Graphene and graphene oxide for desalination
Yi
You
a,
Veena
Sahajwalla
a,
Masamichi
Yoshimura
b and
Rakesh K.
Joshi
*a
aCentre for Sustainable Materials Research and Technology, School of Materials Science and Engineering, University of New South Wales, NSW 2052, Australia. E-mail: veena@unsw.edu.au; r.joshi@unsw.edu.au
bSurface Science Laboratory, Toyota Technological Institute, Nagoya 468-8511, Japan
First published on 20th November 2015
Abstract
There is a huge scope for graphene-based materials to be used as membranes for desalination. A very recent study has confirmed that 100% salt rejection can be achieved for commonly used ions by utilizing single layer nonporous graphene. However, the cost effective fabrication procedure for graphene oxide membranes with precise control of pore size can offer a practical solution for filtration if one can achieve 100% percent salt rejection.
Desalination is defined as the process that isolates pure water from seawater and it is proposed to be an effective solution to water scarcity. In any desalination process, the role of a membrane is crucial. Though various materials have been exploited as membranes for desalination, such as polymers and ceramics (e.g. organosilica, zeolites),1–3 graphene-based materials have recently emerged as potential candidates with excellent desalination characteristics.4–9 Pristine graphene is extremely impermeable to liquid and gases, therefore the possibilities to create water pathways through the material are either the stacking of graphene oxide sheets (Fig. 1a)10–14 or the generation of nanopores in monolayer graphene (Fig. 1b).6,15
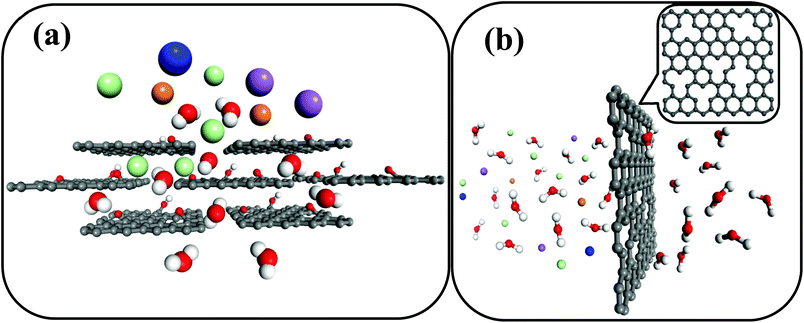 | ||
| Fig. 1 (a) A stacked graphene oxide layer membrane in which water molecules permeate through the developed nanochannels between each graphene oxide layer, while hydrated salt ions are blocked.10 (b) A nanoporous graphene membrane, in which water molecules pass through deliberately created nanopores in the graphene with a specific size, hindering the permeation of large hydrated salt ions.6 (C: black, H: white, O: red, K+: blue, Na+: purple, Mg2+: orange, Cl−: cyan). | ||
The use of graphene oxide as a feasible membrane was firstly developed by Nair et al.,10 and they discovered that stacking the graphene oxide film allows a unique water permeation pathway but selectively hinders the motion of gases and non-aqueous solutions.10 After that, there were several attempts towards the development of graphene oxide based membranes.4,5,11–14,16 Meanwhile, graphene with artificially created nanosized holes finds application as a molecular and ionic sieving membrane, thanks to the development of technology for the fabrication of high quality graphene with a large size.17 After some nanopores are created, the inherent hydrophobia of the parent graphene can exhibit a capillary force for water permeation.6 Recent discoveries have addressed the capability of nanoporous graphene as a reverse osmosis membrane in terms of both mechanical aspects and desalination performances, such as salt rejection and water flux.6,18 Surwade et al. have experimentally demonstrated the possibility of desalination using single layer graphene with a few controllable holes15 and proved the theoretical predictions.6 This is a major breakthrough for the application of graphene in desalination.15 The authors engineered the nanopores by treating the CVD grown graphene layers with oxygen plasma with the nanoporisty being varied by altering the oxygen exposure time, and the nanoporous graphene was then tested for desalination.15 This article has put forth several possibilities to perform more desalination experiments using the membranes for large scale desalination.15 We certainly believe that there is a huge scope for further research on the utilization of single layers with artificially created defects as pores for desalination. The current density measurements performed by the authors are indeed a good tool to qualitatively understand the possible permeation mechanisms. However, at this point, experiments related to the chemical analysis of solutions in feed and permeate are essential to estimate the exact rejection ratios for the application of porous graphene in large scale desalination. One must take a quantity of solutions from feed and permeate at regular intervals in order to perform the chemical analysis of the solutions with the highest possible detection limit. Based on the past investigations on graphene-based desalination membranes, herein we provide brief comparisons between nanoporous graphene and graphene oxide layers employed for desalination.
Nanoporous graphene is expected to excel as a potential candidate due to the following characteristics. Firstly, the sub-nanometre sized pores in the graphene can be defined with high precision by the oxidative etching method and ion bombardment.15,17 Secondly, nanoporous graphene demonstrates the high possibility to achieve 100% salt rejection because the only translocation pathways for water molecules and ions are the nanopores.15 Third, the water permeation rate can be governed by managing the porosity on the graphene. Fourth, various functional groups can be employed to terminate the bare carbon atoms in the holes, thereby diversifying the desalination performance.4,6 However, some disadvantages and experimental challenges are also associated with the nanoporous graphene membrane for desalination. Despite advancements in the methods for producing pores in graphene, it is an extremely daunting challenge to achieve a high density of holes with a narrow size distribution.4 Besides, the tiny nanoscale holes generated in graphene restrict the amount of water permeation. Even if high water permeability is achieved, the high density of holes in the graphene may result in unexpected reductions in mechanical properties or even the destruction of the whole structure. Additionally, the complexity of obtaining continuous large-area perfect graphene and subsequent generation of holes limit the scaling up of production. Furthermore, the whole process of composing the nanoporous graphene should be conducted with extreme caution. Last but not least, the design of high quality graphene, the oxidative etching technique and ion bombardment are expensive methods.
Compared to nanoporous graphene, the stacking of graphene oxide membranes has been proposed to exhibit superiorities in the following aspects. Firstly, unlike the artificially created holes in graphene, graphene oxide membranes rely on tortuous routes in the interconnected nanochannels that serve as a water permeation route.19,20 These nanochannels are actually the spaces between each graphene oxide layer, which can be regulated through the creation of stabilizing force between the layers.11,19 Furthermore, the high surface area in the twisted nanochannels ensures that a high water flux can be achieved. Secondly, the preparation of graphene oxide is cost effective in terms of both raw materials and methodology. For instance, the typical starting material for graphene oxide is graphite, which is abundant and cheap.19 Additionally, the entire synthesis process can be performed in solution. As well as the low cost, this wet chemical synthesis can be performed at the expense of minimum energy and it is facile to control.13 Further, the solution synthesis offers the possibility of surface functionalization with a variety of groups for an efficient catalyst attachment, thereby imparting the graphene oxide membranes with diverse functions, such as sterilization. Besides, these diverse functional groups also provide a foundation for composite constructions such as graphene oxide–polymer composites, which are found to reinforce the mechanical stability and further enhance the membrane properties.16 In addition, the oxygenated functional groups endow graphene oxide with hydrophilicity and pH sensitivity.19,21 Since the hydrophobic region regulates the water flow, the functional group density should be well controlled during the fabrication. The linear distribution of the hydrophobic region will ensure fast water permeation.11 Moreover, despite some defects being introduced during the preparation of the graphene oxide, the stacked layer structure will counter this effect and prevent any unexpected diffusion through these sites.19 On the other hand, as a desalination membrane, stacking graphene oxide necessitates further improvements. The spacing (mesh size) in graphene oxide membranes is the critical parameter that determines the desalination property,20 therefore it should be properly controlled. The uncertainty in the chemical synthesis may introduce several unwanted groups and cause unpredictable swelling effects. Therefore, the challenge of handling functional groups needs to be addressed. On a final note, achieving 100% salt rejection is a daunting task for graphene oxide membranes.11
In summary, graphene-based materials exhibit unlimited potential to be used as membranes for desalination. Recent discovery15 has proved that a membrane with nanoporous graphene can exhibit 100% salt rejection. However, compared to nanoporous graphene, the simple and cost-effective fabrication of graphene oxide membranes with precise control of pore size can be a practical solution for filtration, providing that the salt rejection could match that of nanoporous graphene membranes.
Acknowledgements
This work was supported by the Australian Research Council Laureate Fellowship awarded to Prof. Veena Sahajwalla. RKJ acknowledges Start-Up Research Grant (PS38305) from the University of New South Wales.References
- K. P. Lee, T. C. Arnot and D. Mattia, A review of reverse osmosis membrane materials for desalination–Development to date and future potential, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- Y. Chua, et al., Mesoporous organosilica membranes: Effects of pore geometry and calcination conditions on the membrane distillation performance for desalination, Desalination, 2015, 370, 53–62 CrossRef CAS.
- B. Zhu, et al., Application of robust MFI-type zeolite membrane for desalination of saline wastewater, J. Membr. Sci., 2015, 475, 167–174 CrossRef CAS.
- M. Hu and B. Mi, Enabling graphene oxide nanosheets as water separation membranes, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- Y. Han, Z. Xu and C. Gao, Ultrathin graphene nanofiltration membrane for water purification, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- D. Cohen-Tanugi and J. C. Grossman, Water Desalination across Nanoporous Graphene, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- D. Konatham, et al., Simulation Insights for Graphene-Based Water Desalination Membranes, Langmuir, 2013, 29, 11884–11897 CrossRef CAS PubMed.
- E. N. Wang and R. Karnik, Water desalination: Graphene cleans up water, Nat. Nanotechnol., 2012, 7, 552–554 CrossRef CAS PubMed.
- A. K. Mishra and S. Ramaprabhu, Functionalized graphene sheets for arsenic removal and desalination of sea water, Desalination, 2011, 282, 39–45 CrossRef CAS.
- R. Nair, et al., Unimpeded permeation of water through helium-leak–tight graphene-based membranes, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- R. Joshi, et al., Precise and ultrafast molecular sieving through graphene oxide membranes, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- P. Sun, et al., Selective Trans-Membrane Transport of Alkali and Alkaline Earth Cations through Graphene Oxide Membranes Based on Cation–π Interactions, ACS Nano, 2014, 8, 850–859 CrossRef CAS PubMed.
- P. Sun, et al., Selective ion penetration of graphene oxide membranes, ACS Nano, 2012, 7, 428–437 CrossRef PubMed.
- A. Nicolaï, B. G. Sumpter and V. Meunier, Tunable water desalination across graphene oxide framework membranes, Phys. Chem. Chem. Phys., 2014, 16, 8646–8654 RSC.
- S. P. Surwade, et al., Water desalination using nanoporous single-layer graphene, Nat. Nanotechnol., 2015, 10, 459–464 CrossRef CAS PubMed.
- H. M. Hegab and L. Zou, Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification, J. Membr. Sci., 2015, 484, 95–106 CrossRef CAS.
- S. P. Koenig, et al., Selective molecular sieving through porous graphene, Nat. Nanotechnol., 2012, 7, 728–732 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Mechanical Strength of Nanoporous Graphene as a Desalination Membrane, Nano Lett., 2014, 14, 6171–6178 CrossRef CAS PubMed.
- B. Mi, Graphene oxide membranes for ionic and molecular sieving, Science, 2014, 343, 740–742 CrossRef CAS PubMed.
- D. W. Boukhvalov, M. I. Katsnelson and Y. W. Son, Origin of anomalous water permeation through graphene oxide membrane, Nano Lett., 2013, 13, 3930–3935 CrossRef CAS PubMed.
- B. Ganesh, A. M. Isloor and A. Ismail, Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane, Desalination, 2013, 313, 199–207 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |
