 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymeric near-infrared absorbing dendritic nanogels for efficient in vivo photothermal cancer therapy†
Maria
Molina
,
Stefanie
Wedepohl
and
Marcelo
Calderón
*
Institute of Chemistry and Biochemistry, Freie Universität Berlin, Takustrasse 3, 14195 Berlin, Germany. E-mail: marcelo.calderon@fu-berlin.de; Web: http://www.bcp.fu-berlin.de/chemie/calderon Fax: (+)49-030-838459368
First published on 22nd February 2016
Abstract
In recent years, several near-infrared light absorbing inorganic nanomaterials have been developed for photothermal therapy. However, their biological fate after injection limits their clinical utilization. In this work, we developed a novel polymeric near-infrared light absorbing material based on a biocompatible thermoresponsive nanogel that is semi-interpenetrated with polyaniline, a conjugated polymer with strong near-infrared absorbance. This polymeric nanocomposite generates heat after being irradiated by NIR light, thereby inducing a local hyperthermia that is used for photothermal cancer therapy in vitro and in vivo.
Introduction
Photothermal therapy (PTT) has emerged as promising anticancer therapy due to its high specificity and efficacy. PTT is based on the conversion of light into heat to generate a mild or severe local hyperthermia which leads to different results like cell sensitization or ablation, enhanced tissue penetration by temperature-induced increase in the tissue pore size, and drug triggered release.1 Near-infrared (NIR) light has the key advantage of being minimally absorbed by skin and tissues. Within the NIR window, which comprises wavelengths of about 650 to 900 nm, light can penetrate tissue in the order of hundreds of micrometers to centimeters. In the last decade, different inorganic materials with photothermal properties have been used successfully in anticancer therapy.2 Photoactive materials such as carbon nanotubes,3 magnetic nanomaterials,4 or gold nanoparticles,5 activated with NIR radiation, were shown to be effective in the hyperthermic treatment of different cancers.6 However, there are still some issues related to biocompatibility and biodegradability of those inorganic nanomaterials that limit their applicability.7 As an approach to solve this problem, conjugated polymers have been studied as photothermal agents in the last few years. NIR absorbing, conjugated polymers show excellent photostability, good biocompatibility, and high photothermal conversion.8–10 Because their major drawback is the low solubility in water, different strategies have been followed to increase their solubility, whereby stabilization of the conjugated polymeric nanoparticles with water-soluble polymers has been the most studied one.11 Until now, semi-interpenetration (sIPN) within hydrophilic nanosized networks has not been considered.In order to create well-defined, monodisperse, and stable nanostructures on the molecular level, highly branched dendritic polymers have been successfully employed over the last couple of decades. There has been much interest in the fabrication of dendritic nanogels (NGs), which are high molecular weight crosslinked polymers that combine the characteristics of dendritic polymers with crosslinked macroscopic gels to yield soluble particles with a useful size range between 20 and 200 nm.12 Temperature-sensitive NG systems are versatile in design, their phase transition temperatures can be tuned, and they feature passive targeting ability.13 By combining thermoresponsive polymers with dendritic polyglycerol (dPG), our group has developed a series of dendritic thermoresponsive NGs that are suitable for biomedical applications.14–16 The presence of dPG as a macro-crosslinker, attached via a biodegradable ester linkage, provides high biocompatibility and hydrophilicity and, more importantly, can avoid the precipitation of the NGs in the collapse state above the transition temperature (Tp). In an effort to further control the existing temperature-responsive systems, current studies have combined temperature with other stimuli such as pH and light.17 NIR absorbers have been combined with thermoresponsive gels in order to use NIR light as an external trigger for structural changes in thermoresponsive materials.18,19
Herein, we have synthesized a thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) based NG crosslinked with dPG and semi-interpenetrated with polyaniline (PANI) which generates heat after being irradiated by NIR light, thereby inducing a local hyperthermia (Fig. 1) for its use in PTT. At the body temperature, the shape of the dendritic NG changes because of its thermoresponsive properties, which triggers its shrinkage, but, due to the presence of dPG, the collapsed NG still has good suspension and does not precipitate. In this hydrophobic state, as observed in previous reports, the collapsed NGs can be expected to more easily accumulate in cells and/or penetrate tissues.20
 | ||
| Fig. 1 Schematic representation of the synthesis of a semi-interpenetrating NIR-thermosensitive NG and its application in PTT. | ||
Results and discussion
NGs based on PNIPAM crosslinked with 33 wt% of dPG (PNIPAM-dPG) were synthesized following the procedure previously reported by our group.14 In a further step, PNIPAM-dPG/PANI NGs were synthesized following a two-step semi-interpenetration procedure. Briefly, dry NGs were swollen in a solution of aniline and polymerization was started in situ by the addition of ammonium persulfate. To establish the conditions for the in situ polymerization of PANI inside the NGs to form a nanocomposite, different reaction conditions were screened (Table 1).| NG (mg mL−1) | Aniline (M) | Polymerization time (min) | Results |
|---|---|---|---|
| 10 | 0.05 | 30 | Oligomers |
| 10 | 0.1 | 30 | PANI dispersion |
| 10 | 0.1 | 7 | Not reproducible |
| 10 | 0.5 | 30 | PANI precipitate |
At low concentrations of aniline (0.05 M) or short polymerization times (7 min), oligomers of aniline were obtained. When a high concentration of aniline was used, the PANI started to precipitate out of the solution. Using 0.1 M of aniline and 30 min of polymerization time, a stable PANI dispersion with a reproducible size and thermoresponsive properties was obtained (PNIPAM-dPG/PANI).
The in situ polymerization of aniline was confirmed by 1H-NMR, FTIR, and UV-Vis measurements (ESI†). After the semi-interpenetration of the NIR absorbing polymer into the NGs, two new bands corresponding to the PANI appeared in the FTIR spectrum with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching at 1590 cm−1 and the C–N stretching absorption band at 1310 cm−1, while all the absorption bands of the PNIPAM and dPG remained the same.14 Moreover, the 1H-NMR spectrum confirmed the presence of PANI in the composite with a peak at ∼7.4 ppm corresponding to the aromatic protons. On the other hand, after the in situ polymerization of aniline inside the NG, a typical UV-Vis spectrum of PANI was observed. The spectra changed with the pH in accordance with those previously reported for PANI films in the emeraldine state.21 This result indicates that the PANI chains inserted into the NG were unaltered and responsive to changes in the solution environment.
C stretching at 1590 cm−1 and the C–N stretching absorption band at 1310 cm−1, while all the absorption bands of the PNIPAM and dPG remained the same.14 Moreover, the 1H-NMR spectrum confirmed the presence of PANI in the composite with a peak at ∼7.4 ppm corresponding to the aromatic protons. On the other hand, after the in situ polymerization of aniline inside the NG, a typical UV-Vis spectrum of PANI was observed. The spectra changed with the pH in accordance with those previously reported for PANI films in the emeraldine state.21 This result indicates that the PANI chains inserted into the NG were unaltered and responsive to changes in the solution environment.
Atomic force microscopy (AFM) and cryogenic transmission electronic microscopy (cryoTEM) showed spherical individual nanoparticles and some aggregates. In the AFM micrographs (Fig. 2A) we observed that the NGs appear like core–shell structures. This could be explained by the synthetic procedure in agreement with the results shown by Zhang et al.22 At the beginning of the reaction, due to the interaction of aniline with PNIPAM, the concentration of aniline inside the NG was much higher and the polymerization started inside the network. After that, the reaction mainly took place on the surface of the NG. Thus, the particles underwent structural changes from PNIPAM-dPG to sIPN and finally to the sIPN–PANI core–shell throughout the reaction.
 | ||
| Fig. 2 Characterization of PNIPAM-dPG/PANI NGs. (a) AFM, (b) cryoTEM, and (c) hydrodynamic diameter (Dh) vs. temperature (T) as measured by DLS. | ||
Dynamic light scattering (DLS) measurements revealed an increment of 100 nm in the size of the bare NGs after the semi-interpenetration, while the Tp remained the same (∼34 °C) (Fig. 2C). Above the Tp, the size of the composites decreased from 220 nm to 150 nm. To further prove the colloidal stability of the NGs above the Tp, nanoparticle tracking analysis (NTA) was performed, which made it possible to count the particles at different temperatures. In the case of the PNIPAM-dPG/PANI NGs, the concentration of the particles was constant, ∼1.2 × 109 particles per mL at 25 and 42 °C, which demonstrated that the NGs had neither aggregated nor precipitated. Moreover the amount of the incorporated PANI was studied by UV-Vis and was found to be 0.9 mg of PANI per mg of PNIPAM-dPG.
As the material was supposed to be applied in PTT, we studied the increase in the temperature of the nanocomposites under NIR irradiation. Fig. 3 shows how the increase in temperature depended on the concentration of the nanocomposite and irradiation time. When only water was irradiated, the temperature increased by a maximum of 3 °C while with 20 μg mL−1 of nanocomposites, the temperature increased by 8 °C after 10 min of irradiation, which is enough for mild hyperthermia conditions.23
After studying the outstanding physicochemical properties of the sIPN NGs, further biological characterization was performed in order to use these systems in vivo. The cytotoxicity of PNIPAM and PNIPAM-dPG/PANI on A2780 human ovarian carcinoma cells was determined by the MTT assay. The A2780 cells that had been incubated for 48 hours with PNIPAM-dPG or PNIPAM-dPG/PANI showed no reduction in viability up to 0.1 mg mL−1 and relative viabilities over 50% at a concentration of 1 mg mL−1 (Fig. 4a). The uptake of PNIPAM-dPG/PANI into cells was indirectly monitored by determining the concentration in the cell culture supernatant (Fig. 4b). After 64 h, the concentration was about 30 μg mL−1 less than that initially incubated. In addition, we observed that the cells were dark colored after trypsinization and washing with phosphate buffered saline (PBS) or media (Fig. 4c). The color remained after repeated trypsinization, reculturing, and washing which confirmed that the compound was located inside the cells.
For the study of the photothermal effect in vitro, the cells were released from the cell culture flask, washed, and centrifuged into PCR tubes that allowed a rapid transmission of heat through the walls of the tubes. After removing most of the media, the pellet of 1 million cells had about the volume of a 5 μL drop, which resembled a small tumor tissue. This cluster was irradiated with a NIR laser (785 nm) under constant monitoring with a thermal camera to ensure that the temperature did not exceed 42 °C ± 1 °C. As control for the effect of hyperthermia alone, parallel samples were incubated in a water bath at 42 °C. Irradiation or heating was performed either one time for 5 min or four times for 5 min each with the PNIPAM-dPG/PANI incubated cells as well as untreated cells. The viabilities were measured by the MTT assay 24 h after re-seeding the treated cells into 96-well plates (Fig. 5). The A2780 cells which had taken up PNIPAM-dPG/PANI irradiated with a NIR laser showed a markedly reduced viability after a single treatment for 5 min, compared to 80% of the untreated control cells that had been irradiated for the same time. Moreover, 4 irradiation cycles resulted in a complete loss of the cell viability, whereas the control cells again were not compromised. The effect of hyperthermia for 5 min alone was not different from the controls; we only saw a decrease in viability to about 60% when the hyperthermia was repeated 4 times. As a control for the stress from centrifuging and standing as a cell cluster for a prolonged period of time outside of the incubator, another cell sample was treated under the same conditions but neither heated nor irradiated. The viability of these control cells was not markedly affected and was comparable to the untreated cells in the other series (Fig. 5, columns labeled “no heat”). This result demonstrated the efficacy of the focus heat generation compared to the whole-body hyperthermia.
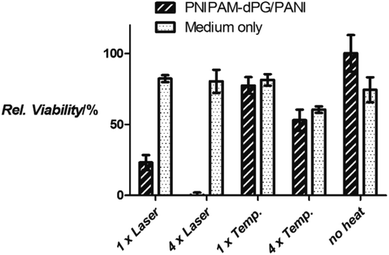 | ||
| Fig. 5 Relative viabilities of A2780 cells treated with PNIPAM-dPG/PANI or untreated, irradiated with NIR laser or heated to 42 °C (“Temp.”). | ||
This promising result encouraged us to further use these systems for localized hyperthermia therapy. Therefore the in vivo tolerability of the PNIPAM-dPG/PANI NGs was studied in female nude (NMRI, nu/nu) mice in a two-step protocol. The mice were first treated with a single injection of increasing doses of PNIPAM-dPG/PANI from 10 to 100 mg kg−1 and the body weight was measured for a period of 3 weeks. The results showed that the mice tolerated up to 100 mg kg−1 without any significant signs of toxicity or drug related body weight loss when given intravenously as a single dose (ESI†). Next, 3 mice were treated with the maximum injected dose, 100 mg kg−1 once per day during 5 consecutive days. The mice were observed for a follow-up period of 18 days. The treatment resulted in blue-stained ear ends and tails, but the color washed out within two days. No significant toxic effects were observed during this period (accumulated dose: 500 mg kg−1) (ESI†).
Once the high in vivo tolerability of this encouraging material was proved, we proceeded to study the therapeutic effect in vivo. A2780 tumor transplants were obtained from tumors, cut into small pieces and transplanted subcutaneously into female nude mice (Taconic). The mean tumor volume at this time was 0.086–0.088 cm3. The mice were injected intratumorally or intravenously with a single dose of 10 or 100 mg kg−1 of PNIPAM-dPG/PANI, respectively, or PBS and PNIPAM-dPG as controls. At the indicated time points, the mice were narcotized and the tumors were exposed to NIR laser light at a distance of about 5 cm for 5 min at a maximum radiation power of 500 mW. The tumor volume and body weight were then monitored two to three times a week until day 39. Fig. 6 summarizes the tumor volume change [treated to control (T/C)] resulting from the different treatments.
There was no or only a weak inhibitory effect of the NGs without NIR irradiation as well as with the control PNIPAM-dPG with irradiation. In contrast, intratumoral injection of PNIPAM-dPG/PANI followed by NIR treatment resulted in a tumor growth inhibition by 55.3%. When injected intravenously, the therapeutic effect was expected to depend on the successful accumulation of the particles in the tumor region in sufficient concentrations. The NGs were injected intravenously and the tumor was irradiated after 6, 24, or 48 h. When NIR treatment was done after 6 h, tumor growth inhibition was about 30%, and after 48 h, only ∼8%. Tumor growth inhibition was optimal when irradiating 24 h after intravenous injection, resulting in a tumor growth inhibition of ∼52.2%, which was nearly as effective as intratumoral injection. Our results indicated that the passive accumulation of the NGs in the tumor was sufficiently concentrated to allow a successful treatment.
Conclusions
In summary, we have formulated NIR light sensitive organic nanoparticles based on PNIPAM-dPG/PANI as a novel photothermal agent and used for highly effective in vivo hyperthermia treatment of cancer cells. By semi-interpenetrating conjugated polymers in thermoresponsive polymeric NGs, a nanocomposite was obtained with excellent compatibility in physiological environments. This platform provides an opportunity to load the NG with anticancer drugs as well as to attach targeting moieties to the dPG. Thus, these nanoparticles appear promising for cancer therapy. Moreover, the studies in vivo showed that the mice tolerated up to 100 mg kg−1 of PNIPAM-dPG/PANI, given at 5 consecutive days (accumulated dose: 500 mg kg−1) without any significant sign of toxicity. Furthermore, the therapeutic effect study indicated that the combination of systemic treatment with PNIPAM-dPG/PANI and NIR exposure resulted in a higher sensitivity of the tumors compared to the controls alone or with NIR. Based on the simplicity and the low cost of synthesis together with the low cytotoxicity, high tolerability, and promising therapeutic effect in vivo, PNIPAM-dPG/PANI has been shown to be an excellent candidate for further studies. This work shows the first approach to use PNIPAM-dPG/PANI in PTT; additional strategies to enhance targeting and synergetic PTT combined with chemotherapy are currently being investigated by our group.Acknowledgements
We gratefully acknowledge financial support from the Bundesministerium für Bildung und Forschung (BMBF) through the NanoMatFutur award (13N12561). M. Molina acknowledges financial support from the Alexander von Humboldt foundation. We gratefully thank Dr P. Winchester for proofreading the manuscript and Dr Böttcher for cryo-TEM measurements.References
- K. F. Chu and D. E. Dupuy, Nat. Rev. Cancer, 2014, 14, 199–208 CrossRef CAS PubMed.
- D. Jaque, L. Martinez Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martin Rodriguez and J. Garcia Sole, Nanoscale, 2014, 6, 9494–9530 RSC.
- R. Singh and S. V. Torti, Adv. Drug Delivery Rev., 2013, 65, 2045–2060 CrossRef CAS PubMed.
- C. S. Kumar and F. Mohammad, Adv. Drug Delivery Rev., 2011, 63, 789–808 CrossRef CAS PubMed.
- W.-S. Kuo, C.-N. Chang, Y.-T. Chang, M.-H. Yang, Y.-H. Chien, S.-J. Chen and C.-S. Yeh, Angew. Chem., Int. Ed., 2010, 49, 2711–2715 CrossRef CAS PubMed.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- M. Molina, M. Asadian-Birjand, J. Balach, J. Bergueiro, E. Miceli and M. Calderon, Chem. Soc. Rev., 2015, 44, 6161–6186 RSC.
- L. Xu, L. Cheng, C. Wang, R. Peng and Z. Liu, Polym. Chem., 2014, 5, 1573–1580 RSC.
- A. Silvestre Bongiovanni, M. Molina, C. Rivarola, M. Kogan and C. Barbero, Nanotechnology, 2014, 25, 495602 CrossRef PubMed.
- J. Yang, J. Choi, D. Bang, E. Kim, E. K. Lim, H. Park, J. S. Suh, K. Lee, K. H. Yoo, E. K. Kim, Y. M. Huh and S. Haam, Angew. Chem., Int. Ed., 2011, 50, 441–444 CrossRef CAS PubMed.
- N. E. Monge, M. a. C. Miras and C. s. A. Barbero, J. Comb. Chem., 2010, 12, 814–817 CrossRef CAS PubMed.
- M. Calderón, M. A. Quadir, S. K. Sharma and R. Haag, Adv. Mater., 2010, 22, 190–218 CrossRef PubMed.
- A. V. Kabanov and S. V. Vinogradov, Angew. Chem., Int. Ed., 2009, 48, 5418–5429 CrossRef CAS PubMed.
- J. C. Cuggino, C. I. Alvarez I, M. C. Strumia, P. Welker, K. Licha, D. Steinhilber, R.-C. Mutihac and M. Calderon, Soft Matter, 2011, 7, 11259–11266 RSC.
- M. Giulbudagian, M. Asadian-Birjand, D. Steinhilber, K. Achazi, M. Molina and M. Calderon, Polym. Chem., 2014, 5, 6909–6913 RSC.
- M. Molina, M. Giulbudagian and M. Calderón, Macromol. Chem. Phys., 2014, 215, 2414–2419 CrossRef CAS.
- M. Bikram and J. L. West, Expert Opin. Drug Delivery, 2008, 5, 1077–1091 CrossRef CAS PubMed.
- M. A. Molina, C. R. Rivarola, M. C. Miras, D. Lescano and C. A. Barbero, Nanotechnology, 2011, 22, 245504 CrossRef CAS PubMed.
- T. Kawano, Y. Niidome, T. Mori, Y. Katayama and T. Niidome, Bioconjugate Chem., 2009, 20, 209–212 CrossRef CAS PubMed.
- M. Witting, M. Molina, K. Obst, R. Plank, K. M. Eckl, H. C. Hennies, M. Calderón, W. Frieß and S. Hedtrich, Nanomedicine, 2015, 11, 1179–1187 CrossRef CAS PubMed.
- W. S. Huang and A. G. MacDiarmid, Polymer, 1993, 34, 1833–1845 CrossRef CAS.
- B. Zhang, S. Sun and P. Wu, Soft Matter, 2013, 9, 1678–1684 RSC.
- C. S. S. R. Kumar and F. Mohammad, Adv. Drug Delivery Rev., 2011, 63, 789–808 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr07587d |
| This journal is © The Royal Society of Chemistry 2016 |



