | 12.37 | 15.91 | 1.035 | |
| DB/Cu2O–Bi2O3/GO | 8.58 | 11.44 | 13.53 | 15.07 | 17.27 | 0.408 | |
| 8 MPa | 10 MPa | 12 MPa | 16 MPa | 20 MPa | (16–22 MPa) | 291 | |
| DBP | 6.49 | 7.81 | 8.99 | 10.30 | 12.24 | 0.783 | |
| DBP/CuO-CNTs | 13.34 | 15.28 | 16.49 | 19.03 | 20.34 | 0.384 | |
| DBP/nano-Bi2O3 | 6.86 | 8.02 | 8.91 | 10.62 | 12.04 | 0.639 | 290 |
| DBP/Bi2O3-CNTs | 9.38 | 10.66 | 11.74 | 13.81 | 15.21 | 0.431 | |
A combination of AC with iron oxide can make the propellant burn at a speed of 50 mm s−1. However, it has an unacceptably large pressure exponent. Later on, it was proven through the analysis of a flame surface by SEM images,292 that a hole in the water encapsulated in AC can reduce the melt flow on the burning surface of the propellant, resulting in a higher pressure exponent. Therefore, it is better to use water-free AC. In addition to AC, CNTs also increased the burn rate of HTPB propellant by 20%, while having practically no effect on its pressure exponents.293 Similar findings were observed for NEPE propellant but the pressure exponent was decreased at low pressures (e.g. <10 MPa), while it was increased at higher pressures (15–18 MPa).294
As shown in Table 8, Cu/CNTs can markedly increase the burning rate and decrease the pressure exponent for AP/HTPB propellant.186 A significant amount of work regarding catalytic effects of CNMs on the combustion behavior of solid rocket propellants was done in China by Zhao's group.295–299 They prepared composite catalysts based on PbO, CuO, Bi2O3 and GO, as well as the “Pb–CB” binary catalytic system, to increase the burn rate of DB propellant. It was shown that “mesa burning” could be achieved in the pressure range 8–14 MPa. The PbO–Cu2O was jointly supported by CB, forming a “Pb–Cu–CB” three-element catalytic system, so that the burning rate of DB propellant at 2 MPa could be elevated from 2.15 to 8.57 mm s−1, with a wider “plateau combustion” pressure range (10–20 MPa).295
These researchers also used a combination of Bi2O3 and GO to replace PbO/GO, as a green combustion catalyst.296,297 It was observed that both Bi2O3/GO and Cu2O–Bi2O3/GO catalysts significantly increased the burning rate of DB propellants and reduced the pressure exponent (Table 8). Although the “mesa burning” disappeared upon replacement of PbO by Bi2O3, the pressure exponents were still found to be acceptable. This was also the case for CNT-based catalysts where the above-mentioned oxides were used. There might be another reason for the enhancement of the combustion of nitrocellulose (NC) by GO. In fact, it was found that the burn rate of NC significantly increased as the GO content increased in GO/NC films.141 The flame structure as well as the burn rate curves are presented in Fig. 39.
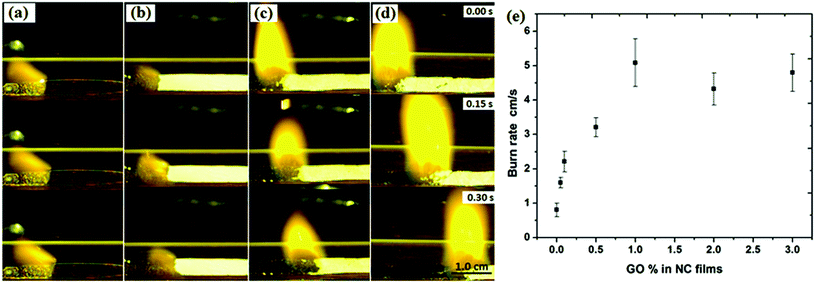 | ||
| Fig. 39 High-speed images of the combustion process of pure NC films and GO-NC films under ambient conditions: (a) pure NC film; (b) 0.05% GO-NC film; (c) 0.5% GO-NC film; and (d) 1% GO-NC film. (e) Comparison of the burn rates of pure NC films and GO-NC films. Reproduced from ref. 164 with permission from the Royal Society of Chemistry. | ||
It is shown in Fig. 39 that CNTs, as a carrier, can prevent aggregation of Bi2O3 or CuO nanoparticles and improve the catalytic efficiency, resulting in a much higher burning rate and a lower pressure exponent.298,299 Later on, Liu et al. found that carbon-coated Cu–Bi/CNTs powders could function as effective catalysts for DB propellants;300 plateau combustion was obtained within a pressure of 10–22 MPa, with an exponent of 0.173, which is very promising for solid rocket motors. In fact, nano-Cu/CNT and nano-Ni/CNT composite catalysts have a similar catalytic effect on AP/HTPB propellants.301,302 It has been stated that when 3 wt% of such catalysts, including nano CNTs, Fe2O3/CNTs and Fe·Cu/CNTs, are used, the burn rate of ADN at 4 MPa increases from 30.49 to 50.59, 39.72 and 38.79 mm s−1 and the corresponding pressure exponents decrease from 0.81 to 0.36, 0.67 and 0.75, respectively.258 In general, the use of modified CNMs can improve the combustion properties of propellants.303 Such modified nanomaterials, which include metal oxides, nanometer-sized rare earth materials and nanometer-sized oxidizers, can increase the burn rates of solid propellants. In addition to increasing the burn rate, the aforementioned CNMs and their derivatives could also improve the energy release rate. For instance, MWCNTs can be used in composition with porous silicon (pSi), impregnated with sodium perchlorate and this makes pSi explode easily.304
As well as propellants and explosives, CNMs can be used in nanothermites. Nanothermite formulations that use Al or Mg as reducing agents and Fe2O3, MoO3 or WO3 as oxidizers, have various applications due to their high energy density and large energy release rate, in comparison to conventional thermites. The addition of boron and CNTs to these nanothermites results in doubling of the gas peak pressure,305 indicating that the boron–CNT mixtures could be useful for a new generation of propellants, explosives and pyrotechnic systems. Recently, MWCNTs were coupled with iron oxide nanoparticles, to form new pyrophoric compositions prepared on the basis of water-dispersed CNTs.306
As mentioned earlier, addition of CNTs to Zr/KClO4 has a significant impact on the light radiation energy of this material. As the CNT content was increased, the combustion rate and exothermic output of these pyrotechnic mixtures were increased correspondingly.206 Flash-ignitable nEMs for underwater explosions or for other purposes could be achieved by addition of SUCNTs282 to Al/CuO nanoparticles or by addition of SWCNTs to pentaerythritol tetranitrate (PETN),307 as an optical sensitizer. The CNMs absorb the irradiated energy and rapidly convert it into thermal energy, greatly improving the ignition and burning properties of the pure parent explosive. The same phenomenon was observed for the case of Zr/oxidant/CNT composites.308 As well as CNTs, functionalized graphene sheet (FGS) is also a promising additive capable of enhancing fuel/propellant combustion. A series of molecular dynamic simulations based on a reactive force field (ReaxFF) show that the catalytic activity of FGS in the combustion of nitromethane is significant.309 The derivatives of fullerene have an even better catalytic effect than C60 and CB on the combustion of DB propellants containing RDX. As mentioned earlier, mNPF is a typical example for demonstration of this effect. The burn rates of RDX-CMDB propellants were greatly improved by addition of mNPF, increasing with the mNPF content. The maximum burning rate can reach up to 19.6 mm s−1 when 1.0 wt% mNPF is used, while the corresponding “plateau combustion” region is in the range from 8 to 22 MPa.310 The unique structure of CNMs and their energetic derivatives can modify the combustion characteristics of propellants, especially when new functional groups or energetic moieties are introduced to their structures. On the one hand, such a catalytic effect could be due to the special carbon structure allowing facile electron and heat transfer, promoting chemical reactions. On the other hand, the metal oxide catalysts could be dispersed in the inner layer or porous structure of the CNMs. Such dispersion can prevent agglomeration of nanoparticles, resulting in more uniform, catalytically active sites and thus better combustion properties. Moreover, the catalytic efficiency could be further improved by synergistic effects between the CNMs and the carried catalysts.
Stabilization and desensitization of EMs by CNMs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 energetic co-crystals of AN with benzo-18-crown-6 (AN-B18C6) avoid this problem.312 Alternatively, in order to achieve both phase and thermal stabilization of AN, a new material, polyvinylpyrrolidone (PVP)-AN glass has been developed, where PVP can separate AN into its ions through an ion–dipole interaction.313
1 energetic co-crystals of AN with benzo-18-crown-6 (AN-B18C6) avoid this problem.312 Alternatively, in order to achieve both phase and thermal stabilization of AN, a new material, polyvinylpyrrolidone (PVP)-AN glass has been developed, where PVP can separate AN into its ions through an ion–dipole interaction.313
It has been reported that ADN could also be stabilized by coating it with other inert materials.314 Recently, a theoretical investigation showed that the stability of EMs could also be improved by encapsulating them inside CNTs or between graphene layers.315 Preparations of a variety of energetic compounds have been attempted, including FOX-7, RDX, HMX, 3,6-di(hydrazino)-1,2,4,5-tetrazine (DHT), 3,6-diazido-1,2,4,5-tetrazine (DiAT) and DAAT, as well as five different N-oxides of DAAT (DAATOn, with n = 1–5). It was found that all of these EMs could be stabilized by 32–53 kcal mol−1 by using CNTs with appropriate diameters. Moreover, FOX-7, RDX and HMX could also be confined between graphene layers, resulting in stabilization by 28–40 kcal mol−1. The stabilization results from dispersion interactions between the molecules and carbon nanostructures, as well as coulombic interactions, due to charge dissipation and intermolecular H-bonding.
In addition to stabilization of normal sensitive energetic compounds, it is also possible to stabilize unstable high-nitrogen compounds using CNTs. Finite temperature simulations demonstrated the stabilization of a polymeric nitrogen (N8) at ambient pressure and room temperature, by confinement inside a CNT. If proven experimentally viable, it may open a new path towards stabilization of polynitrogen or polymeric nitrogen compounds under ambient conditions.316,317 An energy barrier of 1.07 eV was found for the formation of N8/CNT (5,5) from N2/CNT (5,5), while the dissociation barrier was 0.2 eV. Reaction pathway snapshots have shown that the transition state is composed of N2 and N6 inside a CNT (5,5).317
Moreover, the simulation of NM with SWCNTs shows that size-dependent ordered structures of NM with preferred orientations are formed inside the tubular cavities, driven by van der Waals attractions between NM and SWCNT, together with the dipole–dipole interactions of NM, resulting in a higher local mass density than that of bulk NM.318 On the basis of these calculations, the stabilization of nitrogen clusters has been proposed. It was demonstrated that nitrogen clusters could be stabilized by CNTs under various conditions. A linear chain nitrogen cluster could be formed under plasma conditions and it would probably be stabilized inside the walls of the CNTs that were used as substrates for synthesis.319 Later on, a N8 was stabilized on the positively charged sidewalls of MWNTs that were synthesized by cyclic voltammetry (CV) under ambient conditions. It was shown from temperature programmed desorption (TPD) data that MWNT/N8 is thermally stable up to 400 °C.320 Furthermore, an experimental study regarding interaction and energy transport of Al/Teflon nanocomposites with nano-carbon, graphene flakes and CNTs,278 showed that graphene has the greatest influence on the thermal conductivity (increased by 98%), thermal diffusivity and specific heat of the Al/Teflon matrix.
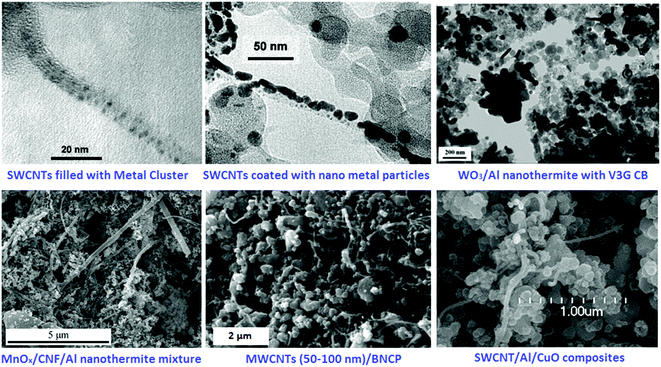 | ||
| Fig. 40 A comparison of SEM morphologies and TEM images of several MWCNTs and CB-based thermites. This combined figure is reproduced from several figures appearing in ref. 58, 60 and 284 with permission. | ||
| Materials | IS/H50 | FS/% | ESD | Ref. |
|---|---|---|---|---|
| IS, impact sensitivity; FS, friction sensitivity; Fs, flame sensitivity; C60-1, commercially available; C60-2, crystallized from pellucid purple carbon disulfide solution; C60-3 brown sheet fullerene obtained from pellucid purple carbon disulfide/petroleum ether solution (80 °C, 10 h); (a) V3G, nanometer-sized graphitized carbon black; RM and AM, carbon milling under air and argon atmospheres; TO, Thermally oxidized carbon nanoparticles. | ||||
| BNCP | 10.6 cm | 24 (swing 70°) | Positive-0.99/negative-1.18 | 52 |
| BNCP/MWCNTs-1 | 17.5 cm | 36 | No fire | |
| BNCP/MWCNTs-2 | 24.7 cm | 4 | No fire | |
| BNCP/SWCNTs | 23.1 cm | 54 | No fire | |
| BNCP/CB | 27.1 cm | 90 | Positive-0.49/negative-0.47 | |
| HMX | 100% | 100% | Note: friction: recorded at 90°, 3.92 MPa; impact: 10 kg hammer, 25 cm, 50 mg; probability out of 30 times | 327 |
| HMX/1%_C60-1 | 100% | 100% | ||
| HMX/1%_C60-2 | 100% | 90% | ||
| HMX/1%_C60-3 | 60% | 70% | ||
| WO3/Al | >50 J | 144 N | <0.14 mJ | 54 |
| WO3/Al/V3G | >50 J | 252 N | <0.14 mJ | |
| WO3/Al/TO | >50 J | 64 N | <0.14 mJ | |
| WO3/Al/RM | >50 J | >360 N | 0.14 mJ | |
| WO3/Al/AM | >50 J | >360 N | <0.14 mJ | |
| MnO2/Al | 31.9 J | >5 N | 1.0 mJ | 330 |
| MnO2/Al/CNF (mix) | 29.4 | >360 N | 1800 | |
| MnO2/Al/CNF | 44.2 J | >360 N | 35 mJ/5400 mJ (fuel rich) | |
| ε-CL-20 | 100% | 100% | Note: the drop hammer is 5 kg, 20 cm height; friction sensitivity of the samples was recorded at 66° ± 1°, 2.45 MPa; [robability out of 30 times | 265 |
| ε-CL-20/glue | 30% | 36% | ||
| ε-CL-20/glue/graphene | 20% | 22% | ||
| ε-CL-20/glue/GO | 25% | 30% | ||
| ε-CL-20/glue/rGO | 22% | 28% | ||
| Laser sensitivity (Sapphire) mJ | Laser sensitivity (K9 glass) mJ | |||
| PETN | 271 | 268 | 329 | |
| PETN/1%CB | 61.6 | 43.3 | ||
| PETN/1%CNTs | 187.9 | 266.6 | ||
Unfortunately, the influence of the CNM additives on the electrostatic discharge sensitivity was very limited, due to the low amount of additive used in the preparation of these nanothermites (<5 wt%). This indicates that ESD desensitization is more related to percolation effects than to the intrinsic conductivity of the CB. In addition to CB, “herringbone” carbon nanofibers (CNFs) are another suitable desensitizer for nanothermites (e.g., MnO2/Al), to allow safer handling.330 A dramatic decrease in the friction sensitivity (>360 N), compared to pure MnO2/Al nanothermite (>5 N), was found and the ESD sensitivity was also considerably reduced (from 1.0 to 5300 mJ). Even though some energetic compounds such as 3-nitro-1,2,4-triazol-5-one (NTO) are quite insensitive, it is still useful to add a certain amount of CNMs (e.g. 1 wt% of graphene) to increase the processing capability of NTO as an ingredient of PBXs.331 It was also shown that the mechanical sensitivities of ε-CL-20/glue can be reduced with the incorporation of graphite, GO and rGO in PBX formulations.265 A later report shows that GO sheets exhibited a better desensitizing effect than C60 and CNTs.166 When 2 wt% GO sheets are added, the impact sensitivity of as-prepared HMX decreases from 100% to 10% and the friction sensitivity reduces from 100% to 32%.
Conclusions and future directions
As summarized in this review, extensive investigations were and are currently being conducted regarding the design, synthesis, characterization and application of various CNMs or functionalized CNMs in EMs. Widely used CNMs include graphene, GO, rGO, CNTs, activated carbon fiber and EG, as well as functionalized graphene and C60-fullerene. Until now, EG had only been used in chemical heat pumps, however, it may have additional uses in the field of EMs, e.g., as an additive in nanothermites and as a promising flame retardant additive in cladding materials for solid propellant charges. In contrast to EG, C60-fullerene can be functionalized with energetic groups, forming a new family of energetic compounds with excellent thermal stability. However, the variety of energetic moieties used for C60-fullerene functionalization is still limited and more exploration is required in this field. C60-fullerene derivatives can either be used as combustion catalysts, energetic binders or additives, depending on the type of functional groups. Their compatibility with other components of explosives and propellants, as well as their sensitivity, also needs to be systematically investigated with respect to their practical applications. Graphene and its aerogels can usually be combined with either catalysts or oxidizers using a sol–gel method. These novel composite materials are likely to be used as advanced oxidizers and combustion catalysts. Among all the CNMs, only GO and rGO were found to be energetic by themselves. They could also be used as matrices to bind many other energetic compounds, including HMX, NC and FOX-7. The common energetic materials could either be deposited on the surface of GO or their crystals/particles could be coated with thin layers of GO (rGO). It should be mentioned that the development of practically all composite materials based on GO or rGO was reported during the last 3 years. Many other new energetic compounds could be deposited on GO or rGO in order to enhance their performance, as potential combustion catalysts of solid propellants or as additives to propellants and explosives. Similarly to C60-fullerene, many functionalized CNTs were developed as combustion catalysts or energetic additives to propellants. Likewise, some of these nanomaterials were also used in new formulations of thermites and ignitors. The preparation procedures of CNM-containing EMs are well developed and include a broad variety of synthetic techniques such as microemulsion, hydrothermal, reductive deposition, ultrasonic compositing and CVD, as well as chemical reaction methods. Combination of CNTs with highly sensitive EMs could provide a way to improve the sensitivity and performances of these EMs. However, the encapsulation of sensitive or unstable energetic molecules into CNTs is still extremely challenging and will require much more theoretical and experimental work. The desensitization mechanism of CNMs on EMs is still not very clear and more theoretical work is needed to address this issue. Although the use of DnDs has become more and more popular in various areas, its application in EMs is only in its infancy. DnDs can be functionalized with energetic or other functional groups, where one of the most challenging issues will be their surface structure characterization.In summary, it is very clear from the information presented in this review that use of various CNMs in energetic applications is extremely promising and rewarding, being capable of addressing numerous problems in this field and having tremendous growth opportunities in the near future.
Acknowledgements
The financial support from the Planning and Budgeting Committee (PBC) of the Council for Higher Education in the Israeli government is greatly appreciated. This work was also partially supported by the Center for Nanoscience and Nanotechnology and by the Faculty of Exact Sciences in Tel Aviv University.References
- J. P. Agrawal, High Energy Materials Propellants, Explosives and Pyrotechnics, Wiley-VCH, 2010, p. 69, ISBN: 978-3-527-32610-5 Search PubMed.
- C. S. David, The Mother of Gunpowder, Oxford University Press, 2013 Search PubMed.
- A. K. Sikder and N. Sikder, A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications, J. Hazard. Mater., 2004, 112, 1–15 CrossRef CAS PubMed.
- T. M. Klapotke, in High Energy Density Materials, ed. T. M. Klapotke, Springer, Heidelberg, 2007, pp. 85–121 Search PubMed.
- J. Ying, X. Guo-gen and W. Xuan-jun, Application of lightweight carbon materials, National Defense Industry Press, Beijing, 2013 Search PubMed.
- M. Lulu, H. C. Amelia, S. O. Hart, V. Robert and M. A. Pulickel, Spiers memorial lecture advances of carbon nanomaterials, Faraday Discuss., 2014, 173, 9 RSC.
- Y. Zhai, Z. Zhu and S. Dong, Carbon-based nanostructures for advanced catalysis, ChemCatChem, 2015, 7(18), 2806–2815 CrossRef CAS.
- M. Comet, V. Pichot, B. Siegert and D. Spitzer, Use of nanodiamonds as a reducing agent in a chlorate-based energetic composition, Propellants, Explos., Pyrotech., 2009, 34, 166–173 CrossRef CAS.
- P. Jiang-feng, Z. Feng-Qi, S. Xiu-duo and Z. Wei, Progress of the application of lightweight carbon materials and their composites in solid rocket propellants, Chin. J. Explos. Propellants, 2014, 2, 1–6 Search PubMed.
- P. Delhaes, Carbon-based Solids and Materials Carbon-based Solids and Materials, 2013, pp. 1–640, ISBN: 9781118557617 Search PubMed.
- W. Han, Z. Feng-qi and L. Shang-wen, Function of carbon materials used in solid propellants and their action mechanism, Chin J. Explos. Propellants, 2005, 29, 32–35 Search PubMed.
- W. Ying-lei, Z. Feng-Qi and Y. Jian-hua, New progress of study combustion catalysts used for solid rocket propellants, Chin. J. Explos. Propellants, 2012, 35, 1–8 Search PubMed.
- V. D. Heijden, R. H. B. Bouma, A. C. Van Der Steen and H. R. Fischer, Application and characterization of nanomaterials in energetic compositions, Mater. Res. Soc. Symp. Proc., 2003, 800, 191–208 Search PubMed.
- Synthesis, characterization and properties of energetic/reactive nanomaterials, Materials Research Society Symposium - Proceedings, 2003, vol. 800, p. 392.
- V. V. Pokropivny and A. L. Ivanovskii, New nanoforms of carbon and boron nitride, Russ. Chem. Rev., 2008, 77, 837–873 CrossRef CAS.
- X. Han and S. Li, Advances in catalytic function of fullerene materials, Prog. Chem., 2006, 18, 715–720 CAS.
- Y.-F. Lan, X.-Y. Li and Y.-J. Luo, Research progress on application of graphene in energetic materials, Chin. J. Explos. Propellants, 2015, 38, 1–7 CAS.
- C. K. Law, Fuel options for next-generation chemical propulsion, AIAA J., 2012, 50, 19–36 CrossRef CAS.
- C. Ren, X.-J. Wang, Y.-X. Li, J.-L. Wang and D.-L. Cao, Research and application of graphene composites, Mod. Chem. Industry, 2015, 35, 32–35 CAS.
- P. M. Ajayan and T. W. Ebbesen, Nanometre-size tubes of carbon, Rep. Prog. Phys., 1997, 60, 1025–1062 CrossRef CAS.
- P. C. Eklund, A. M. Rao, P. Zhou, Y. Wang and J. M. Holden, Photochemical transformation of C60 and C70 films, Thin Solid Films, 1995, 257(2), 185–203 CrossRef CAS.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Carbon Nanotubes Present and Future Commercial Applications, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- M. Zhang, S. Fang, A. A. Zakhidov, S. B. Lee, A. E. Aliev, C. D. Williams, K. R. Atkinson and R. H. Baughman, Strong, Transparent, Multifunctional, Carbon Nanotube Sheets, Science, 2005, 309, 1215–1219 CrossRef CAS PubMed.
- S. Wu, R. Ge, M. Lu, R. Xu and Z. Zhang, Graphene-based nano-materials for lithium-sulfur battery and sodium-ion battery, Nano Energy, 2015, 15, 379–405 CrossRef CAS.
- D. Geng, H. Wang and G. Yu, Graphene single crystals Size and morphology engineering, Adv. Mater., 2015, 27, 2821–2837 CrossRef CAS PubMed.
- D. Tománek, Interfacing graphene and related, 2D materials with the 3D world, J. Phys.: Condens. Matter, 2015, 27, 133203 CrossRef PubMed.
- S. Ge, F. Lan, F. Yu and J. Yu, Applications of graphene and related nanomaterials in analytical chemistry, New J. Chem., 2015, 39, 2380–2395 RSC.
- Y. Li, H. Dong, Y. Li and D. Shi, Graphene-based nanovehicles for photodynamic medical therapy, Int. J. Nanomed., 2015, 10, 2451–2459 CrossRef CAS PubMed.
- W.-B. Ding, L. Wang, Q. Yang, W.-D. Xiang, J.-M. Gao and W. A. Amer, Recent research progress on polymer grafted carbon black and its novel applications, Int. Polym. Process., 2013, 28, 132–142 CrossRef CAS.
- T. Novakov and H. Rosen, The black carbon story Early history and new perspectives, Ambio, 2013, 42, 840–851 CrossRef CAS PubMed.
- X. Li, Z. Li and Y. Xia, Test and calculation of the carbon black reinforcement effect on the hyper-elastic properties of tire rubbers, Rubber Chem. Technol., 2015, 88, 98–116 CrossRef CAS.
- M. Jacobsson, F. Wang, P. Cameron, J. Neilsen, L. Nikiel and W. Wampler, Improvements in carcass carbon blacks to enhance compound performance, Rubber World, 2015, 251, 30–34 CAS.
- V. M. Shopin, Separation of the desired product from aerosol flows in the manufacture of carbon black a review, Solid Fuel Chem., 2014, 48(3), 180–197 CrossRef CAS.
- L. Wang, X. Wang, B. Zou, X. Ma, Y. Qu, C. Rong, Y. Li, Y. Su and Z. Wang, Preparation of carbon black from rice husk by hydrolysis, carbonization and pyrolysis, Bioresour. Technol., 2011, 102, 8220–8224 CrossRef CAS PubMed.
- K.-H. Kuo, Y.-H. Peng, W.-Y. Chiu and T.-M. Don, A novel dispersant for preparation of high loading and photosensitive CB dispersion, Inc, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6185–6197 CrossRef CAS.
- C. M. Karbiwnyk and K. E. Miller, A review of current analytical applications employing graphitized carbon black, Carbon Black Product., Proper. Uses, 2011, 69–92 Search PubMed.
- L. Li, W. Li, D. Qin, S. Jiang and F. Liu, Application of graphitized carbon black to the quechers method for pesticide multiresidue analysis in Spinach, J. AOAC Int., 2009, 92, 538–547 CAS.
- T. Horikawa, Y. Zeng, D. D. Do, K.-I. Sotow and J. R. Alcántara Avila, On the isosteric heat of adsorption of non-polar and polar fluids on highly graphitized carbon black, J. Colloid Interface Sci., 2015, 439, 1–6 CrossRef CAS PubMed.
- X.-Q. Yang, B. Wang, S.-E. Liu and X.-J. Guo, Effect of carbon black on burning properties of modified nitramine double base propellant, Energ. Mater., 2004, 12, 408 CAS.
- E. Papirer, E. Brendle, F. Ozil and H. Balard, Comparison of the surface properties of graphite, CB and fullerene samples, measured by inverse gas chromatography, Carbon, 1999, 37, 1265–1274 CrossRef CAS.
- M. Kruk, Z. Li, M. Jaroniec and W. R. Betz, Nitrogen Adsorption Study of Surface Properties of Graphitized Carbon Blacks, Langmuir, 1999, 15, 1435–1441 CrossRef CAS.
- S.-P. Rwei, F.-H. Ku and K.-C. Cheng, Dispersion of CB in a continuous phase Electrical, rheological, and morphological studies, Colloid Polym. Sci., 2002, 280, 1110–1115 CAS.
- Y.-F. Shih and R.-J. Jeng, Carbon black containing interpenetrating polymer networks based on unsaturated polyester/epoxy. I. Dynamic mechanical properties, thermal analysis, and morphology, J. Appl. Polym. Sci., 2002, 86, 1904–1910 CrossRef CAS.
- G. Wu, S. Asai, M. Sumita and H. Yui, Entropy penalty-induced self-assembly in carbon black or carbon fiber filled polymer blends, Macromolecules, 2002, 35, 945–951 CrossRef CAS.
- P. Kowalczyk, K. Kaneko, A. P. Terzyk, H. Tanaka, H. Kanoh and P. A. Gauden, The evaluation of the surface heterogeneity of carbon blacks from the lattice density functional theory, Carbon, 2004, 42, 1813–1823 CrossRef CAS.
- A. Schröder, M. Klüppel and R. H. Schuster, Characterisation of surface activity of carbon black and its relation to polymer-filler interaction, Macromol. Mater. Eng., 2007, 292, 885–916 CrossRef.
- T. Mathew, R. N. Datta, W. K. Dierkes, J. W. M. Noordermeer and W. J. Van Ooij, A comparative investigation of surface modification of CB and silica by plasma polymerization, Rubber Chem. Technol., 2008, 81, 209–226 CrossRef CAS.
- T. Mathew, R. N. Datta, W. K. Dierkes, W. J. van Ooij, J. W. M. Noordermeer, T. M. Gruenberger and N. Probst, Importance of fullerenic active sites in surface modification of carbon black by plasma polymerization, Carbon, 2009, 47, 1231–1238 CrossRef CAS.
- Q.-L. Yan, S. Zeman and A. Elbeih, Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives PBXs, Thermochim. Acta, 2012, 537, 1–12 CrossRef CAS.
- Y.-L. Duan, Y. Tong, Y. Cao and F.-L. Huang, Phase transition of carbon black shocked by explosion, Acta Armamentarii, 2013, 34, 169–174 Search PubMed.
- E. M. Zhazaeva, A. G. Pshikhachev, T. A. Gubzhev, A. A. Kashirgov, Z. M. Gekkieva and R. B. Tkhakakhov, Surface energetic and strength characteristics of heat treated composites based on butadiene-acrylonitrile elastomer modified with CB, Inter. Polym. Sci. Tech., 2013, 40, T33–T37 Search PubMed.
- L.-K. Chen, D.-L. Sheng, B. Yang, Y.-H. Zhu, M.-H. Xu, Y.-L. Pu and Z.-X. Li, Effects of carbon nanotubes and carbon black on sensitivity performances of BNCP, Chin. J. Energet. Mater., 2013, 21, 35–38 CAS.
- B. Yang, D.-L. Sheng, L.-K. Chen, H.-S. Xu, Y.-Y. Men and Y.-H. Zhu, Performance of carbon black/potassium nitrate propellant for blasting valve, Chin. J. Energet. Mater., 2014, 22, 397–400 CAS.
- A. Bach, P. Gibot, L. Vidal, R. Gadiou and D. Spitzer, Modulation of the Reactivity of a WO3/Al Energetic Material with Graphitized CB as Additive, J. Energ. Mater., 2015, 33, 260–276 CrossRef CAS.
- O. Shenderova, A. M. Panich, S. Moseenkov, S. C. Hens, V. Kuznetsov and H.-M. Vieth, Hydroxylated detonation nanodiamond FTIR, XPS, and NMR studies, J. Phys. Chem. C, 2011, 115, 19005–19011 CAS.
- G. Amaratunga, A. Putnis, K. Clay and W. Milne, Crystalline diamond growth in thin films deposited from a CH4/Arrf plasma, Appl. Phys. Lett., 1989, 55, 634–635 CrossRef CAS.
- B. Wen, J. J. Zhao and T. J. Li, Synthesis and crystal structure of n-diamond, Int. Mater. Rev., 2007, 52, 131–151 CrossRef CAS.
- A. Krishnan, E. Dujardin, M. M. J. Treacy, J. Hugdahl, S. Lynum and T. W. Ebbesen, Graphitic cones and the nucleation of curved carbon surfaces, Nature, 1997, 388, 451–454 CrossRef CAS.
- S. Lijima, P. M. Ajayan and T. Ichihashi, Growth model for carbon nanotubes, Phys. Rev. Lett., 1992, 69, 3100–3103 CrossRef PubMed.
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, C60 buckminsterfullerene, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. B. Howard, J. T. McKinnon, Y. Makarovsky, A. L. Lafleur and M. E. Johnson, Fullerenes C60 and C70 in flames, Nature, 1991, 352, 139–141 CrossRef CAS PubMed.
- M. T. Yin and M. L. Cohen, Will diamond transform under megabar pressures?, Phys. Rev. Lett., 1983, 50, 2006–2009 CrossRef.
- M. Frenklach, R. Kematick, D. Huang, W. Howard, K. E. Spear, A. W. Phelps and R. Koba, Homogeneous nucleation of diamond powder in the gas phase, J. Appl. Phys., 1989, 66, 395–399 CrossRef CAS.
- E. Papirer, E. Brendle, F. Ozil and H. Balard, Comparison of the surface properties of graphite, CB and fullerene samples, measured by inverse gas chromatography, Carbon, 1999, 37, 1265–1274 CrossRef CAS.
- H. Hirai and K.-I. Kondo, Modified phases of diamond formed under shock compression and rapid quenching, Science, 1991, 253, 772–774 CAS.
- J. B. Howard, K. D. Chowdhury and J. B. Vander Sande, Carbon shells in flames, Nature, 1994, 370, 603 CrossRef.
- F. Cataldo, The impact of a fullerene-like concept in carbon black science, Carbon, 2002, 40, 157–162 CrossRef CAS.
- C. Mailhiot and A. K. McMahan, Atmospheric-pressure stability of energetic phases of carbon, Phys. Rev. B: Condens. Matter, 1991, 44, 11578–11591 CrossRef CAS.
- C. S. Yoo, W. J. Nellis, M. L. Sattler and R. G. Musket, Diamond like metastable carbon phases from shock-compressed C60 films, Appl. Phys. Lett., 1992, 61, 273–275 CrossRef CAS.
- J. L. Peng, L. A. Bursill, B. Jiang, J. O. Orwa and S. Prawer, Growth of c-diamond, n-diamond and i-carbon nanophases in carbon-ion-implanted fused quartz, Philosophical Magazine B Physics of Condensed Matter; Statistical Mechanics, Electronic, Opt. Magnet. Proper., 2001, 81, 2071–2087 CAS.
- B. Wen, T. Li, C. Dong, X. Zhang, S. Yao, Z. Cao, D. Wang, S. Ji and J. Jin, Preparation of diamond nanocrystals from catalysed carbon black in a high magnetic field, J. Phys.: Condens. Matter, 2003, 15, 8049–8054 CrossRef CAS.
- B. Wen, J. Zhao, T. Li, C. Dong and J. Jin, n-diamond from catalysed carbon nanotubes synthesis and crystal structure, J. Phys.: Condens. Matter, 2005, 17, L513–L519 CrossRef CAS.
- E. Mironov, E. Petrov and A. Koretz, Chemical aspect of ultradispersed diamond formation, Diamond Relat. Mater., 2003, 12, 1472–1476 CrossRef CAS.
- G. V. Sakovich, A. S. Zharkov and E. A. Petrov, Results of research into the physicochemical processes of detonation synthesis and nanodiamond applications, Nanotechnol. Russ., 2013, 8, 581–591 CrossRef.
- A. A. Gromov, S. A. Vorozhtsov, V. F. Komarov, G. V. Sakovich, Y. I. Pautova and M. Offermann, Ageing of nanodiamond powder Physical characterization of the material, Mater. Lett., 2013, 91, 198–201 CrossRef CAS.
- V. Y. Dolmatov, V. Myllymäki and A. Vehanen, A possible mechanism of nanodiamond formation during detonation synthesis, J. Sup. Hard Mater., 2013, 35, 143–150 CrossRef.
- R. K. Barton and A. J. Barratt, Observations on the Reactivity of Pyrotechnic Compositions Containing Potassium Chlorate and Thiourea, Propellants, Explos., Pyrotech., 1993, 18, 77 CrossRef.
- M. Comet, L. Schreyeck and H. Fuzellier, An efficient composition for bengal lights, J. Chem. Educ., 2002, 79, 70 CrossRef CAS.
- M. Comet, V. Pichot, B. Siegert, D. Spitzer, J.-P. Moeglin and Y. Boehrer, Use of nanodiamonds as a reducing agent in a chlorate-based energetic composition, Propellants, Explos., Pyrotech., 2009, 34, 166–173 CrossRef CAS.
- M. Comet, V. Pichot, F. Schnell and D. Spitzer, Oxidation of detonation n-D in a reactive formulation, Diamond Relat. Mater., 2014, 47, 35–39 CrossRef CAS.
- Y. Tong, R. Liu and T. Zhang, The effect of a detonation nanodiamond coating on the thermal decomposition properties of RDX explosives, Phys. Chem. Chem. Phys., 2014, 16, 17648–17657 RSC.
- M.-Y. Shen, K.-Y. Li, C.-F. Kuan, H.-C. Kuan, C.-H. Chen, M.-C. Yip, H.-W. Chou and C.-L. Chiang, Preparation of expandable graphite via ozone-hydrothermal process and flame-retardant properties of high-density polyethylene composites, High Perform. Polym., 2014, 26, 34–42 CrossRef.
- X.-Y. Pang, M.-W. Duan, Y. Tian and M. Zhai, Preparation of expandable graphite with phosphoric acid as ancillary intercalation reagent and its antiflame property for linear low density polyethylene, Asian J. Chem., 2013, 25, 5390–5394 CAS.
- C.-F. Kuan, K.-C. Tsai, C.-H. Chen, H.-C. Kuan, T.-Y. Liu and C.-L. Chiang, Preparation of expandable graphite via H2O2-hydrothermal process and its effect on properties of high-density polyethylene composites, Polym. Composit., 2012, 33, 872–880 CrossRef CAS.
- W. Meiling, Z. Jinjiang, G. Yunqiao, Y. Xiongbo, G. Yajun and Z. Jianwei, Electrochemical Determination of Sunset Yellow Based on an Expanded Graphite Paste Electrode, J. Electrochem. Soc., 2013, 160(8), H459–H462 CrossRef.
- Y. Wen, K. He, Y. Zhu, F. Han, Y. Xu, I. Matsuda, Y. Ishii, J. Cumings and C. Wang, Expanded graphite as superior anode for sodium-ion batteries, Nat. Commun., 2014, 5, 4033 CAS.
- L. Ji-hui, F. Li-li and J. Zhi-xin, Preparation of expanded graphite with 160 μm mesh of fine flake graphite, Mater. Lett., 2006, 60, 746–749 CrossRef.
- S. Ba, C. Jiang, K. Sun and Z. Sun, Prepared and infrared extinction characteristics of micron expanded graphite, Adv. Mater. Res., 2011, 308–310, 710–714 CrossRef CAS.
- L. Gao, Y.-X. Gong and L. Ma, Effect of expansion time on the microstructure of an expanded natural flake graphite, New Carbon Mater., 2006, 21, 139–143 CAS.
- M. Balat and B. Spinner, Optimization of a chemical heat pump Energetic density and power, Heat Recovery Syst. CHP, 1993, 13, 277–285 CrossRef CAS.
- M. Zamengo, J. Ryu and Y. Kato, Thermochemical performance of magnesium hydroxide-expanded graphite pellets for chemical heat pump, Appl. Therm. Eng., 2014, 64, 339–347 CrossRef CAS.
- X. Wu, L. Wang, C. Wu, G. Wang and P. Jiang, Flammability of EVA/IFR APP/PER/ZB system, and EVA/IFR/synergist CaCO3, NG, and EG, composites, J. Appl. Polym. Sci., 2012, 126, 1917–1927 CrossRef CAS.
- Q. Zhang, J. Yan, T.-P. Li and J. Wang, Preparation of magnetic expanded graphite by Sol-gel method and its electromagnetic properties, Adv. Mater. Res., 2011, 295-297, 93–97 CrossRef CAS.
- P. R. Birkett, Fullerene chemistry, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 1999, 95, 431–451 RSC.
- P. Maurizio, Fullerene chemistry for materials science applications, J. Mater. Chem., 1997, 7, 1097–1109 RSC.
- I. Kenichiro, Molecular Catalysis for Fullerene Functionalization, Chem. Rec. MBLA Spec. Is., 2011, 11(5), 226–235 Search PubMed.
- M. Nazario, S. Nathalie and N. Jean-François, Advances in Molecular and Supramolecular Fullerene Chemistry, Electrochem. Soc. Interface, 2006, 29–33 Search PubMed.
- K. Li, D. I. Schuster, D. M. Guldi, M. A. Herranz and L. Echegoyen, Convergent Synthesis and Photophysics of [60]Fullerene/Porphyrin-Based Rotaxanes, J. Am. Chem. Soc., 2004, 126, 3388 CrossRef CAS PubMed.
- Y. Nakamura, S. Minami, K. Iizuka and J. Nishimura, Preparation of Neutral [60]Fullerene-Based [2]Catenanes and [2]Rotaxanes Bearing an Electron-Deficient Aromatic Diimide Moiety, Angew. Chem., Int. Ed., 2003, 42, 3158 CrossRef CAS PubMed.
- F. Cardinali, H. Mamlouk, Y. Rio, N. Armaroli and J.-F. Nierengarten, Fullerohelicates: a new class of fullerene-containing supermolecules, Chem. Commun., 2004, 1582 RSC.
- D. Felder, B. Heinrich, D. Guillon, J.-F. Nicoud and J.-F. Nierengarten, A Liquid Crystalline Supramolecular Complex of C60 with a Cyclotriveratrylene Derivative, Chem. – Eur. J., 2000, 6, 3501 CrossRef CAS.
- D. M. Guldi and N. Martin, Fullerene architectures made to order; biomimetic motifs — design and features, J. Mater. Chem., 2002, 12, 1978 RSC.
- U. Hahn, M. Elhabiri, A. Trabolsi, H. Herschbach, E. Leize and A. van Dorsselaer, et al., Supramolecular click chemistry with a bisammonium-C60 substrate and a ditopic crown ether host, Angew. Chem., Int. Ed., 2005, 44, 5338 CrossRef CAS PubMed.
- S. Delia and S. Roberto, Coordination Modes and Different Hapticities for Fullerene Organometallic Complexes, Molecules, 2012, 17, 7151–7168 CrossRef.
- S. Osuna, M. Swart and M. Solà, The reactivity of endohedral fullerenes. What can be learnt from computational studies?, Phys. Chem. Chem. Phys., 2011, 13, 3585–3603 RSC.
- S. Yang, F. Liu, C. Chen, M. Jiao and T. Wei, Fullerenes encaging metal clusters-Clusterfullerenes, Chem. Commun., 2011, 47, 11822–11839 RSC.
- Y. Matsuo, K. Kanaizuka, K. Matsuo, Y.-W. Zhong, T. Nakae and E. Nakamura, Photocurrent-Generating Properties of Organometallic Fullerene Molecules on an Electrode, J. Am. Chem. Soc., 2008, 130, 5016–5017 CrossRef CAS PubMed.
- N. Wang, J. Li and G. Ji, Synthesis of trinitrophenyl C60 derivative, Propellants, Explos., Pyrotech., 1996, 21, 317–319 CrossRef CAS.
- B. Jin, R.-F. Peng, Y.-J. Shu, F.-C. Zhong and S.-J. Chu, Study on the synthesis of new energetic fullerene derivative, Chinese, J. High Pressure Phys., 2006, 20, 220–224 CAS.
- B. Jin, R.-F. Peng, B.-S. Tan, Y.-M. Huang, Y.-J. Shu, S.-J. Chu and Y.-B. Fu, Synthesis and characterization of nitro fulleropyrrolidine derivatives, Chin. J. Energet. Mater., 2009, 17, 287–292 CAS.
- W. Tingting and Z. Heping, Synthesis, charge-separated state characterization of N-methyl-2-4′-N-ethylcarbozole,-3- fulleropyrrolidine and its derivatives, Frontiers Chem. China, 2006, 1, 161–169 CrossRef.
- B. Tan, R. Peng, H. Li, B. O. Jin, S. Chu and X. Long, Theoretical investigation of an energetic fullerene derivative, J. Comput. Chem., 2010, 31, 2233–2237 CAS.
- F. Cataldo, O. Ursini and G. Angelini, Synthesis and explosive decomposition of polynitro[60]fullerene, Carbon, 2013, 62, 413–421 CrossRef CAS.
- B.-L. Chen, B. Jin, R.-F. Peng, F.-Q. Zhao, J.-H. Yi, W.-J. Han, H.-J. Guan and S.-J. Chu, Synthesis and characterization of fullerene-ethylenediamine nitrate, Chin. J. Energet. Mater., 2014, 22, 186–191 CAS.
- Q.-Q. Liu, B. Jin, R.-F. Peng, F.-Q. Zhao, S.-Y. Xu and S.-J. Chu, Preparation and characterization of fullerene itaconic acid copolymer lead salt, Chin. J. Explos. Propellants, 2013, 36, 64–69 CAS.
- B.-L. Chen, B. Jin, R.-F. Peng, F.-Q. Zhao, J.-H. Yi, H.-J. Guan, X.-B. Bu and S.-J. Chu, Synthesis, characterization and thermal decomposition of fullerene-ethylenediamine dinitramide, Chin. J. Energet. Mater., 2014, 22, 467–472 CAS.
- H.-J. Guan, R.-F. Peng, B. Jin, H. Liang, F.-Q. Zhao, X.-B. Bu, W.-J. Han and S.-J. Chu, Preparation and thermal performance of fullerene-based lead salt, Bull. Korean Chem. Soc., 2014, 35, 2257–2262 CrossRef CAS.
- Z. Hu, C. H. Zhang, Y. D. Huang, S. F. Sun, W. C. Guan and Y. H. Yao, Photodynamic anticancer activities of water-soluble C60 derivatives and their biological consequences in a HeLa cell line, Chem. – Biol. Interact., 2012, 195, 86–94 CrossRef CAS PubMed.
- H.-J. Guan, B. Jin, R.-F. Peng, F.-Q. Zhao, W.-J. Han, B.-L. Chen and S.-J. Chu, The preparation and thermal decomposition performance of fullerene hydrazine nitrate, Acta Armamentarii, 2014, 35, 1756–1764 Search PubMed.
- W. Gong, B. Jin, R. Peng, N. Deng, R. Zheng and S. Chu, Synthesis and Characterization of [60]Fullerene-Polyglycidyl nitrate, and Its Thermal Decomposition, Ind. Eng. Chem. Res., 2015, 54, 2613–2618 CrossRef CAS.
- T. Huang, B. Jin, R. F. Peng, C. D. Chen, R. Z. Zheng, Y. He and S. J. Chu, Synthesis and characterization of [60]fullerene-glycidyl azide polymer and its thermal decomposition, Polymers, 2015, 7, 896–908 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang and S. V. Dubonos, et al., Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Honeycomb Carbon: A review of graphene, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- Q. Tang, Z. Zhou and Z. Chen, Graphene-related nanomaterials tuning properties by functionalization, Nanoscale, 2013, 5, 4541–4583 RSC.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. R. Ruoff, Large-area synthesis of high-quality and uniform graphene films on copper foils, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- C. Mattevi, H. Kima and M. Chhowalla, A review of chemical vapour deposition of graphene on copper, J. Mater. Chem., 2011, 21, 3324–3334 RSC.
- C. Vallés, C. Drummond, H. Saadaoui, C. A. Furtado, M. He, O. Roubeau and L. Ortolani, et al., Solutions of negatively charged graphene sheets and ribbons, J. Am. Chem. Soc., 2008, 130(47), 15802–15804 CrossRef PubMed.
- T. Morishita, A. J. Clancy and M. S. P. Shaffer, Optimised exfoliation conditions enhance isolation and solubility of grafted graphenes from graphite intercalation compounds, J. Mater. Chem. A, 2014, 2(36), 15022–15028 CAS.
- O. C. Compton and S. T. Nguyen, Graphene Oxide, Highly reduced graphene oxide, and graphene versatile building blocks for carbon-based materials, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- D. Chen, H. Feng and J. Li, Graphene oxide Preparation, functionalization, and electrochemical applications, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, The chemistry of graphene oxide, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- S. Gilje, W. Han, M. Wang, K. L. Wang and R. B. Kaner, A Chemical route to graphene for device applications, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS PubMed.
- J. Pyun, Graphene Oxide as Catalyst Application of Carbon Materials beyond Nanotechnology, Angew. Chem., Int. Ed., 2011, 50, 46–48 CrossRef CAS PubMed.
- T.-F. Yeh, J.-M. Syu, C. Cheng, T.-H. Chang and H. Teng, Graphite Oxide as a Photocatalyst for Hydrogen Production from Water, Adv. Funct. Mater., 2010, 20, 2255–2262 CrossRef CAS.
- D. R. Dreyer, H.-P. Jia and C. W. Bielawski, Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions, Angew. Chem., Int. Ed., 2010, 49, 6813–6816 CAS.
- Y. J. Song, Y. Chen, L. Y. Feng, J. S. Ren and X. G. Qu, Selective and quantitative cancer cell detection using target-directed functionalized graphene and its synergetic peroxidase-like activity, Chem. Commun., 2011, 47, 4436–4438 RSC.
- Y. J. Guo, L. Deng, J. Li, S. J. Guo, E. K. Wang and S. J. Dong, Hemin−Graphene Hybrid Nanosheets with Intrinsic Peroxidase-like Activity for Label-free Colorimetric Detection of Single-Nucleotide Polymorphism, ACS Nano, 2011, 5, 1282–1290 CrossRef CAS PubMed.
- Y. Wang, Z. Li, J. Wang, J. Li and Y. Lin, Graphene and Graphene Oxide biofunctionalization and applications in biotechnology, Trends Biotechnol., 2011, 29, 205–212 CrossRef CAS PubMed.
- V. C. Sanchez, A. Jachak, R. H. Hurt and A. B. Kane, Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review, Chem. Res. Toxicol., 2012, 25, 15–34 CrossRef CAS PubMed.
- J. Kim, L. J. Cote, F. Franklin Kim, W. Yuan, K. R. Shull and J. Huang, Graphene oxide sheets at interfaces, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS PubMed.
- J. Sha, X. Lin-sheng, M. Yu-lu, H. Jing-jie, X. Zhang, Z. Guo-xun, D. Shu-mei and W. Yan-yun, Low-temperature expanded graphite for preparation of graphene sheets by liquid-phase method, J. Phys.: Conf. Ser., 2009, 188, 012040 CrossRef.
- D. Yongqiang, C. Congqiang, Z. Xinting, G. Lili, C. Zhiming, Y. Hongbin, G. Chunxian, C. Yuwu and L. Chang-Ming, One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black, J. Mater. Chem., 2012, 22, 8764–8766 RSC.
- B. C. Brodie, On the atomic weight of graphite, Philos. Trans. R. Soc. London, 1859, 14, 249–259 CrossRef.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun and A. Slesarev, et al., Improved synthesis of graphene oxide, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. Chen, Y. Li, L. Huang, C. Li and G. Shi, High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process, Carbon, 2015, 81, 826–834 CrossRef CAS.
- P. S. V. Jimenez, Thermal Decomposition of Graphite Oxidation Products DSC Studies of Internal Combustion of Graphite Oxide, Mater. Res. Bull., 1987, 22, 601–608 CrossRef.
- C. H. A. Wong, O. Jankovský, Z. Sofer and M. Pumera, Vacuum-assisted microwave reduction/exfoliation of graphite oxide and the influence of precursor graphite oxide, Carbon, 2014, 77, 508–517 CrossRef CAS.
- D. Krishnan, F. Kim, J. Luo, R. Cruz-Silva, L. J. Cote, H. D. Jang and J. Huang, Energetic graphene oxide challenges and opportunities, Nano Today, 2012, 7, 137–152 CrossRef CAS.
- J. C. Garcia, D. B. de Lima, L. V. C. Assali and J. F. Justo, Group IV graphene- and graphane-like nanosheets, J. Phys. Chem. C, 2011, 115, 13242–13246 CAS.
- Y. Yamada, M. Miyauchi and K. Jungpil, et al., Exfoliated graphene ligands stabilizing copper cations, Carbon, 2011, 49, 3375–3378 CrossRef CAS.
- Y. Yamada, Y. Suzuki, H. Yasuda, S. Uchizawa, K. Hirose-Takai, Y. Sato, K. Suenaga and S. Sato, Functionalized graphene sheets coordinating metal cations, Carbon, 2014, 75, 81–94 CrossRef CAS.
- C. Zhang, X. Cao and B. Xiang, Sandwich complex of TATB/graphene: An approach to molecular monolayers of explosives, J. Phys. Chem. C, 2010, 114, 22684–22687 CAS.
- Z.-M. Li, M.-R. Zhou, T.-L. Zhang, J.-G. Zhang, L. Yang and Z.-N. Zhou, The facile synthesis of graphene nanoplatelet-lead styphnate composites and their depressed electrostatic hazards, J. Mater. Chem. A, 2013, 1, 12710–12714 CAS.
- R. Liu, W. Zhao, T. Zhang, L. Yang, Z. Zhou and J. Zhang, Particle refinement and graphene doping effects on thermal properties of potassium picrate, J. Therm. Anal. Calorim., 2014, 118, 561–569 CrossRef CAS.
- X.-B. Wang, J.-Q. Li and Y.-J. Luo, Preparation and thermal decomposition behaviour of ammonium perchlorate/graphene aerogel nanocomposites, Chin. J. Explos. Propellants, 2012, 35, 76–80 CAS.
- X.-B. Wang, J.-Q. Li, Y.-J. Luo and M. Huang, A Novel ammonium perchlorate/graphene aerogel nanostructured energetic composite preparation and thermal decomposition, Sci. Adv. Mater., 2014, 6, 530–537 CrossRef CAS.
- Y. Lan, M. Jin and Y. Luo, Preparation and characterization of graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite, J. Sol-Gel Sci. Technol., 2015, 74, 161–167 CrossRef CAS.
- S. Yang, X. Feng and K. Mullen, Sandwich-like, graphene-based titania nanosheets with high surface area for fast lithium storage, Adv. Mater., 2011, 2331, 3575–3579 CrossRef PubMed.
- Y.-F. Lan and Y.-J. Luo, Preparation and characterization of graphene aerogel/ammonium nitrate nano composite energetic materials, Chin. J. Explos. Propellants, 2015, 38, 15–18 Search PubMed.
- R. Thiruvengadathan, S. W. Chung, S. Basuray, B. Balasubramanian, C. S. Staley, K. Gangopadhyay and S. Gangopadhyay, A versatile self-assembly approach toward high performance nanoenergetic composite using functionalized graphene, Langmuir, 2014, 30(22), 6556–6564 CrossRef CAS PubMed.
- Y. Wang, H. Liu, H. Cheng, M. Zu and J. Wang, Corrosion behavior of pyrocarbon coatings exposed to Al2O3-SiO2 gels containing ammonium nitrate, Corros. Sci., 2015, 94, 401–410 CrossRef CAS.
- H. Cai, L. Tian, B. Huang, G. Yang, D. Guan and H. Huang, 1,1-Diamino-2,2-dintroethene FOX-7, nanocrystals embedded in mesoporous carbon FDU-15, Microporous Mesoporous Mater., 2013, 170, 20–25 CrossRef CAS.
- A. C. Ferrari, F. Bonaccorso and V. Fal'ko, et al., Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems, Nanoscale, 2015, 7, 4598–4810 RSC.
- X. Zhang, W. M. Hikal, Y. Zhang, S. K. Bhattacharia, L. Li, S. Panditrao, S. Wang and B. L. Weeks, Direct laser initiation and improved thermal stability of nitrocellulose/graphene oxide nanocomposites, Appl. Phys. Lett., 2013, 102, 141905 CrossRef.
- P. D. McCrary, P. A. Beasley, S. A. Alaniz, C. S. Griggs, R. M. Frazier and R. D. Rogers, Graphene and graphene oxide can “lubricate” ionic liquids based on specific surface interactions leading to improved low-temperature hypergolic performance, Angew. Chem., Int. Ed., 2012, 51, 9784–9787 CrossRef CAS PubMed.
- Y. Zhang, Z. Shao, K. Gao, X. Wu and Y. Liu, Tensile properties of nitrate glycerol ether cellulose/graphene oxide nanocomposites, Integr. Ferroelectr., 2014, 154, 147–153 CrossRef CAS.
- R. Li, J. Wang, J. P. Shen, C. Hua and G. C. Yang, Preparation and characterization of insensitive HMX/graphene oxide composites, Propellants, Explos., Pyrotech., 2013, 38, 798–804 CrossRef CAS.
- Y. Li, V. Alain-Rizzo, L. Galmiche, P. Audebert, F. Miomandre, G. Louarn, M. Bozlar, M. A. Pope, D. M. Dabbs and I. A. Aksay, Functionalization of Graphene Oxide by Tetrazine Derivatives: A Versatile Approach toward Covalent Bridges between Graphene Sheets, Chem. Mater., 2015, 27, 4298–4310 CrossRef CAS.
- M. Endo, H. Takuya and K. Yoong-Ahm, Large-scale production of carbon nanotubes and their applications, Pure Appl. Chem., 2006, 78, 1703–1713 CrossRef CAS.
- W. Z. Li, S. S. Xie, L. X. Qian, B. H. Chang, B. S. Zou and W. Y. Zhou, et al., Large-Scale Synthesis of Aligned Carbon Nanotubes, Science, 1996, 274, 1701–1703 CrossRef CAS.
- Z. F. Ren, Z. P. Huang, J. W. Xu, J. H. Wang, P. Bush, M. P. Siegal and P. N. Provencio, Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass, Science, 1998, 282, 1105–1107 CrossRef CAS PubMed.
- S. Fan, M. G. Chapline, N. R. Franklin, T. W. Tombler, A. M. Cassell and H. Dai, Self-Oriented Regular Arrays of Carbon Nanotubes and Their Field Emission Properties, Science, 1999, 283, 512–514 CrossRef CAS PubMed.
- B. Zheng, C. Lu, G. Gu, A. Makarovski, G. Finkelstein and J. Liu, Efficient CVD Growth of Single-Walled Carbon Nanotubes on Surfaces Using Carbon Monoxide Precursor, Nano Lett., 2002, 2, 895–898 CrossRef CAS.
- K. Hata, D. N. Futaba, K. Mizuno, T. Namai, M. Yumura and S. Iijima, Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes, Science, 2004, 306, 1362–1364 CrossRef CAS PubMed.
- M. Terrones, N. Grobert, J. Olivares, J. P. Zhang, H. Terrones, K. Kordatos, W. K. Hsu, J. P. Hare, P. D. Townsend, K. Prassides, A. K. Cheetham, H. W. Kroto and D. R. M. Walton, Controlled production of aligned-nanotube bundles, Nature, 1997, 388, 52–55 CrossRef CAS.
- A. Cao, B. Wei, Y. Jung, R. Vajtai, P. M. Ajayan and G. Ramanath, Growth of aligned carbon nanotubes on self-similar macroscopic templates, Appl. Phys. Lett., 2002, 81, 1297–1299 CrossRef CAS.
- B. Q. Wei, R. Vajtai, Y. Jung, J. Ward, R. Zhang, G. Ramanath and P. M. Ajayan, Microfabrication technology Organized assembly of carbon nanotubes, Nature, 2002, 416, 495–496 CrossRef CAS PubMed.
- Y. J. Jung, B. Wei, R. Vajtai, P. M. Ajayan, Y. Homma, K. Prabhakaran and T. Ogino, Mechanism of Selective Growth of Carbon Nanotubes on SiO2/Si Patterns, Nano Lett., 2003, 3, 561–564 CrossRef CAS.
- A. Cao, P. L. Dickrell, W. G. Sawyer, M. N. Ghasemi-Nejhad and P. M. Ajayan, Super-Compressible Foamlike Carbon Nanotube Films, Science, 2005, 310, 1307–1310 CrossRef CAS PubMed.
- A. Cao, V. P. Veedu, X. Li, Z. Yao, M. N. Ghasemi-Nejhad and P. M. Ajayan, Multifunctional brushes made from carbon nanotubes, Nat. Mater., 2005, 4, 540–545 CrossRef CAS PubMed.
- C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Boron nitride substrates for high-quality graphene electronics, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Sorting carbon nanotubes by electronic structure using density differentiation, Nat. Nanotechnol., 2006, 1, 60–65 CrossRef CAS PubMed.
- Y.-M. Lin, C. Dimitrakopoulos, K. A. Jenkins, D. B. Farmer, H.-Y. Chiu, A. Grill and Ph. Avouris, 100-GHz transistors from wafer-scale epitaxial graphene, Science, 2010, 327, 662 CrossRef CAS PubMed.
- K. S. Novoselov, V. I. Fal'Ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, A roadmap for graphene, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- S. V. Morozov, K. S. Novoselov, M. I. Katsnelson, F. Schedin, D. C. Elias, J. A. Jaszczak and A. K. Geim, Giant intrinsic carrier mobilities in graphene and its bilayer, Phys. Rev. Lett., 2008, 100, 016602 CrossRef CAS PubMed.
- P. W. Sutter, J.-I. Flege and E. A. Sutter, Epitaxial graphene on ruthenium, Nat. Mater., 2008, 7, 406–411 CrossRef CAS PubMed.
- T. Utschig, M. Schwarz, G. Miehe and E. Kroke, Synthesis of carbon nanotubes by detonation of, 2,4,6-triazido-1,3,5-triazine in the presence of transition metals, Carbon, 2004, 42, 823–828 CrossRef CAS.
- E. Kroke, M. Schwarz, V. Buschmann, G. Miehe, H. Fuess and R. Riedel, Nanotubes formed by detonation of C/N precursors, Adv. Mater., 1999, 11, 158–161 CrossRef CAS.
- S. J. Shearer, G. C. Turrell, J. I. Beyant and R. L. Brooks III, Vibrational spectra of cyanuric triazide, J. Chem. Phys., 1968, 48, 1138–1144 CrossRef CAS.
- C. Ye, H. Gao, J. A. Boatz, G. W. Drake, B. Twamley and J. M. Shreeve, Polyazidopyrimidines High-energy compounds and precursors to carbon nanotubes, Angew. Chem., Int. Ed., 2006, 45, 7262–7265 CrossRef CAS PubMed.
- E.-C. Koch, Metal/fluorocarbon pyrolants VI. Combustion behaviour and radiation properties of magnesium/polycarbon monofluoride, pyrolant, Propellants, Explos., Pyrotech., 2005, 30, 209–215 CrossRef CAS.
- G. Ćirić-Marjanovic, I. Pašti and S. Mentus, One-dimensional nitrogen-containing carbon nanostructures, Prog. Mater. Sci., 2014, 69, 61–182 CrossRef.
- C. Zhang, J. Li, Y. Luo, X. Zhang and B. Zhai, Preparation and properties of carbon nanotubes modified glycidyl azide polymer binder film, Polym. Mater. Sci. Eng., 2013, 29, 105–108 CAS.
- C. Zhang, J. Li, Y.-J. Li and Z. B. Zhang, Preparation and property studies of carbon nanotubes covalent modified BAMO-AMMO energetic binders, J.Energet Mater, 2015, 33, 305–314 CrossRef CAS.
- Q.-L. Yan, S. Zeman and A. Elbeih, Thermal behavior and decomposition kinetics of Viton A bonded explosives containing attractive cyclic nitramines, Thermochim. Acta, 2013, 562, 56–64 CrossRef CAS.
- Q.-L. Yan, S. Zeman, T.-L. Zhang and A. Elbeih, Non-isothermal decomposition behavior of Fluorel bonded explosives containing attractive cyclic nitramines, Thermochim. Acta, 2013, 574, 10–18 CrossRef CAS.
- C.-M. Lin, J.-H. Liu, S.-J. Liu, Z. Huang, Y.-B. Li and J.-H. Zhang, Characterization viscoelastic properties of multi-walled carbon Nanotubes/F2314 composites using DMA method, Chin. J. Energet. Mater., 2015, 23, 140–145 Search PubMed.
- X.-M. Qian, N. Deng, S.-F. Wei and Z.-Y. Li, Catalytic effect of carbon nanotubes on pyrotechnics, Chin. J. Energet. Mater., 2009, 17, 603–607 CAS.
- R. Guo, Y. Hu, R. Shen, Y. Ye and L. Wu, A micro initiator realized by integrating KNO3/CNTs nanoenergetic materials with a Cu microbridge, Chem. Eng. J., 2012, 211-212, 31–36 CrossRef CAS.
- H. Yan, G. Rui, Y. Yinghua, S. Ruiqi, W. Lizhi and Z. Peng, Fabrication and electro-explosive performance of carbon nanotube energetic igniter, Sci. Technol. Energ. Mater., 2012, 73, 116–123 Search PubMed.
- R. Guo, Y. Hu, R. Shen and Y. Ye, Electro-explosion performance of KNO3-filled carbon nanotubes initiator, J. Appl. Phys., 2014, 115, 174901 CrossRef.
- L. J. Currano, W. Churaman, C. Becker, C. J. Morris, L. M. Reddy, A. Ihnen, P. Ajayan and W. Y. Lee, Energetic materials for integration on chip, Proceedings - 14th International Detonation Symposium, IDS, 2010, pp. 30–36.
- V. Pelletier, S. Bhattacharyya, I. Knoke, F. Forohar, M. Bichay and Y. Gogotsi, Copper azide confined inside templated carbon nanotubes, Adv. Funct. Mater., 2010, 20, 3168–3174 CrossRef CAS.
- W. Choi, S. Hong, J. T. Abrahamson, J.-H. Han, C. Song, N. Nair, S. Baik and M. S. Strano, Chemically driven carbon-nanotube- guided thermopower waves, Nat. Mater., 2010, 9, 423–429 CrossRef CAS PubMed.
- L.-M. Liu, X.-L. Kang, Y. Yi, H.-F. Zhang, J.-S. Luo and Y.-J. Tang, Influence of CNTs on thermal behavior and light radiation properties of Zr/KClO4 pyrotechnics, Chin. J. Energet. Mater., 2014, 22, 75–79 CAS.
- N. Labhsetwar, P. Doggali, S. Rayalu, R. Yadav, T. Mistuhashi and H. Haneda, Ceramics in environmental catalysis Applications and possibilities, Chin. J. Catalyst., 2012, 33, 1611–1621 CrossRef CAS.
- X.-F. Dong, Y. Li, X.-F. Xiong and D.-L. Cao, Preparation and performance of copper-lead-carbon composite burning rate catalyst, Chin. J. Explos. Propellants, 2011, 34, 69–73 CAS.
- B. Van Devener, J. P. L. Perez, J. Jankovich and S. L. Anderson, Oxide-free, catalyst-coated, fuel-soluble, air-stable boron nanopowder as combined combustion catalyst and high energy density fuel, Energy Fuels, 2009, 23, 6111–6120 CrossRef CAS.
- Y. Liu, W. Jiang, J.-X. Liu, Y. Wang, G.-P. Liu and F.-S. Li, Study of catalyzing thermal decomposition and combustion of AP/HTPB propellant with nano Cu/CNTs, Acta Armamentarii, 2008, 29, 1029–1033 CAS.
- X. Liu, W.-L. Hong, F.-Q. Zhao, D.-Y. Tian, J.-X. Zhang and Q.-S. Li, Synthesis of CuO/CNTs composites and its catalysis on thermal decomposition of FOX-12, J. Solid Rocket Technol., 2008, 31, 508–511 CAS.
- I. G. Assovskiy and A. A. Berlin, Metallized carbon nanotubes, Int. J. Energ. Mater. Chem. Propul., 2009, 8, 281–289 Search PubMed.
- F. Wang, S. Arai and M. Endo, Metallization of multi-walled carbon nanotubes with copper by an electroless deposition process, Electrochem. Commun., 2004, 6, 1042–1044 CrossRef CAS.
- R. Wang, Z. Li, Y. Ma, F. Zhao and C. Pei, Preparation of high filling ratio Fe2O3/MWCNTs composite particles and catalytic performance on thermal decomposition of ammonium perchlorate, Micro. Nano Lett., 2014, 9, 787–791 Search PubMed.
- J.-X. Zhang, W.-L. Hong, F.-Q. Zhao, J.-H. Liu, D.-Y. Tian, X.-Y. Zhu and Y.-Q. Ma, Synthesis of SnO2-Cu2O/CNTs catalyst and its catalytic effect on thermal decomposition of FOX-12, Chin. J. Explos. Propellants, 2011, 34, 47–51 Search PubMed.
- H. Ren, Y.-Y. Liu, Q.-J. Jiao, X.-F. Fu and T.-T. Yang, Preparation of nanocomposite PbO·CuO/CNTs via microemulsion process and its catalysis on thermal decomposition of RDX, J. Phys. Chem. Solids, 2010, 71, 149–152 CrossRef CAS.
- G. Fan, H. Wang, X. Xiang and F. Li, Co-Al mixed metal oxides/carbon nanotubes nanocomposite prepared via a precursor route and enhanced catalytic property, J. Solid State Chem., 2013, 197, 14–22 CrossRef CAS.
- W. Pang, X. Fan, F. Zhao, H. Xu, W. Zhang, H. Yu, Y. Li, F. Liu, W. Xie and N. Yan, Effects of different metal fuels on the characteristics for HTPB-based fuel rich solid propellants, Propellants, Explos. Pyrotech., 2013, 386, 852–859 CrossRef.
- Q.-L. Yan, X.-G. Wu, X. Guo, X.-F. Qi, X.-J. Li and K.-Q. Wang, Combustion efficiency and pyrochemical properties of micron-sized metal particles as the components of modified double-base propellant, Acta Astro., 2011, 687–8, 1098–1112 Search PubMed.
- D. Sylvain, B. Patric, G. Nicole, C. Sebastien and T. Serge, Flash-ignitable energetic material, US Patent, 20040040637A1 , Mar 4, 2004 Search PubMed.
- T. An, H.-Q. Cao, F.-Q. Zhao, X.-N. Ren, D.-Y. Tian, S.-Y. Xu, H.-X. Gao, Y. Tan and L.-B. Xiao, Preparation and characterization of Ag/CNTs nanocomposite and its effect on thermal decomposition of cyclotrimethylene trinitramine, Acta Physo.–Chim. Sin., 2012, 28, 2202–2208 CAS.
- H. Y. Jeong, K. P. So, J. J. Bae, S. H. Chae, T. H. Ly, T. H. Kim, D. H. Keum, C. K. Kim, J. S. Hwang, Y. J. Choi and Y. H. Lee, Tailoring oxidation of Al particles morphologically controlled by carbon nanotubes, Energy, 2013, 55, 1143–1151 CrossRef CAS.
- A. Rai, D. Lee, K. Park and M. R. Zachariah, Importance of phase change of aluminum in oxidation of aluminum nanoparticles, J. Phys. Chem. B, 2004, 108, 14793–14795 CrossRef CAS.
- K. J. Blobaum, M. E. Reiss, J. M. Plitzko Lawrence and T. P. Weihs, Deposition and characterization of a self-propagating CuOx/Al thermite reaction in a multilayer foil geometry, J. Appl. Phys., 2003, 94, 2915–2922 CrossRef CAS.
- A. M. K. Esawi, K. Morsi, A. Sayed, M. Taher and S. Lanka, Effect of carbon nanotube CNT, content on the mechanical properties of CNT-reinforced aluminium composites, Compos. Sci. Tech., 2010, 70, 2237–2241 CrossRef CAS.
- Y. Wang, S. Malhotra and Z. Iqbal, Nanoscale energetics with carbon nanotubes, Mater. Res. Soc. Symp. Proc., 2003, 800, 351–359 CrossRef CAS.
- F. Forohar, C. M. Whitaker, I. C. Uber and V. Bellitto, Synthesis and Characterization of Nitro-Functionalized Single-Walled Carbon Nanotubes, J. Energet. Mater., 2012, 30, 55–71 CrossRef CAS.
- X.-T. Ji, J.-H. Bu, Z.-X. Ge, T.-Q. Li, H.-P. Su, Q. Liu, Y. Zhu and X. Xiao, Preparation and characterization of tetrazolyl-functionalized single walled carbon nanotubes, Chin. J. Energet. Mater., 2015, 23, 99–102 Search PubMed.
- J. T. Abrahamson, C. Song, J. H. Hu, J. M. Forman, S. G. Mahajan, N. Nair, W. Choi, E.-J. Lee and M. S. Strano, Synthesis and energy release of nitrobenzene-functionalized single-walled carbon nanotubes, Chem. Mater., 2011, 23, 4557–4562 CrossRef CAS.
- L. Wang, C. Yi, H. Zou, H. Gan, J. Xu and W. Xu, Adsorption of the insensitive explosive TATB on single-walled carbon nanotubes, Mol. Phys., 2011, 109, 1841–1849 CrossRef CAS.
- L. Wang, J. Wu, C. Yi, H. Zou, H. Gan and S. Li, Assemblies of energetic 3,3′-diamino-4,4′-azofurazan on single-walled carbon nanotubes, Comput. Theor. Chem., 2012, 982, 66–73 CrossRef CAS.
- Y. Zhong, M. Jaidann, Y. Zhang, G. Zhang, H. Liu, M. Ioan Ionescu, R. Li, X. Sun, H. Abou-Rachid and L.-S. Lussier, Synthesis of high nitrogen doping of carbon nanotubes and modeling the stabilization of filled DAATO/CNTs 10,10, for nanoenergetic materials, J. Phys. Chem. Solids, 2010, 71, 134–139 CrossRef CAS.
- M. A. Hiskey, D. E. Chavez and D. Naud, Preparation of 3,3′-azobis6-amino-1,2,4,5-tetrazine, US Patent, 6342589, 2002, United States Department of Energy, USA Search PubMed.
- C. C. Tang, Y. Bando, D. Golberg and F. F. Xu, Structure and nitrogen incorporation of carbon nanotubes synthesized by catalytic pyrolysis of dimethylformamide, Carbon, 2004, 42, 2625–2633 CrossRef CAS.
- K. Huang, S. Pisharath and S.-C. Ng, Preparation of polyurethane-carbon nanotube composites using ‘click’ chemistry, Tetrahedron Lett., 2015, 56, 577–580 CrossRef CAS.
- K. A. Jiang, L. S. Eitan, P. M. Schadler, R. W. Ajayan, N. Siegel and Grobert, et al., Selective attachment of gold nanoparticles to nitrogen-doped carbon nanotubes, Nano Lett., 2003, 3, 275–277 CrossRef CAS.
- H. Abou-Rachid, A. Hu, V. Timoshevskii, Y. Song and L.-S. Lussier, Nanoscale high energetic materials A polymeric nitrogen chain N8 confined inside a carbon nanotube, Phys. Rev. Lett., 2008, 100, 196401 CrossRef PubMed.
- G.-Q. Zhang, X.-B. Liu and M. Huang, Review on energetic nitroguanidine derivatives, Chin. J. Energet. Mater., 2013, 21, 668–674 CAS.
- H. Gao and J. M. Shreeve, Azole-based energetic salts, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- U. R. Nair, S. N. Asthana, A. S. Rao and B. R. Gandhe, Advances in high energy materials, Defense Sci. J., 2010, 60, 137–151 CrossRef CAS.
- V. P. Sinditskii, V. Y. Egorshev, V. V. Serushkin, A. I. Levshenkov, M. V. Berezin, S. A. Filatov and S. P. Smirnov, Evaluation of decomposition kinetics of energetic materials in the combustion wave, Thermochim. Acta, 2009, 496, 1–12 CrossRef CAS.
- M. B. Talawar, R. Sivabalan, T. Mukundan, H. Muthurajan, A. K. Sikder, B. R. Gandhe and A. S. Rao, Environmentally compatible next generation green energetic materials GEMs, J. Hazard. Mater., 2009, 161, 589–607 CrossRef CAS PubMed.
- O. P. Korobeinichev, A. A. Paletskii and E. N. Volkov, Flame structure and combustion chemistry of energetic materials, Russ. J. Phys. Chem. B, 2008, 2, 206–228 CrossRef.
- M. W. Beckstead, K. Puduppakkam, P. Thakre and V. Yang, Modeling of combustion and ignition of solid-propellant ingredients, Prog. Energy Combust. Sci., 2007, 33, 497–551 CrossRef CAS.
- S. Zeman, Q.-L. Yan and M. Vlcek, Recent advances in the study of the initiation of energetic materials using characteristics of their thermal decomposition, Part I. cyclic nitramines, Cent. Eur. J. Energ. Mater., 2014, 112, 173–189 Search PubMed.
- S. Zeman, Q.-L. Yan and A. Elbeih, Recent Advances in the study of the initiation of energetic materials using the characteristics of their thermal decomposition, Part II. Using simple differential thermal analysis, Cent. Eur. J. Energ. Mater., 2014, 113, 285–294 Search PubMed.
- Q.-L. Yan, S. Zeman, J.-G. Zhang, P. He, T. Musil and M. Bartošková, Multi-stage decomposition of 5-aminotetrazole derivatives kinetics and reaction channels for the rate-limiting steps, Phys. Chem. Chem. Phys., 2014, 16, 24282–24291 RSC.
- G.-Y. Zeng, C.-M. Lin and J.-H. Zhou, Influences of Carbon Nanotubes on the Thermal Decomposition Behavior of HMX, Chin. J. Explos. Propellants, 2012, 356, 55–57 Search PubMed.
- Yu Xian-feng, The effect of carbon nanotubes on the thermal decomposition of CL-20, Chin. J. Explos. Propellants, 2004, 273, 78–80 Search PubMed.
- W. Zhang, M.-M. Li and J. Li, Effect of carbon nanotubes on thermal decomposition of aminomitrobenzodifuroxan, J. Combus.t Sci. Tech., 2004, 101, 92–95 Search PubMed.
- K.-Z. Gu, X.-D. Li and R.-J. Yang, Catalytic action on combustion and thermal decomposition of AP with CNTs, Chin. J. Explos. Propellants, 2006, 291, 48–51 Search PubMed.
- L.-X. Wang, Z.-B. Wu, X.-L. Tuo, H.-T. Zou, J. Xu, C.-H. Yi and W.-L. Xu, Theoretical study on thermal decomposition of nitromethane confined inside an armchair 5, 5, single-wall carbon nanotube, Chin. J. Energet. Mater., 2009, 17(5), 518–522 CAS.
- L. Wang, J. Xu, C. Yi, H. Zou and W. Xu, Theoretical study on the thermal decomposition of nitromethane encapsulated inside single-walled carbon nanotubes, J. Mol. Struct.: THEOCHEM, 2010, 940, 76–81 CrossRef CAS.
- L. Wang, C. Yi, H. Zou, J. Xu and W. Xu, Rearrangement and thermal decomposition of nitromethane confined inside an armchair 5, 5, single-walled carbon nanotube, Chem. Phys., 2010, 367, 120–126 CrossRef CAS.
- L. Wang, H. Zou, C. Yi, J. Xu and W. Xu, Finite-Length Effect of Carbon Nanotubes on the Encapsulation and Decomposition of Nitromethane ONIOM Calculation, Curr. Nanosci., 2011, 7, 1054–1060 CrossRef CAS.
- L. Wang, C. Yi, H. Zou, J. Xu and W. Xu, On the isomerization and dissociation of nitramide encapsulated inside an armchair 5,5, single-walled carbon nanotube, Mater. Chem. Phys., 2011, 127, 232–238 CrossRef CAS.
- S. Reshmi, K. B. Catherine and C. P. Reghunadhan Nair, Effect of carbon nanotube on the thermal decomposition characteristics of selected propellant binders and oxidisers, Int. J. Nanotechnol., 2011, 8, 979–987 CrossRef CAS.
- X.-D. Li and R.-J. Yang, Combustion and thermal decomposition of ammonium dinitramide catalyzed by carbon nanotubes, New Carbon Mater., 2010, 25, 444–448 CAS.
- N. Li, M. Cao and Q. Wu, A facile one-step method to produce Ni/graphenenanocomposites and their application to the thermal decomposition of ammonium perchlorate, CrystEngComm, 2012, 142, 428–434 RSC.
- N. Li, Z. Geng and M. Cao, Well-dispersed ultrafine Mn3O4 nanoparticles on graphene as a promising catalyst for the thermal decomposition of ammonium perchlorate, Carbon, 2013, 54, 124–132 CrossRef CAS.
- J. Zhu, G. Zeng and F. Nie, Decorating graphene oxide with CuO nanoparticles in a water-isopropanol system, Nanoscale, 2010, 26, 988–994 RSC.
- W. Zhang, Q. Luo and X. Duan, et al., Nitrated graphene oxide and its catalytic activity in thermal decomposition of ammonium perchlorate, Mater. Res. Bull., 2014, 50, 73–78 CrossRef CAS.
- Y.-I. Izato, A. Miyake and H. Echigoya, Influence of the physical properties of carbon on the thermal decomposition behavior of ammonium nitrate and carbon mixtures, Sci. Technol. Energ. Mater., 2009, 70, 101–104 CAS.
- A. Miyake and Y.-I. Izato, Thermal decomposition behaviors of ammonium nitrate and carbon mixtures, Int. J. Energ. Mater. Chem. Propul., 2010, 9, 523–531 Search PubMed.
- L. Yu, H. Ren, X.-Y. Guo, X.-B. Jiang and Q.-J. Jiao, A novel ε-HNIW-based insensitive high explosive incorporated with reduced graphene oxide, J.Therm Anal Calorim, 2014, 117, 1187–1199 CrossRef CAS.
- V. V. Chaban, E. E. Fileti and O. V. Prezhdo, Buckybom, reactive molecular dynamics simulation, J. Phys. Chem. Lett., 2015, 6, 913–917 CrossRef CAS PubMed.
- H. Fazhoğlu and J. Hacaloğlu, The effect of triethanolamine on thermal decomposition of GAP, J. Macromol. Sci., Part A: Pure Appl.Chem., 2002, 7, 759–768 CrossRef.
- H. Fazlıoğlu and J. Hacaloğlu, Thermal decomposition of glycidyl azide polymer by direct insertion probe mass spectrometry, J. Anal. Appl. Pyrolysis, 2002, 2, 327–338 CrossRef.
- B. Jin, J. Shen, R. F. Peng, Y. J. Shu, B. S. Tan, S. J. Chu and H. S. Dong, Synthesis, characterization, thermal stability and sensitivity properties of the new energetic polymer through the azidoacetylation of polyvinyl alcohol, Polym. Degrad. Stab., 2012, 4, 473–480 CrossRef.
- I. Levin and D. Brandon, Thermodynamic stability of Metastable alumina polymorphs crystal structures and transition sequences, J. Am. Ceram. Soc., 1998, 81, 1995–2012 CrossRef CAS.
- L. Jeurgens, W. Sloof, F. Tichelaar and E. Mittemeijer, Amorphous oxidefilms on metals application to aluminum oxide films on aluminum substrates, Phys. Rev. B: Condens. Matter, 2000, 62, 4707–4719 CrossRef CAS.
- L. Ren, E. Chu, G. Zhang, Y. Bai, C. Shi and L. Sun, Development discussion on metrology of initiators and pyrotechnics, Proceedings - 4th International Conference on Computational and Information Sciences, ICCIS, 2012, 6300819, pp. 1120–1122.
- P. Zhu, X. Zhou, R.-Q. Shen, Y.-H. Ye and Y. Hu, Electrical-explosion performance of dielectric structure pyrotechnic initiators prepared by Al/CuO reactive multilayer films, Chin. J. Energet. Mater., 2011, 19, 366–369 CAS.
- P. Zhu, R. Shen, N. N. Fiadosenka, Y. Ye and Y. Hu, Dielectric structure pyrotechnic initiator realized by integrating Ti/CuO-based reactive multilayer films, J. Appl. Phys., 2011, 109, 084523 CrossRef.
- T. J. Hinkel and F. Salazar, Material properties effects on pyrotechnic initiator output, 44th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, 2008.
- M. R. Manaa, A. R. Mitchell, P. G. Garza, P. F. Pagoria and B. E. Watkins, Flash ignition and initiation of explosives-nanotubes mixture, J. Am. Chem. Soc., 2005, 127, 13786–13787 CrossRef CAS PubMed.
- K. Kappagantula, M. L. Pantoy and E. M. Hunt, Impact ignition of aluminum-teflon based energetic materials impregnated with nano-structured carbon additives, J. Appl. Phys., 2012, 112, 024902 CrossRef.
- K. Kappagantula and M. L. Pantoya, Experimentally measured thermal transport properties of aluminum- polytetrafluoroethylene nanocomposites with graphene and carbon nanotube additives, Int. J. Heat Mass Transfer, 2012, 55, 817–824 CrossRef CAS.
- W. Hong and N. Tai, Investigations on the thermal conductivity of composites reinforced with carbon nanotubes, Diamond Relat. Mater., 2008, 17, 1577–1581 CrossRef CAS.
- J. H. Kim, J. Y. Ahn, H. S. Park and S. H. Kim, Optical ignition of nanoenergetic materials: the role of single-walled carbon nanotubes as potential optical igniters, Combust. Flame, 2013, 160, 830–834 CrossRef CAS.
- E. S. Collins, B. R. Skelton, M. L. Pantoya, F. Irin, M. J. Green and M. A. Daniels, Ignition sensitivity and electrical conductivity of an aluminum fluoropolymer reactive material with carbon nanofillers, Combust. Flame, 2015, 162, 1417–1421 CrossRef CAS.
- J. H. Kim, S. B. Kim, M. G. Choi, D. H. Kim, K. T. Kim, H. M. Lee, H. W. Lee, J. M. Kim and S. H. Kim, Flash-ignitable nanoenergetic materials with tunable underwater explosion reactivity: the role of sea urchin-like carbon nanotubes, Combust. Flame, 2015, 162, 1448–1454 CrossRef CAS.
- Y.-L. Wang, Z.-X. Wei and L. Kang, Progress on combustion catalysts of solid propellant, Chin. J. Energet. Mater., 2015, 23, 89–98 Search PubMed.
- Y.-P. Chai and T.-L. Zhang, Advances on burning rate catalyzer of composite solid propellant at home and abroad, J. Solid Rocket Technol., 2007, 30, 44–47 CAS.
- X. Han and S. Li, Advances in catalytic function of fullerene materials, Prog. Chem., 2006, 18, 715–720 CAS.
- J. T. Abrahamson, N. Nair and M. S. Strano, Modelling the increase in anisotropic reaction rates in metal nanoparticle oxidation using carbon nanotubes as thermal conduits, Nanotechnol., 2008, 19, 195701 CrossRef PubMed.
- J. L. Sabourin, D. M. Dabbs, R. A. Yetter, F. L. Dryer and I. A. Aksay, Functionalized graphene sheet colloids for enhanced fuel/propellant combustion, ACS Nano, 2009, 3, 3945–3954 CrossRef CAS PubMed.
- X. Han, T. F. Wang, Z. K. Lin, D. L. Han, S. F. Li, F. Q. Zhao and L. Y. Zhang, RDX/AP-CMDB Propellants Containing Fullerenes and Carbon Black Additives, Def. Sci. J., 2009, 59, 284–293 CrossRef CAS.
- C. Bice Charles, Propellants with improved burning rate, US patent, US3018204A, 1962 Search PubMed.
- K. Ives Edwin, Catalyzed propellant compositions of high burning rate, US patent, US3240640A, 1966 Search PubMed.
- S. Verma and P. A. Ramakrishna, Activated charcoal A novel burn rate enhancer of aluminized composite propellants, Combust. Flame, 2010, 157, 1202–1210 CrossRef CAS.
- S. Verma and P. A. Ramakrishna, Investigations on activated charcoal, a burn-rate enhancer in composite solid propellant, J. Propul. Power, 2013, 295, 1214–1219 CrossRef.
- X. D. Li, R.-J. Yang and X.-X. Li, The functional additveis CNTs, conference paper in Propellants Explos. Proced., Xi'an Modern Chemistry Research Institute, 2004, pp. 228–231.
- R.-Z. Hu; Y. Wang; and J. Zheng, The application of nano-sized materials in solid propellants, The, 24th annual conference in Professional Committee of solid rocket propellant of Chinese Society of Astronautics, Xiang Fan, China, 2003, pp. 144–149.
- F.-Q. Zhao and J.-H. Yi, Preparation method of Cu2O-PbO/GO composites, Chinese patent, CN1003007947A, 2013 Search PubMed.
- Q.-L. Tan, W.-L. Hong and X.-B. Xiao, Preparation of Bi2O3/GO and its combustion catalytic performance on double-base propellant, Nanosci. Nanotechnol., 2013, 106, 22–27 Search PubMed.
- W.-L. Hong, H.-B. Shi and F.-Q. Zhao, Preparation method of Cu2O-Bi2O3/GO composites, Chinese patent, CN102895979A, 2013 Search PubMed.
- W.-L. Hong, Y.-F. Xue and F.-Q. Zhao, Preparation of Bi2O3/CNTs Composite and Its Combustion Catalytic Effecton Double-base Propellant, Chin. J. Explos. Propellants, 2012, 6, 7–11 Search PubMed.
- W.-L. Hong, X.-Y. Zhu and F.-Q. Zhao, Preparation of CuO/CNTs and its combustion catalytic activity on double-base propellant, Chin. J. Explos. Propellants, 2010, 336, 83–86 Search PubMed.
- J.-H. Liu, W. L. Hong and X.-Y. Zhu, A preparation method for Cu-Bi/CNTs composite coated by carbon particles, Chinese patent, CN101757927A, 2010 Search PubMed.
- Y. Liu, W. Jiang and J.-X. Liu, A study of catalyzing thermal decomposition and combustion of AP/HTPB propellant with nano Cu/CNTs, Acta Armamentarii, 2008, 299, 1029–1033 Search PubMed.
- X.-J. Zhang, W. Jiang, D. Song and J.-X. Liu, Preparation and catalytic activity of Ni/CNTs nanocomposites using microwave irradiation heating method, Mater. Lett., 2008, 621, 2343–2346 CrossRef.
- F. Li, W. Jiang, X. Guo, L. Liu, M. Li, W. Chen and S. Wu, Application of nanometer materials for solid propellants, Int. J. Energ. Mater. Chem. Propul., 2011, 10, 67–83 Search PubMed.
- C. D. Malec, N. H. Voelcker, J. G. Shapter and A. V. Ellis, Carbon nanotubes initiate the explosion of porous silicon, Mater. Lett., 2010, 64, 2517–2519 CrossRef CAS.
- K. S. Martirosyan, L. Wang and D. Luss, Tuning the gas pressure discharge of nanoenergetic materials by boron and carbon nanotubes additives, Technical Proceedings of the, 2011 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech, 2011, 1, 323–326 CAS.
- Z. Doorenbos, L. Groven, C. Haines, D. Kapoor and J. Puszynski, Novel pyrophoric substrates with a tunable energetic output, AIChE Annual Meeting, Conference Proceedings, 2010.
- M. R. Manaa, A. R. Mitchell, P. G. Garza, P. F. Pagoria and B. E. Watkins, Flash ignition and initiation of explosives-nanotubes mixture, J. Am. Chem. Soc., 2005, 127, 13786 CrossRef CAS PubMed.
- C. Zhang, Y. Wen and X. Xue, Self-enhanced catalytic activities of functionalized graphene sheets in the combustion of nitromethane Molecular dynamic simulations by molecular reactive force field, ACS Appl. Mater. Interfaces, 2014, 6, 12235 CAS.
- X. Xiang, S.-B. Xiang, Z. Wang, X. Wang and G. Hua, Photo-responsive behaviors and structural evolution of carbon-nanotube-supported energetic materials under a photoflash, Mater. Lett., 2012, 88, 27–29 CrossRef CAS.
- B. Jin, R. Peng, F. Zhao, J. Yi, S. Xu, S. Wang and S. Chu, Combustion effects of nitrofulleropyrrolidine on RDX-CMDB propellants, Propellants, Explos., Pyrotech., 2014, 39, 874–880 CrossRef CAS.
- M. Huagn and B.-S. Tan, Some strategies on the stabilization of high-energy explosives, Chin. J. Energet. Mater., 2014, 22, 134–135 Search PubMed.
- T. Lee, J. W. Chen, H. L. Lee, T. Y. Lin, Y. C. Tsai, S.-L. Cheng, S.-W. Lee, J.-C. Hu and L.-T. Chen, Stabilization and spheroidization of ammonium nitrate Co-crystallization with crown ethers and spherical crystallization by solvent screening, Chem. Eng. J., 2013, 225, 809–817 CrossRef CAS.
- A. J. Lang and S. Vyazovkin, Phase and thermal stabilization of ammonium nitrate in the form of PVP-AN glass, Mater. Lett., 2008, 62, 1757–1760 CrossRef CAS.
- Z. Zhang, B. Wang, Y. Ji, Z. Liu and Q. Liu, Thermal Stabilization of Ammonium Dinitramide ADN, Proceedings of the, International Autumn Seminar on Propellants, Explosives and Pyrotechnics, IASPEP, Theory Practice Energet. Mater., 2003, 5, 259–262 Search PubMed.
- M. Smeu, F. Zahid, W. Ji, H. Guo, M. Jaidann and H. Abou-Rachid, Energetic molecules encapsulated inside carbon nanotubes and between graphene layers DFT calculations, J. Phys. Chem. C, 2011, 115, 10985–10989 CAS.
- H. Abou-Rachid, A. Hu, V. Timoshevskii, Y. Song and L.-S. Lussier, Nanoscale high energetic materials: A polymeric nitrogen chain N8 confined inside a carbon nanotube, Phys. Rev. Lett., 2008, 100, 196401 CrossRef PubMed.
- W. Ji, V. Timoshevskii, H. Guo, H. Abou-Rachid and L. Lussier, Thermal stability and formation barrier of a high-energetic material N8 polymer nitrogen encapsulated in 5,5, carbon nanotube, Appl. Phys. Lett., 2009, 95, 021904 CrossRef.
- Y. Liu, W. Lai, T. Yu, Z. Ge and Y. Kang, Structural characteristics of liquid nitromethane at the nanoscale confinement in carbon nanotubes, J. Mol. Model., 2014, 20, 8 Search PubMed.
- E. M. Benchafia, C. Yu, M. Sosnowski, N. M. Ravindra and Z. Iqbal, Plasma synthesis of nitrogen clusters on carbon nanotube sheets, JOM, 2014, 66, 608–615 CrossRef CAS.
- Z. Wu, E. M. Benchafia, Z. Iqbal and X. Wang, N8 polynitrogen stabilized on multi-wall carbon nanotubes for oxygen-reduction reactions at ambient conditions, Angew. Chem., Int. Ed., 2014, 53, 12555–12559 CAS.
- Q.-L. Yan and S. Zeman, Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods a brief review, Int. J. Quantum Chem., 2013, 113, 1049–1061 CrossRef CAS.
- A. K. Pandey, Damage prediction of RC containment shell under impact and blast loading, Struct. Eng. Mech., 2010, 366, 729–744 CrossRef.
- P. A. Urtiew and C. M. Tarver, Shock initiation of energetic materials at different initial temperatures review, Combust., Explos. Shock Waves, 2005, 416, 766–776 CrossRef.
- M. H. Keshavarz, Overview on theoretical prediction of 3,6-bis-3,5-dinitro-1,2,4-triazolyl’-1,2,4,5-tetrazine as a high performance explosive, Chin. J. Energet. Mater., 2007, 155, 551–554 Search PubMed.
- S. M. Walley, J. E. Field and M. W. Greenaway, Crystal sensitivities of energetic materials a review, Mater. Sci. Technol., 2006, 22, 402–413 CrossRef CAS.
- C. J. Wu, T. Piggott, J. Yoh and J. Reaugh, Numerical Modeling of Impact Initiation of High Explosives, UCRL-TR-221760, June 1, 2006.
- B. Jin, R. Peng, S. Chu, Y. Huang and R. Wang, Study of the desensitizing effect of different [60]fullerene crystals on cyclotetramethylene tetranitramine HMX, Propellants, Explos., Pyrotech., 2008, 33, 454–458 CrossRef CAS.
- I. G. Assovskiy, Metallized SWCNT-promising way to low sensitive high energetic nanocomposites, Propellants, Explos., Pyrotech., 2008, 33, 51–54 CrossRef CAS.
- J. Xu, L.-Z. Wu, R.-Q. Shen, Y.-H. Ye and Y. Hu, Effects of dopants and confined windows on laser initiation sensitivity of explosives, Chin. J. Explos. Propellants, 2011, 34, 77–79 Search PubMed.
- B. Siegert, M. Comet, O. Muller, G. Pourroy and D. Spitzer, Reduced-sensitivity nanothermites containing manganese oxide filled carbon nanofibers, J. Phys. Chem. C, 2010, 114, 19562–19568 CAS.
- T. Zecheru, T. Rotariu, L.-C. Matache, A.-C. Sava, R.-M. Lungu, A. Voicu and L. Cosereanu, Synthesis and applications of 3-nitro-1,2,4-triazol-5-one based hybrid energetic compositions, Rev. Chim., 2014, 65, 1186–1189 CAS.
- H. Xu, J. Guo and K. S. Suslick, Porous carbon spheres from energetic carbon precursors using ultrasonic spray pyrolysis, Adv. Materials, 2012, 24, 6028–6033 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |