Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diterpenoids of terrestrial origin
James R.
Hanson
Department of Chemistry, University of Sussex, UK
First published on 28th July 2016
Abstract
Covering January to December 2015. Previous review; Nat. Prod. Rep., 2015, 32, 1654–1663.
This review covers the isolation and chemistry of diterpenoids from terrestrial as opposed to marine sources and includes labdanes, clerodanes, abietanes, pimaranes, kauranes, cembranes and their cyclization products. There are 214 references.
1 Introduction
This report covering the period January to December, 2015, follows the pattern of its predecessors1,2 and includes the identification and chemistry of diterpenoids of terrestrial as opposed to marine origin. The latter are covered in the articles on marine natural products.3 Traditional Chinese medicines have continued to be a treasure trove of novel diterpenoids. Many of these warrant a further examination of their structure–biological activity relationships in order to identify the pharmacophores. There are a number of diterpenoids that are sufficiently abundant to provide suitable starting materials for partial synthesis. Several studies in this context have been reported during the year including work on the abietane diterpenoids. This has been reviewed.4,5Advances in the synthesis of multi-functionalized decalins,6 the bicyclo[3,2,l]-octane system of the ent-kauranoids,7,8 the atisane diterpenoids,9 the neodolastanes,10 the pseudopterosin aglycones,11 and vinigrol,12 together with the use of dehydrogenation to access diterpenoid degradation products that are found in the environment,13 have all been reviewed. Other articles on the diterpenoid composition of Pinus sibirica14 and various aspects of the diterpene biosynthetic synthases including their genetics, have appeared.15,16 Genetic studies have led to the identification of diterpene gene clusters17 and of silent pathways that can be activated to produce diterpenoids.18
2 Acyclic and related diterpenoids
The syntheses of C-8, C-9 and C-10 deuteriated geranylgeraniols have been reported.193 Bicyclic diterpenoids
3.1 Labdanes
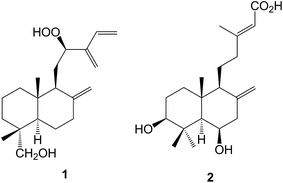
Labdanolic acids have been identified as biomarkers for the botanical origin of French ambers20 whilst copalic acid and its relatives have been associated21 with the biological activity of the resins from Copaifera species. The lanceolatanols, including some rare hydroperoxides such as the 12-hydroperoxide 1, have been isolated22 from the Chinese fir, Cunninghamia lanceolata (Cupressaceae) which is grown in plantations for timber production. The lanceolatins A–G (e.g. A, 2) are a group of labdanes and abietanes which were obtained23 from Cephalotaxus lanceolata (Cephalotaxaceae). Some of the abietanes described in this paper were formulated without comment, as a dienone tautomer of a phenol.A number of the labdanes which have been found in members of the Zingiberaceae, e.g. Alpinia japonica,24Curcuma longa25 and Hedychium longipetalum26 such as the hedylongnoids A–C (e.g. A, 3), inhibit the production of nitric oxide. The paraguhenryisins A–D (e.g. C, 4) which were isolated27 from the capitate glandula trichomes of Paragutzlaffi henryi (Acanthaceae), have phytotoxic properties and may provide a defensive measure against other invasive plants. The absolute configuration at C-14 of the labdanes from Physalis nicandroides (Solanaceae) has been established.28
Labdane glycosides have been reported29 as constituents of Diplopterygium rufopilosum (Gleicheniaceae) whilst examination of the seeds and leaves of Colophospermum mopane [Fabaceae (Leguminosae)]30 and Leonotis leonurus (Lamiaceae)31 has yielded some further labdanes and clerodanes. Continued examination of Leonurus japonicus gave leojaponin D32 whilst the macranthins (e.g. A, 5) were anti-inflammatory labdanes which were obtained33 from L. macranthus. Further studies on white horehound (Marrubium vulgare, Lamiaceae) afforded34 12(S)-hydroxymarrubiin. The structure 6 has been assigned35 to cinereanoid A which was isolated from Roylea cinerea (Lamiaceae) whilst the vitexolides A–E were similar anti-bacterial γ-hydroxy-αβ-unsaturated 15→16-lactones which were obtained36 from Vitex vestita (Lamiaceae).
Isodon species (Lamiaceae) are renowned for the tetracyclic diterpenoid constituents of their leaves. However examination of the roots of I. adenantha afforded37 the labdanes, adenanthic acids A and B (e.g. A, 7) and the adenanthosides A–C whilst two other labdanes were obtained38 from I. yuennanensis.
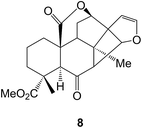
A large number of labdanes have been detected in Chinese liverworts including the haplomintrins (e.g. A, 8) from Haplomitrium minioides39 in which a cyclobutane ring has been formed by the possible photochemical addition of a side-chain furan across the double bond of a 7,8-en-6-one. Other examples of labdanes which have been isolated include the ptychantins P and R from Ptychanthus striatus40 and the scapairrins A–Q from Scapania irrigua.41
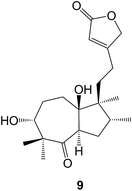
Various methods for the recovery of useful compounds from Stevia rebaudiana (Asteraceae) have been reviewed.42 The fermentation of S. rebaudiana with the yeast Saccharomyces cerevisiae has led43 to the formation of some modified sterebins arising from the selective epoxidation and hydration of the 14,15-double bond. Examination of the Taiwanese shrub, Callicarpa randaiensis (Verbenaceae) in the search for anti-inflammatory agents afforded44 the randainins A–D. As an alternative to the biosynthesis suggested in the original paper, their structures (e.g. A, 9) may arise by the oxidative cleavage of a 5,10-double bond of a halimane followed by aldol condensations of the 5,10-diketone to form the trans 7/5 and 5/7 ring systems of these diterpenoids.
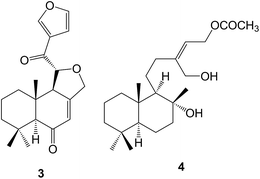
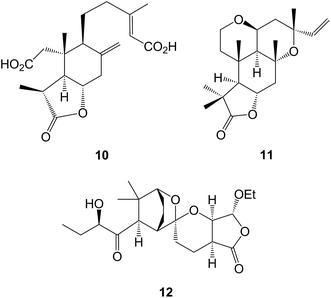
The cleavage of ring A has been encountered in a number of diterpenoids including labdanes. A 3-nor-2,3-secolabdane structure 10 has been assigned45 to penioxalicin which was obtained from the fungus, Penicillium oxalicum and to paecilomycine A from an insect pathogenic fungus, Paecilomyces sp.46 Further examination47 of the stems of the highly poisonous mangrove plant, Excoecaria agallocha (Euphorbiaceae) gave excolide 11 in a study which also led to a revision of the structure of rhizophorin A. The structure of the spiro-ketal, leonuketal 12 obtained48 from Leonurus japonicus, may be derived by a retro-Prins cleavage of the 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-bond in a 7,9-dihydroxylabdane followed by a cyclization involving the 3-hydroxyl group, the 9-ketone and C-15.
9-bond in a 7,9-dihydroxylabdane followed by a cyclization involving the 3-hydroxyl group, the 9-ketone and C-15.
Derivatives of the readily available labdanes such as polyalthic acid49 and copalic acid50 have been evaluated for the treatment of various diseases. The merosesquiterpenes, neopterosiquinones A and B have been synthesized51 from trans-communic acid. The anti-fungal activity of sclareol52 and some derivatives53 has been examined. Sclareol has been used54 as the starting material for the synthesis of the sesterterpenes, luffarin L and 16-epiluffarin and for a biomimetic synthesis of two salmahyrtisanes.55 Work has continued56–58 to prepare derivatives of andrographolide in order to study structure–biological activity relationships. The enzyme systems responsible for the biosynthesis of 13(R)-manoyl oxide have been transferred59 from Sitka spruce to a cyanobacterium, Synechocystis sp.
3.2 Halimanes and clerodanes
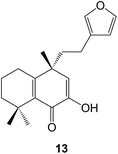
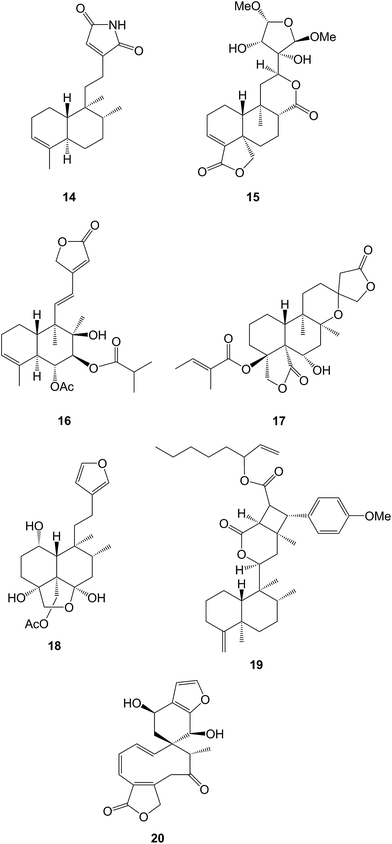
18-Hydroxy-ent-halima-1(10),13(E)-dien-15-oic acid has been isolated60 from Hymenaea stigonocarpa (Fabaceae) whilst investigations of the anti-microbial activity of Vellozia kolbekii (Velloziaceae) afforded61 15,16-dihydroxy-ent-halima-1(10)-ene. The halimane structure 13 has been assigned62 to isoleojaponin from Leonurus japonicus.The clerodane pyrrole 14 was amongst63 the anti-plasmodial constituents of Polyalthia longifolia varn.pendula (Annonaceae), the bark of which is used in West African folk medicine for the treatment of malaria. 2β-Acetoxyhardwickiic acid and a number of other clerodanes were anti-bacterial constituents of Salvia adenophora (Lamiaceae)64 and S. buchananii.65 Further structural modifications of salvinorin have been made66 including some 2-alkyl-2-methoxymethyl ethers in order to examine its structure–activity relationships as a selective κ-opioid receptor agonist. ent-2β,18,19-Trihydroxycleroda-3,13-dien-15,16-olide has been obtained67 from Crassocephalum bauchiense (Asteraceae) which is a herb that is used in Cameroon folk medicine. The dichrocephnoids A–E (e.g. A, 15) which possess anti-HIV integrase activity, were isolated68 from Dichrocephala benthamii (Asteraceae). Further examination of the Chinese medicinal herb, Scutellaria barbata (Lamiaceae) has led to the isolation of the anti-viral scutolides A–L (e.g. A, 16)69 and the scutebatins A–C70 which inhibit NO production. The scutefolides (e.g. A, 17) were obtained71 from S. coleifolia whilst the teufruintins A–G (e.g. A, 18) were obtained72 from the aerial parts of the herb, Teucrium fruticans (Lamiaceae) which had been cultivated in China. Examination of Isodon scoparius (Lamiaceae) afforded73 the scopariusicides A 19 and B which were unusual cyclobutane derivatives which were formed by the cycloaddition of an ester of 4-hydroxycinnamic acid to the clerodane side chain. The unusual spirocyclic ring system 20 which may be derived from a clerodane, has been assigned74 to teotihuacanin, which was obtained from the Mexican plant, Salvia amarissima. It has shown activity against multi-drug resistance in cancer cells.
A number of new cis-clerodanes have been isolated including linarenone A from Linaria japonica (Plantaginaceae),75 the crotocurins A–C from Croton europhyllus (Euphorbiaceae)76 and the graveopenes A–J (e.g. A, 21) from Casearia graveolens (Flacourtiaceae).77 The latter were shown to stimulate NGF-mediated neurite out-growth which is of interest in the context of neuron degeneration in Alzheimer's disease. The related caseagrewifolins were obtained78 from C. grewifolia. Further examination of Tinospora sagittata (Menispermaceae) gave79 tinosporin A and B.
4 Tricyclic diterpenoids
4.1 Pimaranes
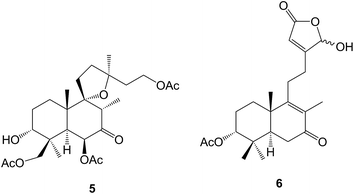
The arabinofuranoside of isopimara-7,15-dien-19-ol has been identified80 amongst the anti-fungal constituents of Sagittaria latifolia (Alismataceae). A series of isopimaranes known as the kaempulchraols A–I have been obtained81–83 from the rhizomes of Kaempferia pulchra (Zingiberaceae). Some constituents of the roots of Oryza sativa (rice) including momilactone D 22 have been shown84 to inhibit the production of NO. Further studies85–87 on the tubers of the Nigerian herbal medicine Icacina trichantha (Icacinaceae) have revealed the presence of more 9β-H-pimaranes and some aromatic 17-norpimaranes, the icacinlactones A–H (e.g. A, 23). The xylabisboeins A and B were anti-bacterial pimarane 14,16-ethers which were isolated88 from an endophytic Xylaria sp. obtained from the leaves of Bisboeckiera microcephala. Isopimaranes (e.g.24) were amongst89 the anti-inflammatory constituents of Dysoxylum gotadhora. A group of 16-norditerpenoids have been obtained90 from the aerial parts of Flickingeria fimbriata (Orchidaceae) whilst some pentahydroxypimaranes were isolated91 from Aerva lanata (Amaranthaceae), a plant which is used in Ayurvedic medicine. The dienone 25 and the corresponding 11,14-α- and β-epidioxides and diols were isolated92 from the stems of Croton insularis which was found in the Australian rain forest. A group of rosanes, e.g. stachyrosane I 26 have been reported93–95 as constituents of Stachys parviflora (Lamiaceae).4.2 Abietanes
The intervention of quinone methides in the anti-oxidant activity of the phenolic diterpenoids ferruginol96 and carnosic acid97 has been described. The anti-fungal activity of some abietic acid esters in the context of their use as wood preservatives98 and the anti-viral activity of podocarpic acid derivatives99 have been examined. The action of a visible light LED on the N-chlorosulfonamide of dehydroabietylamine afforded100 a 6α-chloro compound. Dehydroabietic acid has been shown101 to regulate liver glucose levels which may be linked to the use of Abies balsamea in a folk medicine treatment for type 2 diabetes. The brevistylumsides A and B, obtained from Illicium brevistylus have been identified102 as glycosides of dehydroabietic acid. The structure 27 has been assigned103 to the o-quinone teuvisone which was obtained from Teucrium viscidum. The dimeric biteuvisones A and B arose by addition of the corresponding o-catechol across the 8,9-bond.Further examples of diterpenoids in which ring A has been cleaved have been isolated from Salvia species. Thus salviaprione 29 was isolated104 as a racemate from S. prionitis. It may be formed by the cyclization of salvisyrianone 28. 1-Ketoethiopinone 30 was obtained105 from the roots of S. sahendica. The diacetate of dihydroethiopinone inhibits the growth of breast cancer cells. The roots of S. grandifolia yielded106 the grandifolias A–F (e.g. A, 31). These include some modified abietanes arising from the cleavage of ring C. The quinone, caryopterone A possesses a methylcyclopropane ring in place of the isopropyl group of the abietanes. It was isolated107 from the roots of Caryopteris mongolica (Lamiaceae). Some of the abietanes from this plant showed108 cholinesterase inhibitory activity. The macrophypenes A–E, obtained109 from Callicarpa macrophylla (Verbenaceae) included some rearranged abietanes (e.g. A, 32).
The constituents of the genus Chloranthus have been reviewed.110ent-Abietanes have been obtained111 from C. henryi whilst the chlorabietols A–C isolated112 from C. oldhamii included an unusual phloroglucinol-abietane adduct (A, 33). Rings B and C have been cleaved in the formation of various sessilifols A–N (e.g. C, 34) obtained113 from C. sessilifolius.
Further studies on the traditional Chinese medicine Tripterygium wilfordii (Celastraceae) have afforded more highly oxidized 18(4→3)-abeo-abietanoid derivatives including the triptergulides A and B (e.g. B, 35),114 the wilfordosides A and B,115 and the tripterlides A–F (e.g. A, 36)116 in which ring C has also undergone rearrangement. A range of abietanes, pimaranes and kauranes have been isolated117 from T. hypoglaucom.
Examination of the anti-proliferative constituents of Ethiopean Podocarpus falcatus led118 to the isolation of 16-hydroxynagilactone F and a revision of the stereochemistry of 2α-hydroxynagilactone F to the 2β-epimer. The botryosphaerins G and H (e.g. G, 37) which were obtained119 from a Botryospbaeria endophyte of Huperzia serrata, have a seco ring C structure or they may be tetranorlabdanes.
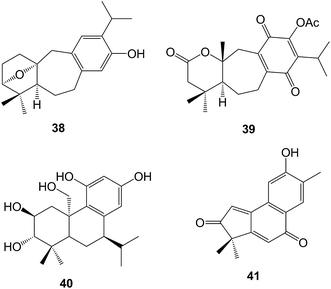
Some further icetexanes have been isolated120 from Perovskia atriplicifoiia (Lamiaceae). The hispidanols A and B (e.g. B, 38) were sempervirane relatives which were obtained121 from the rhizomes of Isodon hispida. The roots of Pygmacopremna herbacea (Verbenaceae), which have been used in Ayurvedic medicine as an anti-inflammatory agent, yielded a quinone which was assigned122 the somewhat unusual structure 39 in which ring B has been expanded but the C-20 methyl group has been retained. Another unusual structure 40 involving a curious biosynthesis, has been assigned123 to plebeianiol A which was obtained from Salvia plebeia. Neoboutomannin A 41 isolated124 from the West African Neoboutonia macrocalyx (Euphorbiaceae) may be a degraded abietane.
4.3 Cassanes
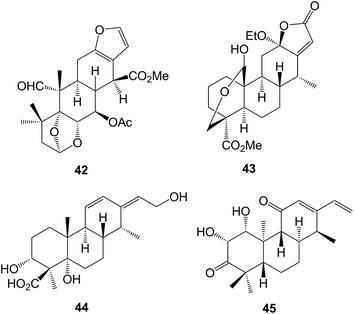
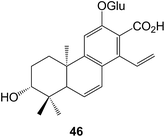
Cassane diterpenoids are characteristic constituents of the Caesalpiniaceae. Further examination of Caesalpinia bonducella has yielded125 the bonducellpins H–P whilst C. crista afforded126 the phangininoxys D and E. The echinalides H–U were obtained127 from C. echinata. Many of these compounds have been shown to possess anti-inflammatory and anti-oxidative properties. The cassanes from several studies128–130 of C. minax have been examined in this context. These included the caesalmins I–M128 and a seco ring A compound neocaesalminin A 42.130 The caesalsappanins A–L (e.g. A, 43) which were isolated131 from C. sappan, have been studied for their anti-malarial and anti-proliferative activity. Other cassanes have been isolated from Erythrophleum suaveolens132 and Swartzia simplex.133 The activation by epigenetic mining of a silent biosynthetic pathway in the fungus Calcarisporium arbuscula led to the isolation18 of arbusculic acid A 44. A new phytoalexin phytocassane F 45 has been identified134 in rice leaves that had been subjected to UV radiation.The cleistanthanes phyllanembloids A–F (e.g. A, 46) have been obtained135 from the roots of Phyllanthus emblica which are used in Chinese traditional medicine.
5 Tetracyclic diterpenoids
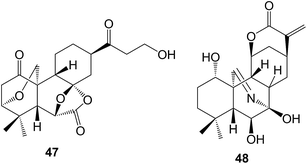
The ent-kauranes which have been obtained136 from the fruits of Annona glabra (Annonaceae) as part of a study of their anti-inflammatory activity, include 7β,16α,17-trihydroxy-ent-kauran-19-oic acid. 4β,16α,17,19-Tetrahydroxy-18-nor-ent-kaurane has been isolated137 from Wedelia trilobata (Asteraceae) whilst some ent-kaurenoic acid glycosides have been found138 in Ageratina cylindrica (Asteraceae). It has been estimated that over a thousand diterpenoids, many of which possess interesting biological activity, have been detected in Isodon (Lamiaceae) species. Further examples include the neolaxiflorins I–Y139 and the unusual laxiflorol A 47140 from I. eriocalyx varn.laxiflora. Extraction of I. excisoides,141I. parvifolius142 and I. scoparius143 provided other examples. The 7,20-azakaurenes, kaurines A and B (e.g. A, 48) were isolated144 from I. rubescens together with a compound containing an unusual 17-succinimide moiety. Xerophilusin B has been shown145 to induce cell cycle arrest and apoptosis in esophageal squamosus cell carcinoma. The total synthesis of maoecrystal V has been described.146The helikaurolides A–D which were obtained from Helianthus annuus varn.arianna, have been assigned147 structures based on a combination of a sesquiterpene lactone (helivypolide L) and an ent-kauranoic acid.
The steviol glycosides from Stevisa rebaudiana have continued to attract attention particularly in the food industry. The relationship between the conformation of rebaudioside A in solution and its sweetness148 and molecular modeling studies of the docking of rebaudioside A with the human sweet taste receptor149 have been reported. Dereplication of the NMR profiles of mixed steviol glycosides150 and the application of new separation techniques151 have been described. 15α-Hydroxyrebaudioside M has been isolated152 from S. rebaudiana. The ready availability of isosteviol from the acid-hydrolysis of the mixed glycosides from S. rebaudiana has led to its use as the starting material for a number of studies.153–156 The X-ray crystal structure of a dimeric isosteviol sulfite has been described.157
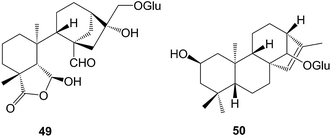
The prinsosides A–C which were isolated158 from Prinsepia utilis (Rosaceae) included the seco-ring B ent-kaurane (C, 49). Two glycosides, ranunculosides A and B that were isolated159 from Ranunculus muricatus (Ranunculaceae), were described as ent-kaurane glycosides but were drawn with an ent-phyllocladene structure 50.
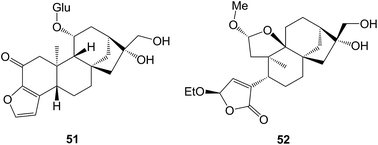
Cafestol has been shown160 to stimulate insulin production and it has been suggested that cafestol may contribute to the effect of coffee on reducing type 2 diabetes. The known furanokaurane, mozambioside 51 has been identified161 as a bitter tasting glycoside that is specific to Coffea arabica as opposed to C. robusta. The tricalysins A–H that were found162 in Tricalysia fruticosa (Rubiaceae), have been shown to be cafestol relatives and to have anti-inflammatory properties. The frutilactones A and B (e.g. A, 52) were 2,3-seco-cafestol relatives that were isolated163 from the same plant.
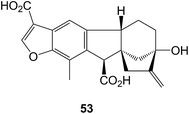
The gastroprotective activity of some derivatives of 18-hydroxybeyerenes has been described.164 2β,12β-Dihydroxygibberellin A12 has been isolated165 from the leaves of Scheffiera sessiliflora (Araliaceae) in a surprisingly high amount for a gibberellin. The synthesis of pharbinilic acid 53 starting from gibberellic acid has been described.166 This is an allogibberic acid relative which had been obtained from Morning Glory (Pharbitis nil).
Further grayane diterpenoids have been obtained from the leaves of Rhododendron micranthum (Ericaceae)167 and R. molle168 both of which are used in Chinese traditional medicine. The total synthesis of atisane diterpenoids has been reported.169 The biological activity of scopadulciol has been examined.170
6 Macrocyclic diterpenoids and their cyclization products
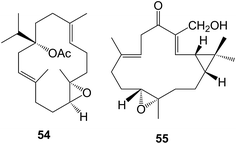
Diterpenoids with the cembrane skeleton have been found in various resins. Thus the resin of the North American Bursera microphylla (Burseraceae) afforded171 microphyllanin 54 whilst the frankincense from Boswellia carterii (Burseraceae) yielded172 a further group of cembranoids including the boscartins. The regioselective oxidation of the tobacco component, β-cembranediol at C-9 and C-10 using cytochrome P450 variants has been studied.173 Some casbanes, e.g. pekinenin G 55 have been isolated174 from Euphorbia pekinensis. A relative, sapidisin A was obtained175 from Sapium discolor (Euphorbiaceae).Preparative methods have been described176 for the isolation of paclitaxel (taxol®) and of further taxanes from Taxus chinensis (T. wallichiana varn.mairei).177 The modification of the structure of ring D-seco-taxanes in the context of microtubule interactions has been examined.178 Further synthetic studies directed at paclitaxel continue to be reported.179
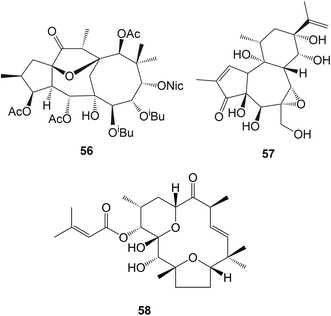
A large number of esters of jatrophanes, lathyranes, myrsinanes, daphnanes, tiglianes and ingenanes have been isolated, particularly from members of the Euphorbiaceae and Thymelaeaceae. Care must be taken in the separation of these compounds to avoid conditions that may lead to acyl migration and transesterification. The juxtaposition and consequent interactions between functional groups provide ample opportunities for these reactions in this closely related series of compounds and indeed may contribute to their biological activity. Molecular modeling and advanced NMR methods have been used in locating esters in the jatrophanes of Euphorbia amydaloides.180 Some cytotoxic lathyranes including lathyranlactone and jatrocurcusenone A have been isolated181 from Jatropha curcas cv. nigroviens rugosus whilst further jatrophane and myrsinane esters have been obtained from E. connata,182E. dracunculoides,183–185E. exigua.186E. osyridea,187E. prolifera,188 and E. wallichii.189 A 12,17-cyclojatrophane, euphowelwitschine A 56 was isolated190 from E. weiwitschii. Venenatin 57 which had been obtained from Excoecaria venenta (Euphorbiaceae), showed191 inhibitory effects on human leukaemia cells. Further daphnane esters have been isolated from Trigonostemon xyphophylloides (Euphorbiaceae),192Daphne genkwa (Thymelaeaceae),193Gnidia polycephela (Thymelaeaceae),194 and Stellera chamaejasme (Thymelaeaceae)195 whilst some more tigliane (phorbol) esters with cytotoxic and anti-viral activity were obtained from Croton tiglium (Euphorbiaceae),196,197Daphne aurantica,198 and Stillingia lineata (Euphorbiaceae).199 The anti-viral activity of these compounds (e.g. 58) against the chikungunya virus was examined. The evaluation of epoxylathyrol derivatives in combating multi-drug resistance200 and the anti-viral activity of a number of phorbol and ingenol esters201 has been reported. Some synthetic studies directed towards jatrophanes have been described.202
7 Miscellaneous diterpenoids
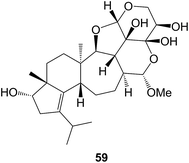
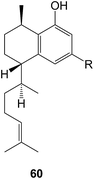
The synthesis of the 5,6,7-ring system of the mulinane diterpenoids has been achieved.203 A number of new cyathane diterpenoids have been isolated including the striatoids A–F (e.g. A, 59) from the basidiomycetes, Cyathus striatus,204 and from C. africanus205 and Hericium erinaceus.206 The total synthesis of the guanacastepenes N and O,207 dolestatrienol,208 and the trichoaurantiolides C and D209 have been reported. Further derivatives of pleuromutilin have been prepared210 in the context of their potential anti-tubercular activity.Dehydrovibsanin G has been isolated211 from Viburnum odoratissimum and a total synthesis of vibsanin which unambiguously defines its stereochemistry has been reported.212 8-Hydroxyserrulat-14-en-19-oic acid 60 (R = CO2H) which was isolated from the Australian medicinal plant, Eremophila neglecta (Scrophulariaceae) has been shown213 to break-up and disperse bacterial biofilms with the potential for use in wound management. A number of modified serrulatanes have been synthesized214 from leubethanol 60 (R = Me) and examined for activity against Mycobacterium tuberculosis.
8 References
- J. R. Hanson, Nat. Prod. Rep., 2015, 32, 76–87 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2015, 32, 1654–1663 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzere, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2016, 33, 382–431 RSC.
- M. A. Gonzalez, Nat. Prod. Rep., 2015, 32, 684–704 RSC.
- M. A. Gonzalez, Tetrahedron, 2015, 71, 1883–1908 CrossRef CAS.
- S. Dhambri, S. Mohammad, O. Nguyen van Buo, G. Galvani, Y. Meyer, M.-I. Lannou, G. Sorin and J. Ardisson, Nat. Prod. Rep., 2015, 32, 841–864 RSC.
- L. Zhu, S.-H. Huang, J. Yu and R. Hong, Tetrahedron Lett., 2015, 56, 23–31 CrossRef CAS.
- P. S. Riehl, Y. C. DePorre, A. M. Armaly, E. J. Groso and C. S. Schindler, Tetrahedron, 2015, 71, 6629–6650 CrossRef CAS.
- G. Zhu, R. Liu and B. Liu, Synthesis, 2015, 47, 2691–2708 CrossRef CAS.
- D. Markovic, M. Kolympadi, B. Deguin, F.-H. Poree and M. Turks, Nat. Prod. Rep., 2015, 32, 230–255 RSC.
- C. G. Newton and M. S. Sherburn, Nat. Prod. Rep., 2015, 32, 865–876 RSC.
- C. Draghici and J. T. Njardarson, Tetrahedron, 2015, 71, 3775–3793 CrossRef CAS PubMed.
- J. R. Hanson, J. Chem. Res., 2015, 39, 127–133 CAS.
- A. D. Rogachev and N. F. Salakhutdinov, Chem. Biodiversity, 2015, 12, 1–53 CAS.
- P. Zerbe and J. Bohlmann, Trends Biotechnol., 2015, 33, 419–428 CrossRef CAS PubMed.
- Y. J. Hong and D. J. Tantillo, Org. Biomol. Chem., 2015, 13, 10273–10278 CAS.
- C. Nakano, M. Oshima, N. Kurashima and T. Hoshino, ChemBioChem, 2015, 16, 772–781 CrossRef CAS PubMed.
- X.-M. Mao, W. Xu, D. Li, W.-B. Yin, Y.-H. Chooi, Y. O. Li, Y. Tang and Y. Hu, Angew. Chem., Int. Ed., 2015, 54, 7591–7592 Search PubMed.
- Y. Totsuka, S. Ueda, T. Kuzuyama and T. Shinada, Bull. Chem. Soc. Jpn., 2015, 88, 575–577 CrossRef CAS.
- Y. A. Nohra, V. Perrichot, L. Jeanneau, L. LePolles and D. Azar, J. Nat. Prod., 2015, 78, 1284–1293 CrossRef CAS PubMed.
- F. de. S. Vargas, P. D. O. de Almeida, E. S. P. Aranha, A. P. de A. Boleti, P. Newton, M. C. de Vasconcelios, V. F. Veiga Jnr and E. S. Lima, Molecules, 2015, 20, 6194–6210 CrossRef CAS PubMed.
- S. Zhao, J. Ling, Z. Li, S. Wang, J. Hu and N. Wang, Bioorg. Med. Chem. Lett., 2015, 25, 1483–1489 CrossRef CAS PubMed.
- Y.-R. He, Y.-H. Shen, L. Shan, X. Yang, B. Wen, J. Ye, X. Yuan, H.-L. Li, X.-K. Xu and W. D. Zhang, RSC Adv., 2015, 5, 4126–4134 RSC.
- G.-M. Li, J.-G. Luo, M.-H. Yang and L.-Y. Kong, Chem. Biodiversity, 2015, 12, 388–396 Search PubMed.
- J. Xu, F. Ji, J. Kang, H. Wang, S. Li, D.-Q. Jin, Q. Zhang, H. Sun and Y. Guo, J. Agric. Food Chem., 2015, 63, 5805–5812 CrossRef CAS PubMed.
- H. Zhao, G. Zeng, S. Zhao, J. Xu, L. Kong, Y. Li, N. Tan and S. Yang, Bioorg. Med. Chem. Lett., 2015, 25, 4572–4575 CrossRef CAS PubMed.
- Y. Wang, S.-H. Luo, J. Hua, Y. Liu, S.-X. Jing, X.-N. Li and S.-H. Li, J. Agric. Food Chem., 2015, 63, 10004–10012 CrossRef CAS PubMed.
- E. Maldonado, A. L. Perez-Castorena, Y. Romero and M. Martinez, J. Nat. Prod., 2015, 78, 202–207 CrossRef CAS PubMed.
- J. Hu, Y. Song, H. Li, X. Mao and X.-D. Shi, J. Asian Nat. Prod. Res., 2015, 17, 262–267 CrossRef CAS PubMed.
- K. Du, M. De Mieri, M. Neuburger, P. C. Zietsman, A. Marston, S. F. van Vuuren, D. Ferreira, M. Hamburger and J. H. van der Westhuizen, J. Nat. Prod., 2015, 78, 2494–2504 CrossRef CAS PubMed.
- Y. Narukawa, M. Komori, A. Niimura, H. Naguchi and F. Kiuchi, J. Nat. Med., 2015, 69, 130–134 CrossRef CAS PubMed.
- W.-M. Zhong, Z.-M. Cui, Z.-K. Liu, Y.-J. Dang and W.-L. Xiao, Chin. Chem. Lett., 2015, 26, 1000–1003 CrossRef CAS.
- Z. Huang, Z.-X. Zhu, Y.-T. Li, D.-R. Pang, J. Zheng, Q. Zhang, Y.-F. Zhao, D. Ferreira, J. K. Zjawlony, P.-F. Tu and J. Li, J. Nat. Prod., 2015, 78, 2276–2285 CrossRef CAS PubMed.
- M. Masoodi, Z. Ali, S. Liang, H. Yin, W. Wang and I. A. Khan, Phytochem. Lett., 2015, 13, 275–279 CrossRef CAS.
- R. Sharma, R. Chebolu and P. C. Ravikumar, Phytochem. Lett., 2015, 13, 187–193 CrossRef CAS.
- N. Corlay, M. Lesco-Bornet, E. Leborgne, F. Blanchard, X. Cachet, J. Bignon, F. Roussi, M.-J. Butel, K. Awang and M. Litauden, J. Nat. Prod., 2015, 78, 1348–1356 CrossRef CAS PubMed.
- L.-B. Wu, C.-J. Xiao, X. Jiang, L. Qiu, X. Dong and B. Jiang, Chem. Biodiversity, 2015, 12, 1229–1236 CAS.
- Z.-Y. Huang, B. Huang, C.-J. Xiao, X. Dong and B. Jiang, Nat. Prod. Res., 2015, 29, 628–632 CrossRef CAS PubMed.
- J. Zhou, J. Zhang, A. Cheng, Y. Xiong, L. Liu and H. Lou, Org. Lett., 2015, 17, 3560–3563 CrossRef CAS PubMed.
- J.-Y. Wu, J.-Z. Zhang, Y.-Q. Kang, X. Wang, P.-H. Fan, J.-C. Zhou and H.-X. Lou, J. Asian Nat. Prod. Res., 2015, 17, 462–467 CrossRef CAS PubMed.
- J. Zhang, Y. Li, R. Zhu, Z. Zhang and H. Lou, J. Nat. Prod., 2015, 78, 2087–2094 CrossRef CAS PubMed.
- M. Koubaa, E. Roseilo-Solo, J. Sic Ziabur, A. R. Jambrak, M. Brncke, N. Grimi, N. Boussetta and F. J. Baiba, J. Agric. Food Chem., 2015, 63, 6835–6846 CrossRef CAS PubMed.
- H. Kamauchi, T. Kon, K. Kinoshita, K. Takatori, K. Takahashi and K. Koyama, Tetrahedron Lett., 2015, 56, 4377–4382 CrossRef CAS.
- H.-H. Cheng, Y.-B. Cheng, T.-L. Hwang, T.-H. Kuo, C.-H. Chen and Y.-C. Shen, J. Nat. Prod., 2015, 78, 1823–1828 CrossRef CAS PubMed.
- X. Bian, J. Bai, X. Hu, X. Wu, C. Xue, A. Han, G. Su, H. Hua and Y. Pei, Tetrahedron Lett., 2015, 56, 5013–5016 CrossRef CAS.
- K. Zhou, X.-L. Zhao, L.-P. Han, M.-M. Cao, C. Chen, B.-Z. Shi and D.-Q. Luo, Helv. Chim. Acta, 2015, 98, 642–649 CrossRef CAS.
- S. C. V. A. R. Annam, M. Ankireddy, M. B. Sura, M. G. Ponnapalli, A. V. S. Sarma and J. Basha, Org. Lett., 2015, 17, 2840–2843 CrossRef PubMed.
- L. Xiong, G.-M. Zhou, Y. Zou, M.-H. Chen, L. Guo, G.-Y. Hu, Z.-H. Liu and C. Peng, Org. Lett., 2015, 17, 6238–6241 CrossRef CAS PubMed.
- C. S. Mizuno, A. B. Souza, B. L. Tekwani, S. R. Ambrosio and R. C. S. Veneziani, Bioorg. Med. Chem. Lett., 2015, 25, 5529–5531 CrossRef CAS PubMed.
- P. M. Matos, B. Mahoney, Y. Chan, D. P. Day, M. M. W. Cabral, C. H. G. Martins, R. A. Santos, J. K. Bastos, P. C. Bulman-Page and V. C. G. Heleno, Molecules, 2015, 20, 18264–18278 CrossRef CAS PubMed.
- I. Chayboun, E. Boulifa, A. I. Mansour, F. Rodriguez-Serrano, E. Carrasco, P. J. Alvarez, R. Chahboun and E. Alvarez-Manzaneda, J. Nat. Prod., 2015, 78, 1026–1036 CrossRef CAS PubMed.
- L. Mendoza, C. Sepulveda, R. Melo and M. Coloras, J. Chil. Chem. Soc., 2015, 60, 3024–3038 CrossRef CAS.
- M. Ma, J. Feng, R. Li, S.-W. Chen and H. Xu, Bioorg. Med. Chem. Lett., 2015, 25, 2773–2777 CrossRef CAS PubMed.
- A. Urosa, I. S. Marcos, D. Diez, J. M. Padron and P. Basabe, J. Org. Chem., 2015, 80, 6447–6455 CrossRef CAS PubMed.
- M. Martin, A. Urosa, I. S. Marcos, D. Diez, J. M. Padron and P. Basabe, J. Org. Chem., 2015, 80, 4566–4572 CrossRef CAS PubMed.
- Y. Luo, K. Wang, M.-H. Zhang, D.-Y. Zhang, Y.-C. Wu, X.-M. Wu and W.-Y. Hua, Bioorg. Med. Chem. Lett., 2015, 25, 2421–2424 CrossRef CAS PubMed.
- V. S. Nguyen, X. Y. Loh, H. Wijaya, J. Wang, Q. Lin, Y. Lam, W.-S. F. Wong and Y. K. Mok, J. Nat. Prod., 2015, 78, 208–217 CrossRef CAS PubMed.
- S. G. S. Kandanur, W. R. Golakoti and S. Nanduri, Bioorg. Med. Chem. Lett., 2015, 25, 5781–5786 CrossRef CAS PubMed.
- E. Englund, J. Andersen-Ranberg, R. Miao, B. Hamberger and P. Lundberg, ACS Synth. Biol., 2015, 4, 1270–1278 CrossRef CAS PubMed.
- A. F. Monteiro, J. M. Batista, M. A. Machado, R. P. Severino, E. W. Bianah, V. S. Bolzani, P. C. Vieira and V. G. P. Severino, J. Nat. Prod., 2015, 78, 1451–1455 CrossRef CAS PubMed.
- C. G. Silva, H. M. Santos Jnr, J. P. Barbosa, G. L. Costa, F. A. R. Rodrigues, D. F. Oliveira, L. V. Costa-Lotufa, R. J. Alves, E. C. A. Eleutherio and C. M. Rezende, Chem. Biodiversity, 2015, 12, 1891–1901 CAS.
- H. Wu, S. Wang, Z. Xu, S. Sun, H. Liu, J. Wang, Y. Lv, X. Dong, G. Li, L. Zhang and Y. Shi, Molecules, 2015, 20, 839–845 CrossRef PubMed.
- K. Annan, E. Ekuadzi, C. Asare, K. Sarpong, D. Pistorius, L. Oberer, B. A. Gyan and M. Ofori, Phytochem. Lett., 2015, 11, 28–31 CrossRef CAS.
- A. Bisio, A. M. Schito, S. N. Ebrahimi, M. Hamburger, G. Mele, G. Piatti, G. Romussi, F. Dai Paz and N. De Tommasi, Phytochemistry, 2015, 110, 120–132 CrossRef CAS PubMed.
- A. Bisio, A. M. Schito, A. Parricchi, G. Mele, G. Romussi, N. Malafronte, P. Oliva and N. De Tommasi, Phytochem. Lett., 2015, 14, 170–177 CrossRef CAS.
- D. Y. W. Lee, G. Deng, Z. Ma, W. Xu, L. Yang, L. Liu, R. Dai and L. Y. Liu-Chen, Bioorg. Med. Chem. Lett., 2015, 25, 4689–4692 CrossRef CAS PubMed.
- A. T. Tchinda, S. R. Mouokeu, R. A. N. Ngono, M. R. E. Ebelle, A. L. K. Mokale, D. K. Nono and M. Frederich, Nat. Prod. Res., 2015, 29, 1990–1994 CrossRef CAS PubMed.
- B. Song, G. Ding, X.-H. Tian, L. Li, C. Zhao, Q.-B. Zhang, M.-H. Wang, T. Zhang and Z.-M. Zou, Phytochem. Lett., 2015, 14, 249–253 CrossRef CAS.
- T. Wu, Q. Wang, C. Jiang, S. L. Morris-Natschke, H. Cui, Y. Wang, Y. Yan, J. Xu, K.-H. Lee and Q. Gu, J. Nat. Prod., 2015, 78, 500–509 CrossRef CAS PubMed.
- E. T. Yeon, J. W. Lee, C. Lee, Q. Jin, H. Jang, D. Lee, J. S. Ahn, J. T. Hong, Y. Kim, M. K. Lee and B. Y. Hwang, J. Nat. Prod., 2015, 78, 2292–2296 CrossRef CAS PubMed.
- S.-I. Kurimoto, J.-X. Pu, H. D. Sun, Y. Takaishi and Y. Kashiwada, Phytochemistry, 2015, 116, 298–304 CrossRef CAS PubMed.
- H.-W. Lv, J.-G. Luo, M.-D. Zhu, H.-J. Zhao and L. Y. Kong, Phytochemistry, 2015, 119, 26–31 CrossRef CAS PubMed.
- M. Zhou, X. R. Li, J.-W. Tang, Y. Liu, X.-N. Li, B. Wu, H.-B. Qin, X. Du, L.-M. Li, W.-G. Wang, J.-X. Pu and H. D. Sun, Org. Lett., 2015, 17, 6062–6065 CrossRef CAS PubMed.
- E. Bautista, M. Fragoso-Serrano, R. A. Toscano, M. R. Garcia-Peno and A. Ortega, Org. Lett., 2015, 17, 3280–3282 CrossRef CAS PubMed.
- A. Widyowati, S. Sugimoto, Y. Yamano, H. Otsuka, S. Sukardiman and K. Matsunami, Phytochem. Lett., 2015, 14, 56–62 CrossRef.
- Z. Pan, D. Ning, X. Wu, S. Huang, D. Li and S. Lv, Bioorg. Med. Chem. Lett., 2015, 25, 1329–1332 CrossRef CAS PubMed.
- J. Xu, F. Ji, X. Sun, X. Cao, S. Li, Y. Ohizumi and Y. Guo, J. Nat. Prod., 2015, 78, 2648–2656 CrossRef CAS PubMed.
- H. T. T. Nguyen, N. B. Truong, H. T. M. Doan, M. Litaudon, P. Retailleau, T. T. Do, H. V. Nguyen, M. V. Chau and C. V. Pham, J. Nat. Prod., 2015, 78, 2726–2730 CrossRef CAS PubMed.
- N.-B. Qin, A.-L. Wang, D.-H. Li, K. B. Wang, B. Lin, Z.-L. Li and H.-M. Hua, Phytochem. Lett., 2015, 12, 173–176 CrossRef CAS.
- R. R. Ravu, M. Jacob, C. Jefferies, Y. Tu, S. I. Khao, A. K. Agarwal, R. K. Guy, L. A. Walker, A. M. Clark and X. C. Li, J. Nat. Prod., 2015, 78, 2255–2259 CrossRef CAS PubMed.
- N. N. Win, T. Ito, S. Armati, H. Imagawa, H. Ngwe, I. Abe and H. Morita, J. Nat. Prod., 2015, 78, 1113–1118 CrossRef CAS PubMed.
- N. N. Win, T. Ito, S. Armati, T. Kodama, H. Imagawa, H. Ngwe, Y. Asakawa, I. Abe and H. Morita, Tetrahedron, 2015, 71, 4707–4713 CrossRef CAS.
- N. N. Win, T. Ito, S. Armati, T. Kodama, T. Kodama, H. Ngwe, Y. Asakawa, I. Abe and H. Morita, J. Nat. Prod., 2015, 78, 2306–2309 CrossRef CAS PubMed.
- J.-G. Cho, B.-J. Cha, S. M. Lee, S. Shrestha, R. H. Jeong, D. S. Lee, Y.-C. Kim, D.-G. Lee, H.-C. Kang, J. Kim and N.-I. Baek, Chem. Biodiversity, 2015, 12, 1356–1364 CAS.
- M. Zhao, M. M. Onakpa, W.-L. Chen, B. D. Santarsiero, S. M. Swanson, J. E. Burdette, I. U. Asuzu and C.-T. Che, J. Nat. Prod., 2015, 78, 789–796 CrossRef CAS PubMed.
- M. Zhao, M. M. Onakpa, W.-L. Chen, B. D. Santarsiero, X.-J. Huang, X.-Q. Zhang, J. Chen, J.-J. Cheng, R. Longnecker and C.-T. Che, Org. Lett., 2015, 17, 3834–3837 CrossRef CAS PubMed.
- M. Zhao, M. M. Onakpa, W.-L. Chen, B. D. Santarsiero, K. M. Szymulanska-Ramamurthy, S. M. Swanson, J. E. Burdette and C.-T. Che, J. Nat. Prod., 2015, 78, 2731–2737 CrossRef CAS PubMed.
- J. Sorres, C. Nirma, S. Toure, V. Eparvier and D. Stien, Tetrahedron Lett., 2015, 56, 4596–4598 CrossRef CAS.
- K. Jiang, L.-L. Chen, S.-F. Wang, Y. Wang, Y. Li and K. Gao, J. Nat. Prod., 2015, 78, 1037–1044 CrossRef CAS PubMed.
- H. Li, J.-J. Zhao, J.-L. Chen, L.-P. Zhu, D.-M. Wang, L. Jiang, D. Yang and Z.-M. Zhao, Phytochemistry, 2015, 117, 400–409 CrossRef CAS PubMed.
- Y. P. Bharitkar, A. Hazra, N. S. Apoorva Podun, A. Ash, P. R. Maulik and N. B. Mondal, Nat. Prod. Res., 2015, 29, 253–261 CrossRef CAS PubMed.
- L. A. Maslovskaya, A. I. Savchenko, V. A. Gordon, P. W. Reddell, C. J. Pierce, P. G. Parson and C. M. Williams, Aust. J. Chem., 2015, 68, 652–659 CrossRef CAS.
- U. Farooq, S. Naz, R. Sarwar, A. Khan, A. Rauf, H. Chan, M. Ahmad, A. H. Ali Shah and S. Hameed, Phytochem. Lett., 2015, 14, 198–202 CrossRef CAS.
- U. Farooq, K. Ayub, M. A. Hashmi, A. Khan and M. Ali, Rec. Nat. Prod., 2015, 9, 329–335 CAS.
- U. Farooq, K. Ayub, M. A. Hashmi, R. Sarwar, A. Khan, M. Ali, M. Ahmmad and A. Khan, Nat. Prod. Res., 2015, 29, 813–819 CrossRef CAS PubMed.
- H. Saijo, H. Kofujita, K. Takahashi and T. Ashitani, Nat. Prod. Res., 2015, 29, 1739–1743 CrossRef CAS PubMed.
- S. Birtic, P. Dussort, F.-X. Pierre, A. C. Bily and M. Rollei, Phytochemistry, 2015, 115, 9–19 CrossRef CAS PubMed.
- H. Wang, T. T. H. Nguyen, S. Li, T. Liang, Y. Zhang and J. Li, Bioorg. Med. Chem. Lett., 2015, 25, 347–354 CrossRef CAS PubMed.
- C.-H. Chen and L. Huang, ACS Med. Chem. Lett., 2015, 6, 355–358 CrossRef PubMed.
- Q. Qin and S. Yu, Org. Lett., 2015, 17, 1894–1897 CrossRef CAS PubMed.
- A. Nachar, A. Saleem, J. T. Arnason and P. S. Haddad, Phytochemistry, 2015, 117, 373–379 CrossRef CAS PubMed.
- Y. Chen, Z. Dong, D. Huang, W. Chen, F. Yao, D. Xue and L. Sun, Phytochem. Lett., 2015, 14, 27–30 CrossRef CAS.
- C. Gao, L. Han, D. Zheng, H. Jin, C. Gai, J. Wang, H. Zhang, L. Zhang and H. Fu, J. Nat. Prod., 2015, 78, 630–634 CrossRef CAS PubMed.
- Y.-J. Jiang, Y. Zhang, J. He, X.-D. Wu, L.-D. Shao, X.-N. Lim, J. Su, L.-Y. Peng, C. K. Li and Q.-S. Zhao, Tetrahedron Lett., 2015, 56, 5457–5459 CrossRef CAS.
- V. Kafil, M. Eskandani, Y. Omidi, H. Nazamiyeh and J. Barar, RSC Adv., 2015, 5, 18041–18051 RSC.
- J. Kang, L. Li, D. Wang, H. Wang, C. Liu, B. Li, Y. Yan, L. Fang, G. Du and R. Chen, Phytochemistry, 2015, 116, 337–348 CrossRef CAS PubMed.
- E. Saruul, T. Murata, E. Selenge, K. Sasaki, F. Yoshizaki and J. Batkhuu, Bioorg. Med. Chem. Lett., 2015, 25, 2555–2558 CrossRef CAS PubMed.
- T. Murata, E. Selenge, S. Oikawa, K. Sasaki and F. Yoshizaki, J. Nat. Med., 2015, 69, 471–478 CrossRef CAS PubMed.
- J. Xu, Y. Sun, M. Wang, Q. Ren, S. Li, H. Wang, X. Sun, D.-Q. Jin, H. Sun, Y. Ohizumi and Y. Guo, J. Nat. Prod., 2015, 78, 1563–1569 CrossRef CAS PubMed.
- A.-R. Wang, H.-C. Song, H.-M. An, Q. Huang, X. Luo and J.-Y. Dong, Chem. Biodiversity, 2015, 12, 451–473 CAS.
- C. Xie, L. Sun, K. Liao, S. Liu, M. Wang, J. Xu, M. Bartiam and Y. Guo, J. Nat. Prod., 2015, 78, 2800–2807 CrossRef CAS PubMed.
- J. Xiong, Z.-L. Hong, L.-X. Gao, J. Shen, S.-T. Liu, G.-X. Yang, J. Li, H. Zeng and J.-F. Hu, J. Org. Chem., 2015, 80, 11080–11085 CrossRef CAS PubMed.
- L.-J. Wang, J. Xiong, S.-T. Liu, L.-L. Pan, G.-X. Yang and J.-F. Hu, J. Nat. Prod., 2015, 78, 1635–1646 CrossRef CAS PubMed.
- L. Ni, J. Ma, C.-J. Li, L. Li, J.-M. Guo, S.-P. Yuan, Q. Hou, Y. Guo and D.-M. Zhang, Tetrahedron Lett., 2015, 56, 1239–1243 CrossRef CAS.
- H.-M. Li, D.-W. Wan and R.-T. Li, J. Asian Nat. Prod. Res., 2015, 17, 761–766 CrossRef CAS PubMed.
- C. Wang, C.-J. Li, J. Ma, J.-Z. Yang, X.-G. Chen, L. Li and D.-M. Zhang, RSC Adv., 2015, 5, 30046–30052 RSC.
- X.-L. Li, L.-H. Gao, H.-M. Li, L.-T. Wang, K.-H. Lee and R.-T. Li, Phytochem. Lett., 2015, 12, 84–89 CrossRef CAS.
- E. M. Addo, H.-B. Chai, A. Hymete, M. Y. Yeshak, C. Slebodnick, D. G. I. Kingston and L. H. Rakotondraibe, J. Nat. Prod., 2015, 78, 827–835 CrossRef CAS PubMed.
- Y.-M. Chen, Y.-H. Yang, X.-N. Li, C. Zou and P.-J. Zhao, Molecules, 2015, 20, 16924–16932 CrossRef CAS PubMed.
- Z.-Y. Jiang, Y.-J. Yu, C.-G. Huang, X.-Y. Zhang and G.-P. Li, Planta Med., 2015, 81, 241–246 CrossRef CAS PubMed.
- B. Huang, Z.-Y. Huang, C.-J. Xiao, X. Dong and B. Jiang, Helv. Chim. Acta, 2015, 98, 527–533 CrossRef CAS.
- K. Satish, G. Srihari, G. Sudhakar, K. Narsimha, T. P. Rao and M. M. Murthy, Phytochem. Lett., 2015, 12, 129–132 CrossRef CAS.
- B.-B. Zhang, B.-Q. He, J.-B. Sun, B. Zeng, X.-J. Shi, Y. Zhou, Y. Niu, S.-Q. Nie, F. Feng, Y. Liang and F.-H. Wu, Molecules, 2015, 20, 14879–14888 CrossRef CAS PubMed.
- T. Maffo, P. Wafo, R. S. T. Kamdem, R. Melong, P. F. Uzor, P. Mkounga, Z. Ali and B. T. Ngadjui, Phytochem. Lett., 2015, 12, 323–331 Search PubMed.
- P. H. Dang, M. T. T. Nguyen, H. X. Nguyen, D. T. T. Vu, S. V. Truong and N. T. Nguyen, Phytochem. Lett., 2015, 13, 99–102 CrossRef CAS.
- Q.-B. Liu, L. Huang, L. Zhang, Q. Liu, Q.-Q. Xu, X.-J. Qin, X. Y. Fang and Y. Zeng, J. Asian Nat. Prod. Res., 2015, 17, 1073–1078 CrossRef CAS PubMed.
- T. Mitsui, R. Ishihara, K. Hayashi, N. Matsuura, H. Akashi and H. Nozaki, Phytochemistry, 2015, 116, 349–358 CrossRef CAS PubMed.
- J.-S. Zhang, Y.-Q. Guo, J.-M. Bao, M.-H. Jiang, S.-L. Lin, Z.-Y. Su, G.-H. Tang and S. Yin, Helv. Chim. Acta, 2015, 98, 1387–1394 CrossRef CAS.
- L. Lian, X.-B. Li, J.-Z. Yuan, L. Cheng, Z. Wu and H. Gao, J. Asian Nat. Prod. Res., 2015, 17, 893–899 CrossRef CAS PubMed.
- R. Dong, J. Yuan, S. Wu, J. Huang, X. Xu, Z. Wu and H. Gao, Phytochemistry, 2015, 117, 325–331 CrossRef CAS PubMed.
- G. Ma, H. Wu, D. Chen, N. Zhu, Y. Zhu, Z. Sun, P. Li, J. Yang, J. Yuan and X. Xu, J. Nat. Prod., 2015, 78, 2364–2371 CrossRef CAS PubMed.
- J. M. E. Dade, L. A. Kablan, T. Okpekon, M. Say, K. D. Yapo, G. Komlaga, J. B. Boti, A. P. Koffi, L. E. Guei, L. A. Djakoure and P. Champy, Phytochem. Lett., 2015, 12, 224–231 CrossRef CAS.
- Q. Favre-Godal, S. Dorsaz, E. F. Queiroz, L. Marcourt, S. N. Ebrahimi, P.-M. Allard, F. Voinesco, M. Hamburger, M. P. Gupta, K. Gindro, D. Sanglard and J.-L. Wolfender, J. Nat. Prod., 2015, 78, 2994–3004 CrossRef CAS PubMed.
- K. Horie, Y. Inoue, M. Sakai, Q. Yuo, Y. Tanimoto, J. Koga, H. Toshima and M. Hasegawa, J. Agric. Food Chem., 2015, 63, 4050–4059 CrossRef CAS PubMed.
- J.-J. Lv, S. Yu, Y. Xin, H.-T. Zhu, D. Wang, R.-R. Cheng, C.-R. Yang, M. Xu and Y.-J. Zhang, RSC Adv., 2015, 5, 29096–29107 Search PubMed.
- N. X. Nhiem, N. T. T. Hien, B. H. Tai, H. L. T. Ahn, D. T. T. Hang, T. H. Quang, P. V. Kiem, C. V. Minh, W. Ko, S. Lee, H. Oh, S. H. Kim and Y. H. Kim, Bioorg. Med. Chem. Lett., 2015, 25, 254–258 CrossRef PubMed.
- H. Ren, Q. L. Xu, Y. Luo, M. Zhang, Z.-Y. Zhou, L.-M. Dong and J. W. Tan, Phytochem. Lett., 2015, 11, 260–263 CrossRef CAS.
- C. Bustos-Brito, M. Sanchez-Castellanos, B. Esquivel, J. S. Calderon, F. Calzada, L. Yepez-Mulia, P. Joseph-Nathan, G. Cuevas and L. Quijano, J. Nat. Prod., 2015, 78, 2580–2587 CrossRef CAS PubMed.
- W.-G. Wang, J. Yang, H.-Y. Wu, L.-M. Kong, J. Su, X.-N. Li, X. Du, R. Zhan, M. Zhou, Y. Li, J.-X. Pu and H. D. Sun, Tetrahedron, 2015, 71, 8161–8171 Search PubMed.
- W.-G. Wang, J.-W. Tang, Y.-M. Shi, X. Du, X.-N. Li, H. Y. Wu, Y. Li, H.-Y. Jiang, J.-X. Pu and H. D. Sun, RSC Adv., 2015, 5, 6132–6135 RSC.
- L.-P. Dai, C. Li, H.-Z. Yang, Y.-Q. Lu, H.-Y. Yu, H.-M. Gao and Z.-M. Wang, Molecules, 2015, 20, 17544–17556 CrossRef CAS PubMed.
- L. Yang, S. Liu, H. Wang and Y. Suo, Rec. Nat. Prod., 2015, 9, 526–529 CAS.
- M. Zhou, X.-R. Li, J.-W. Tang, J.-X. Pu and H. D. Sun, Org. Lett., 2015, 17, 6062–6065 CrossRef CAS PubMed.
- X. Liu, J. Yang, W.-G. Wang, Y. Li, J.-Z. Wu, J.-X. Pu and H. D. Sun, J. Nat. Prod., 2015, 78, 196–201 CrossRef CAS PubMed.
- R. Yao, Z. Chen, C. Zhou, M. Luo, X. Shi, J. Li, Y. Gao, F. Zhou, J. Pu, H. D. Sun and J. He, J. Nat. Prod., 2015, 78, 10–16 CrossRef CAS PubMed.
- W.-B. Zhang, G. Lin, W.-B. Shao, J.-X. Gong and Z. Yang, Chem.–Asian J., 2015, 10, 903–909 CrossRef CAS PubMed.
- A. Torres, J. M. G. Molinillo, R. M. Varela, L. Casas, C. Mantell, E. J. Martinez de la Ossa and F. A. Macias, Org. Lett., 2015, 17, 4730–4733 CrossRef CAS PubMed.
- P. D. Chopade, B. Sarma, E. E. Santiso, J. Simpson, J. C. Fry, N. Yurttas, K. L. Biermann, J. Chen, B. L. Trout and A. S. Myerson, J. Chem. Phys., 2015, 143, 244–301 CrossRef PubMed.
- Mayank and V. Jaitak, Phytochemistry, 2015, 116, 12–20 CrossRef CAS PubMed.
- J. G. Napolitano, C. Simmler, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2015, 78, 658–665 CrossRef CAS PubMed.
- J. Hubert, N. Borie, S. Chollet, J. Dayde and J. H. Renault, Planta Med., 2015, 81, 1614–1620 CrossRef CAS PubMed.
- I. Prakesh, G. Ma, C. Bunders, T. M. Snyder and C. Priedemann, Nat. Prod. Commun., 2015, 10, 1159–1161 Search PubMed.
- B. F. Garifullin, R. R. Sharipova, I. Yu Strobykina, M. A. Kravchenko and V. E. Kataev, Russ. J. Org. Chem., 2015, 51, 1488–1498 CrossRef CAS.
- O. V. Andreeva, R. R. Sharipova, I. Yu Strobykina, R. Musin and V. E. Kataev, Russ. J. Org. Chem., 2015, 51, 1324–1333 CrossRef CAS.
- I. Yu Strobykina, M. G. Belenkov, M. N. Semenova, V. F. Mironov and V. E. Kataev, J. Nat. Prod., 2015, 78, 1300–1318 CrossRef PubMed.
- B. F. Garifullin, R. R. Sharipova, I. Yu Strobykina, O. V. Andreeva and V. E. Kataev, Chem. Nat. Compd., 2015, 51, 886–889 CrossRef CAS.
- A. G. Dobrynin, O. V. Andreeva and I. A. Litvinov, J. Struct. Chem., 2015, 56, 477 CrossRef.
- Q. Zhang, H.-X. Liu, H. B. Tan and S.-X. Qiu, Tetrahedron, 2015, 71, 9415–9419 CrossRef CAS.
- B. L. Wu, H. L. Zou, F.-M. Qin, H.-Y. Li and G.-X. Zhou, Molecules, 2015, 20, 22445–22453 CrossRef CAS PubMed.
- F. B. Mellbye, P. B. Jeppesen, K. Hermansen and S. Gregersen, J. Nat. Prod., 2015, 78, 2447–2451 CrossRef CAS PubMed.
- R. Lang, S. Klade, A. Beusch, A. Dunkel and T. Hofmann, J. Agric. Food Chem., 2015, 63, 10492–10499 CrossRef CAS PubMed.
- C. P. Shen, J. G. Luo, M.-H. Yang and L.-Y. Kong, J. Nat. Prod., 2015, 78, 1322–1329 CrossRef CAS PubMed.
- C. P. Shen, J. G. Luo, M.-H. Yang and L.-Y. Kong, Tetrahedron Lett., 2015, 56, 1328–1331 CrossRef CAS.
- T. Parra, J. Benites, L. M. Ruiz, B. Sepulveda, M. Simirgiotis and C. Areche, Bioorg. Med. Chem. Lett., 2015, 25, 2813–2817 CrossRef CAS PubMed.
- T. P. Nguyen, T. T. V. Tran, D. T. Mai, N. M. Phan and T. D. Bui, Nat. Prod. Res., 2015, 29, 1432–1436 CrossRef CAS PubMed.
- J. R. Annand, P. A. Bruno, A. K. Mapp and C. S. Schindler, Chem. Commun., 2015, 51, 8990–8993 RSC.
- M. Zhang, Y. Xie, G. Zhan, L. Lei, P. Shu, Y. Chen, Y. Xue, Z. Luo, Q. Wan, G. Yao and Y. Zhang, Phytochemistry, 2015, 117, 107–115 CrossRef CAS PubMed.
- Y. Li, Y.-B. Liu, J.-J. Zhang, Y. Liu, S.-G. Ma, J. Qu, H.-N. Lv and S. S. Yu, J. Nat. Prod., 2015, 78, 2887–2895 CrossRef CAS PubMed.
- L. Song, G. Zhu, Y. Liu, B. Liu and S. Qin, J. Am. Chem. Soc., 2015, 137, 13706–13714 CrossRef CAS PubMed.
- R. G. Puentes, K. Toume, M. A. Aral, S. K. Sadhu, F. Ahmed and M. Ishibashi, J. Nat. Prod., 2015, 78, 864–872 CrossRef PubMed.
- F. Messina, M. Curini, C. D. Sano, C. Zadra, G. Gigliarelli, L. A. Rascon-Valenzuela, R. E. Robles-Zepeda and M. C. Marcotullio, J. Nat. Prod., 2015, 78, 1184–1188 CrossRef CAS PubMed.
- J. Ren, Y.-G. Wang, A.-G. Wang, L.-G. Wu, H.-J. Zhang, W.-J. Wang, Y.-L. Su and H.-L. Qin, J. Nat. Prod., 2015, 78, 2322–2331 CrossRef CAS PubMed.
- P. Le-Huu, T. Heidt, B. Claasen, S. Laschat and V. B. Urlacher, ACS Catal., 2015, 5, 1772–1780 CrossRef CAS.
- K. Wang, H. Yu, H. Wu, X. Wang, Y. Pan, Y. Chen, L. Liu, Y. Jin and C. Zhang, Nat. Prod. Res., 2015, 29, 1456–1460 CrossRef CAS PubMed.
- H.-B. Liu, H. Zhang, J.-H. Yu and J.-M. Yue, J. Asian Nat. Prod. Res., 2015, 17, 1117–1128 CrossRef CAS PubMed.
- Z.-K. Liang, R.-G. Huang, Z.-S. Kie and X.-J. Xu, Nat. Prod. Res., 2015, 29, 327–330 CrossRef CAS PubMed.
- Z.-H. Sun, Y. Chen, Y.-Q. Guo, J. Qiu, C.-G. Zhu, J. Jin, G.-H. Tang, X.-Z. Bu and S. Yin, Bioorg. Med. Chem. Lett., 2015, 25, 1240–1243 CrossRef CAS PubMed.
- S.-R. Wang, C.-G. Yang, P. A. Sanchez-Murcia, J. P. Snyder, N. Yan, G. Saez-Calve, J. F. Diaz, F. Gago and W. S. Fang, Org. Lett., 2015, 17, 6098–6101 CrossRef CAS PubMed.
- K. Fukaya, K. Kodama, Y. Tanaka, H. Yamazaki, T. Sugai, Y. Yamaguchi, H. Watanabe, T. Oishi, T. Sato and N. Chida, Org. Lett., 2015, 17, 2574–2577 CrossRef CAS PubMed.
- L.-F. Nothias-Scaglia, J.-F. Gallard, V. Dumontet, F. Roussi, J. Costa, B. I. Iorga, J. Paolini and M. Litaudon, J. Nat. Prod., 2015, 78, 2423–2431 CrossRef CAS PubMed.
- J. Q. Liu, J. F. Yang, J. J. Xia, X. Y. Li, Z. R. Li, L. Zhou and M. Qiu, Phytochemistry, 2015, 117, 462–468 CrossRef CAS PubMed.
- S. Shadi, H. Saeidi, M. Ghanadian, M. R. Rahimnejad, M. Aghaei, S. M. Ayarollahi and M. I. Choudhary, Nat. Prod. Res., 2015, 29, 607–614 CrossRef CAS PubMed.
- L. Wang, Y.-T. Ma, Q.-Y. Sun, X.-N. Li, Y. Yan, J. Yang, F.-M. Yang, F.-Y. Liu, Z. Zang, X.-H. Wu, S.-X. Huang and Y. Zhao, Tetrahedron, 2015, 71, 5485–5493 Search PubMed.
- L. Wong, J. Yang, Y.-Q. Chi, W.-B. Ouyang, Z. Zang, S.-X. Huang, P. Cao and Y. Zhao, Nat. Prod. Res., 2015, 29, 1406–1413 CrossRef PubMed.
- L. Wang, Z. Zang, J. Zhang, P. Cao and Y. Zhao, Rec. Nat. Prod., 2015, 9, 374–378 CAS.
- D. Redei, K. Bores, P. Forgo, J. Molnar, Z. Kele, I. Palinko, C. Pinke and J. Hohmann, Chem. Biodiversity, 2015, 12, 1214–1221 CAS.
- M. Ghanadian, H. Saeidi, M. Aghaei, M. R. Rahiminejad, E. Ahmadi, S. M. Ayatollahi, M. I. Choudhary and B. Bahmani, Phytochem. Lett., 2015, 12, 302–307 CrossRef CAS.
- J. Xu, J. Kang, X. Cao, X. Sun, S. Yu, X. Zhang, H. Sun and Y. Guo, J. Agric. Food Chem., 2015, 63, 5902–5910 CrossRef CAS PubMed.
- D. S. Yang, W.-B. Peng, Y.-P. Yang, K.-C. Liu, X.-L. Li and W.-L. Xiao, J. Asian Nat. Prod. Res., 2015, 17, 946–951 CrossRef CAS PubMed.
- M. A. Reis, V. Andre, M. T. Duarte, H. Lage and M. J. U. Ferreira, J. Nat. Prod., 2015, 78, 2684–2690 CrossRef CAS PubMed.
- D.-S. Ning, L.-Y. Peng, S.-H. Lv, D.-P. Li and Z.-H. Pan, Nat. Prod. Res., 2015, 29, 524–528 CrossRef CAS PubMed.
- B. Yang, Z. Meng, Z. Li, L. Sun, Y. Hu, Z. Wang, G. Ding, W. Xiao and C. Han, Phytochem. Lett., 2015, 11, 270–274 CrossRef CAS.
- H.-L. Jiang, R. Wang, J.-Y. Li and Y.-P. Shi, Nat. Prod. Res., 2015, 29, 1878–1883 CrossRef CAS PubMed.
- H. De Mieri, K. Du, M. Neuburger, P. Saxena, P. Zietsman, S. Hering, J. H. van der Westhuizen and M. Hamburger, J. Nat. Prod., 2015, 78, 1697–1707 CrossRef PubMed.
- M. Yan, Y. Lu, C.-H. Chen, Y. Zhao, K.-H. Lee and D.-F. Chen, J. Nat. Prod., 2015, 78, 2712–2718 CrossRef CAS PubMed.
- J.-F. Wang, S.-H. Yang, Y.-Q. Liu, D.-X. Li, W.-J. He, X.-X. Zhang, Y.-H. Liu and X.-J. Zhou, Bioorg. Med. Chem. Lett., 2015, 25, 1986–1989 CrossRef CAS PubMed.
- D.-D. Zhang, B. Zhou, J.-H. Yu, C.-H. Xu, J. Ding, H. Zhang and J.-M. Yue, Tetrahedron, 2015, 71, 9638–9644 CrossRef CAS.
- S.-Z. Huang, X. Zhang, Q.-Y. Ma, Y.-T. Zheng, H.-F. Dai, Q. Wang, J. Zhou and Y.-X. Zhao, RSC Adv., 2015, 5, 80254–80263 RSC.
- F. Oiivon, H. Palenzuela, E. Girard-Valenciennes, J. Neyts, C. Pannecouque, F. Roussi, I. Grondin, P. Leyssen and M. Litaudon, J. Nat. Prod., 2015, 78, 1119–1128 CrossRef PubMed.
- A. M. Matos, M. Reis, N. Duarte, G. Spengler, J. Molnar and M. J. U. Ferreira, J. Nat. Prod., 2015, 2215–2228 CrossRef CAS PubMed.
- L.-F. Nothias-Scaglia, C. Pannecouque, F. Renucci, L. Delang, J. Neyts, F. Roussi, J. Costa, P. Leyssen, M. Litaudon and J. Paolini, J. Nat. Prod., 2015, 78, 1277–1283 CrossRef CAS PubMed.
- P. Mohan, K. Koushik and M. J. Fuertes, Tetrahedron Lett., 2015, 56, 61–65 CrossRef CAS.
- K. P. Reber, J. Xu and C. A. Guerrero, J. Org. Chem., 2015, 80, 2397–2406 CrossRef CAS PubMed.
- R. Bai, C.-C. Zhang, X. Yin, J. Wei and J.-M. Gao, J. Nat. Prod., 2015, 78, 783–788 CrossRef CAS PubMed.
- J.-J. Han, L. Zhang, J.-K. Xu, L. Bao, F. Zhao, Y.-H. Chen, W. K. Zhang and H.-W. Liu, J. Asian Nat. Prod. Res., 2015, 17, 541–549 CrossRef CAS PubMed.
- Z. Zhang, R.-N. Liu, Q.-J. Tang, J.-S. Zhang, Y. Yang and X.-P. Shang, Phytochem. Lett., 2015, 11, 151–156 CrossRef CAS.
- S.-Z. Peng and C.-K. Sha, Org. Lett., 2015, 17, 3486–3489 CrossRef CAS PubMed.
- L. T. Leung and P. Chiu, Chem.–Asian J., 2015, 10, 1042–1049 CrossRef CAS PubMed.
- D. R. Williams, P. T. Gladen and J. R. Pinchman, J. Org. Chem., 2015, 80, 5474–5493 CrossRef CAS PubMed.
- Y.-J. Dong, Z.-H. Meng, Y.-Q. Mi, C. Zhang, Z.-H. Cui, P. Wang and Z.-B. Xu, Bioorg. Med. Chem. Lett., 2015, 25, 1799–1803 CrossRef CAS PubMed.
- F.-J. Li, J.-H. Yu, G.-C. Wang, H. Zhang and J.-M. Yue, J. Asian Nat. Prod. Res., 2015, 17, 475–481 CrossRef CAS PubMed.
- K.-I. Takao, K. Tsunoda, T. Kurisu, A. Sakama, Y. Nishimura, K. Yoshida and K.-I. Tadano, Org. Lett., 2015, 17, 756–759 CrossRef CAS PubMed.
- H. H. Mon, S. N. Christo, C. F. Ndi, H. J. Griesser and S. J. Semple, J. Nat. Prod., 2015, 78, 3031–3034 CrossRef CAS PubMed.
- R. Escarcena, J. Perez-Meseguer, E. del Olmo, B. Alanis-Garza, E. Garza-Gonzalez, R. Salazar-Aranda and N. Waksman de Torres, Molecules, 2015, 20, 7245–7262 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |