Open Access Article
Open Access ArticleRecent advances in research on lignans and neolignans
Rémy Bertrand
Teponno†
ab,
Souvik
Kusari†
 a and
Michael
Spiteller
*a
a and
Michael
Spiteller
*a
aInstitute of Environmental Research (INFU), Department of Chemistry and Chemical Biology, Chair of Environmental Chemistry and Analytical Chemistry, TU Dortmund, Otto-Hahn-Str. 6, 44221 Dortmund, Germany. E-mail: michael.spiteller@tu-dortmund.de; Fax: +49-231-755-4085; Tel: +49-231-755-4080
bDepartment of Chemistry, Faculty of Science, University of Dschang, P. O. Box 67, Dschang, Cameroon
First published on 9th May 2016
Abstract
Covering: 2009 to 2015
Lignans and neolignans are a large group of natural products derived from the oxidative coupling of two C6–C3 units. Owing to their biological activities ranging from antioxidant, antitumor, anti-inflammatory to antiviral properties, they have been used for a long time both in ethnic as well as in conventional medicine. This review describes 564 of the latest examples of naturally occurring lignans and neolignans, and their glycosides in some cases, which have been isolated between 2009 and 2015. It comprises the data reported in more than 200 peer-reviewed articles and covers their source, isolation, structure elucidation and bioactivities (where available), and highlights the biosynthesis and total synthesis of some important ones.
1 Introduction
The last decade has witnessed a renewed interest in biologically active compounds isolated from a plethora of natural resources, in addition to impressive progress in allied methodologies such as combinatorial chemistry and high-throughput screening. Given the advantages of formulations containing compounds obtained from natural resources over compounds obtained by total synthesis, including relatively low toxicity, complete biodegradability, and in some cases, lower production costs, an emerging global impetus towards natural product research is presently observed.1 Lignans and neolignans represent an enormous class of pharmacologically active compounds. However, there is currently insufficient updated information on this important class of natural products, with a comprehensive review on their chemistry, bioactivity and synthesis published long back in 2009.2 Remarkable advances have been made in the isolation and identification of lignans and neolignans in the recent years, which has already led to about 500 new congeners and most of which possess beneficial pharmacological effects. Among these, dibenzocyclooctadiene lignans isolated principally from plants of the Schisandraceae family (Kadsura and Schisandra genera) have been the most abundant class with about 131 new compounds. However, there is currently a dearth of a unifying review that provides a comprehensive account of lignans and neolignans reported in the recent years. Therefore, the objective of the present review is to compile the source, identification, bioactivities, biosynthesis and total synthesis of bioactive lignans and neolignans isolated and characterized between 2009 and 2015, a period that marks enormous progress in the discovery of this interesting class of compounds.1.1 Definition
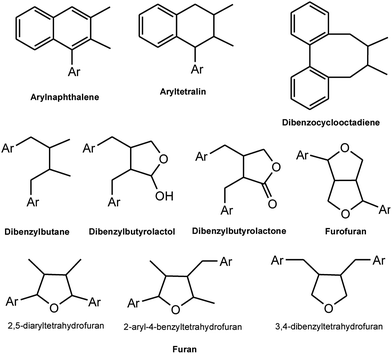
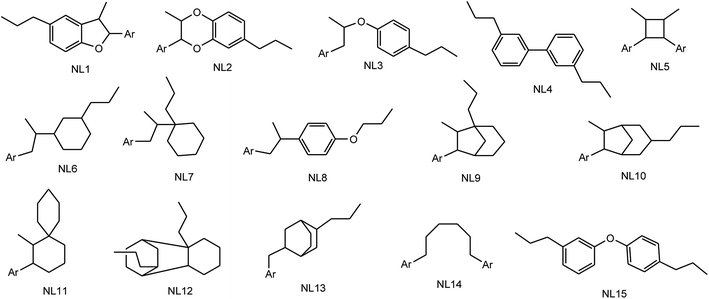
Lignans and neolignans are a large group of naturally occurring phenols which are widely spread within the plant kingdom and are derived from the shikimic acid biosynthetic pathway. These compounds show dimeric structures formed by a β,β′-linkage between two phenyl propane units with a different degree of oxidation in the side-chain and a different substitution pattern in the aromatic moieties.3 Similar to the ecological functions of several other secondary metabolites, lignans represent a means of protection against herbivores and microorganisms for the plants that synthesize them. For nomenclature purposes, the C6–C3 unit is treated as propylbenzene and numbered from 1 to 6 in the ring, starting from the propyl group, and with the propyl group numbered from 7 to 9, starting from the benzene ring. With the second C6–C3 unit, the numbers are primed. When the two C6–C3 units are linked by a bond between positions 8 and 8′, the compound is named as a “lignan”. In the absence of the C–8 to C–8′ bond, the dimer formed from the two C6–C3 units is a “neolignan”.2,4 Compounds in which an ether oxygen atom provides the linkage between the two C6–C3 units, are also classified under neolignans and are called oxyneolignans. Norlignans are defined as a group of related natural compounds, usually found to co-occur with lignans or neolignans, which have a C16 to C17 core structure. From the biosynthetic point of view, these compounds are plausibly derived from two arylpropane units by the loss of one or two carbons, probably through decarboxylations.As depicted in Fig. 1, lignans are classified into eight groups on the basis of their structural patterns, including their carbon skeletons, the way in which oxygen is incorporated into the skeletons, and the cyclization pattern. These are arylnaphthalene, aryltetralin, dibenzocyclooctadiene, dibenzylbutane, dibenzylbutyrolactol, dibenzylbutyrolactone, furan, and furofuran.5,6 Neolignans consist of fifteen subtypes designated as NL1 to NL15 (see Fig. 2) and no special names have been assigned to them.2,7
1.2 Biosynthesis and distribution of lignans and neolignans
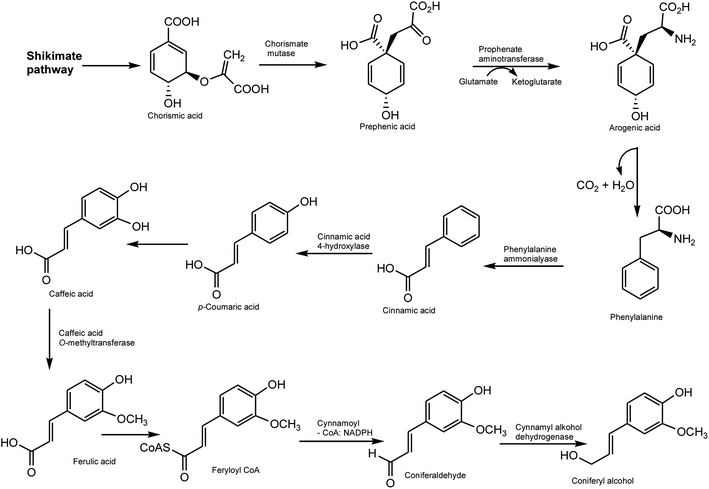
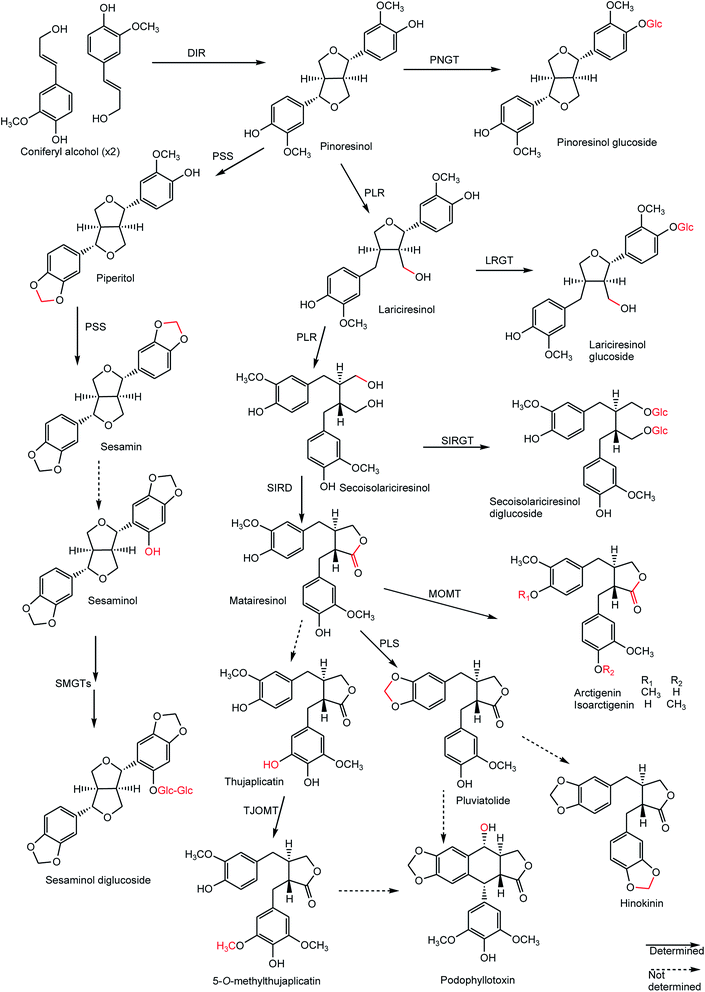
Both lignans and neolignans originate from cinnamic acid derivatives which are related biochemically to the metabolism of phenylalanine. Chorismic acid is transformed into prephenic acid via a Claisen rearrangement, which transfers the phosphoenolpyruvate derived side-chain so that it becomes directly bonded to the carbocycle, and thus builds up the basic carbon skeleton of phenylalanine. Decarboxylative aromatization of prephenic acid yields phenylpyruvic acid, and pyridoxal phosphate-dependent transamination leads to L-phenylalanine.8,9 The biosynthesis of coniferyl alcohols is initiated with deamination of phenylalanine by phenylalanine ammonialyase to form cinnamic acid, which is then hydroxylated by a P450 enzyme, cinnamate 4-hydroxylase, to form p-coumaric acid. Coniferyl alcohol is derived from the reduction of coumaric acid via coenzyme A ester to an aldehyde, which is further reduced in the presence of a NADPH molecule. Formation of the coenzyme A ester facilitates the first reduction step by introducing a better leaving group (CoAS−) for the NADPH-dependent reaction (Scheme 1).8,9The biosynthesis of lignans with 9(9′)-oxygen is very well studied that is clearly revealed by some important results obtained in the last couple of years. This type of lignan is formed by enantioselective dimerization of two coniferyl alcohol units with the aid of a dirigent protein (DIR) to give rise to pinoresinol (furofuran). Pinoresinol is then reduced to secoisolariciresinol (dibenzybutane) by pinoresinol/lariciresinol reductase (PLR), via lariciresinol (furan), which is in turn oxidized to afford matairesinol (dibenzylbutyrolactone) by secoisolariciresinol dehydrogenase (SIRD) (Scheme 2). The conversion from coniferyl alcohol to matairesinol has been demonstrated in various plant species, which strongly suggests that this is the general biosynthetic pathway of lignans.5,6,9 Pinoresinol also undergoes glucosylation by a putative pinoresinol glucosyltranferase (PNGT). Such glycosylation is highly likely not only to suppress the chemical reactivity of a phenolic hydroxyl group of pinoresinol but also to potentiate high water solubility of pinoresinol aglycone, resulting in large and stable amounts of pinoresinol.10 Similar to pinoresinol, lariciresinol and secoisolariciresinol can be glycosylated by the action of lariciresinol glycosyltransferase (LRGT) and secoisolariciresinol glycosyltransferase (SIRGT), respectively (Scheme 2). In various plants such as Forsythia koreana, Carthamus tinctorius, and Anthriscus sylvestris, matairesinol is metabolized to arctigenin/isoarctigenin by matairesinol O-methyltransferase (MOMT) via methylation of a phenolic hydroxyl group.11,12 Additionally, 70–90% of matairesinol is glucosylated throughout the year in the Forsythia leaves, although no matairesinol-glucosylating enzymes have been identified.13 In Linum, Anthriscus, and Podophyllum plants, matairesinol is also converted into hinokinin, yatein or podophyllotoxin via multiple biosynthetic pathways, even though all relevant enzymes have not yet been identified.6 The isolation and characterization of a thujaplicatin-specific plant O-methyltransferase (TJOMT) from A. sylvestris that exclusively catalyzed regioselective methylation of thujaplicatin to produce 5-O-methylthujaplicatin, a biosynthetic precursor of the lignan podophyllotoxin, was recently reported by Ragamustari et al.14 Furthermore, next generation sequencing (NSG) coupled to bioinformatics and metabolome analyses of Podophyllum tissues led to the discovery of two putative unknown genes in the podophyllotoxin biosynthetic pathway.15 The cytochrome P450s CYP719A23 from P. hexandrum and CYP719A24 from P. peltatum were capable of specifically converting (−)-matairesinol to (−)-pluviatolide by catalyzing methylenedioxy bridge formation and did not act on other possible substrates that were tested. On one hand, the enzymes described in this study were highly similar to methylenedioxy bridge-forming enzymes from alkaloid biosynthesis. On the other hand, candidates more similar to lignan biosynthetic enzymes were catalytically inactive with the substrates employed. Pinoresinol is metabolized into piperitol, followed by further conversion into (+)-sesamin by piperitol/sesamin synthase (PSS), a cytochrome P450 family enzyme, CYP81Q1, which is responsible for the formation of two methylenedioxy bridges (Scheme 2). Although sesamin is anticipated to be further metabolized into sesaminol, the underlying molecular mechanisms have not yet been elucidated. Sesaminol is glucosylated at its 2-hydroxyl position by sesaminol 2-O-glucosyltransferase (SMGT) in Sesamum radiatum, Sesamum indicium, and Sesamum alatum.6
Schisandraceae plants are rich in lignans, especially dibenzocyclooctadiene lignans,16–18 which show varied beneficial pharmacological effects including anti-HIV, antitumor-promoting, calcium antagonistic, and anti-lipid peroxidative actions.18,19 Furofuran type lignans are widely distributed in edible plants [flaxseed, sesame (Pedaliaceae), seeds, cereal products (Poaceae or Gramineae), and Brassica vegetables (Brassicaceae)]. Most of these dietary lignans are metabolized by the gut microflora to enterolactone and enterodiol, also known as enterolignans, and are traditionally classified as phytoestrogens20 which have demonstrated protective efficacies against prostate and breast cancer.21,22
1.3 Hyphenated techniques used for the isolation, identification and quantification of lignans and neolignans
Phenolic compounds such as lignans and neolignans are commonly extracted using organic and/or inorganic solvents. Several parameters may influence the extraction efficacy and yield, including extraction time, temperature, solvent-to-sample ratio, the number of repeat extractions of a sample, in conjunction with the type of solvent employed. Furthermore, the optimum recovery is mostly different when comparing one sample to the other and relies on the type of plant tissue and its active constituents. The choice of extraction solvents such as water, acetone, ethyl acetate, alcohols (such as methanol, ethanol and propanol) and their mixtures have been shown to influence the yield.23 The remarkable improvement in hyphenated analytical techniques in the recent years has significantly broadened the scope and application of these techniques in the analysis of natural products. High-performance liquid chromatography (HPLC) with photodiode array detector (PDA) and tandem mass spectrometry (MSn) has facilitated qualitative and quantitative evaluation of lignans.24,25 For example, an accurate, sensitive and reliable method was developed in 2012 using HPLC coupled to PDA and a mass spectrometer for quantitative analysis of twelve lignans in the fruit of the Chinese medicinal plant Schisandra sphenanthera.26 The chemodiversity of Linum plants (Linaceae) was explored by Schmidt et al.27 who identified 64 different lignans of the aryltetralin-, arylnaphthalene-, aryldihydronaphthalene-, dibenzylbutyrolactone-, and furofuran-types from 41 Linum species by means of HPLC-ESI-MS/MS.Although HPLC-MSn allows identification of compounds by taking into account retention times and mass spectral data, gas chromatography (GC) coupled with electron capture detection (ECD) has been shown to be another highly sensitive technique that can detect specific substances in complex matrices up to the picogram or even femtogram level. A new method for lignan analysis in wheat bran using GC with ECD was presented by Cukelj et al.28 The method was relatively simple, requiring methanolysis and enzymatic hydrolysis, followed by solid-phase extraction as the sole technique for sample concentration and purification. Furthermore, lignans were detected and quantified for the first time in pomegranate (Punica granatum L.) and in commercial pomegranate juices by means of GC-MS.29 Furthermore, a rapid and sensitive micellar electrokinetic capillary chromatography method has recently been developed for the simultaneous quantitative analysis of eight lignans in the seed, pulp, stem, rattan and leaf tissues of Schisandra sphenanthera.30
2 Lignans
2.1 Dibenzocyclooctadiene derivatives
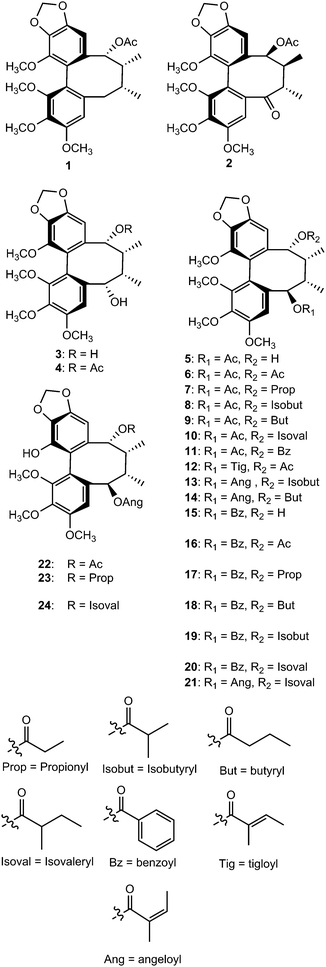
About 131 dibenzocyclooctadiene lignans, isolated mainly from the plant genera Kadsura and Schisandra belonging to the family Schisandraceae, were published as natural products between 2009 and 2015. Strikingly, this class of lignans has seen remarkable growth in the number of new examples reported recently, with more than 130 in the period 2009 to 2015 compared to only 32 in the previous four years. The structures and absolute configurations of fourteen new dibenzocyclooctadiene lignans, ananolignans A–N (1–14) isolated from the seeds of Kadsura ananosma, were established using a combination of spectroscopic methods and CD techniques. Ananolignans F and ananolignan L showed the most promising in vitro cell survival data against oxidative stress-induced neurotoxicity.31Further investigation on the seeds of this plant led to isolation of ananonins A–N (15–28). Ananonin M (27) was shown to exhibit moderate neuroprotective effects in an in vitro assay.32 The dibenzocyclooctadiene lignans polysperlignans A–K (29–39) were isolated from the stems of Kadsura polysperma. Polysperlignans A, B, D and E showed statistically significant neuroprotective effects against β-amyloid or hydrogen peroxide-induced neurotoxicity on PC12 cells.33
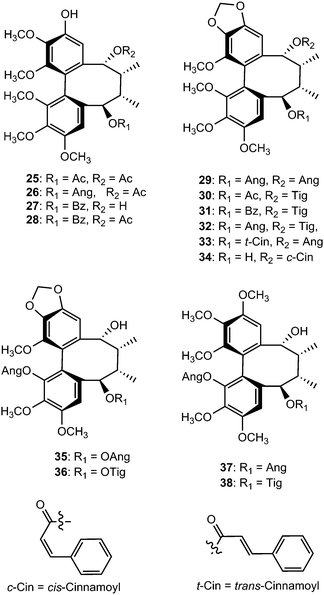
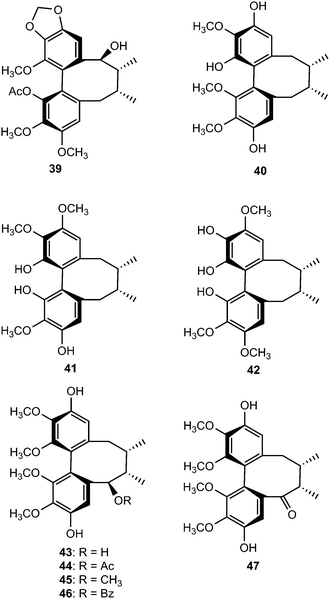
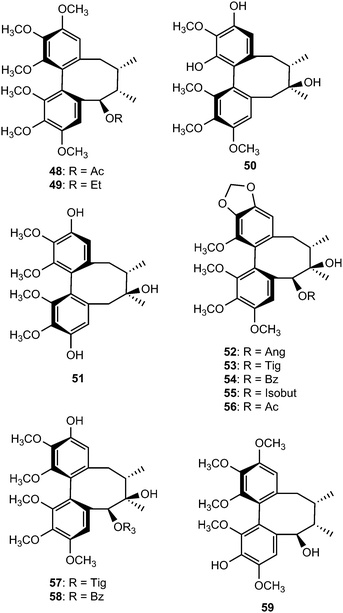
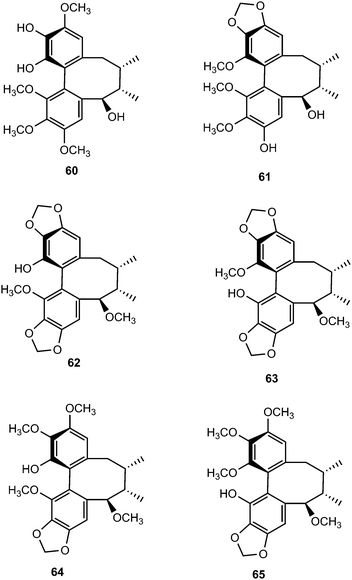
Chemical investigation of the 70% aqueous acetone extract of leaves and stems of Schisandra wilsoniana led to the isolation of marlignans A–L (40–51).34 These compounds were evaluated for their anti-HIV activities, in which marlignans C, E, H, and L showed moderate activities with therapeutic index values of 13, 16, 18, and 16, respectively. Schisanwilsonins A–C (52–54), D (57), E (55), F (56) and G (58) have been reported from the same plant species originating from the Yunnan province in People's Republic of China.35 More recently, Yang et al.36 investigated the fruits of S. wilsoniana that further led to the isolation of the dibenzocyclooctadiene lignans, marlignans M–S (59–65). Their cytotoxicity was measured in addition to determining their antiviral activity, using AZT as a positive control (EC50 = 0.0043 μg mL−1 and CC50 > 200 μg mL−1). They showed anti-HIV-1 activity with EC50 values in the range 3–6 μg mL−1 and therapeutic index (TI) values of 5–23.36
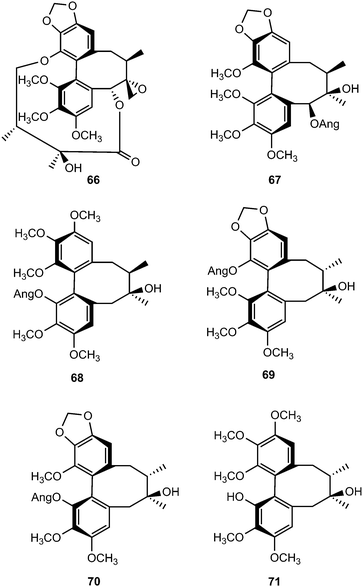
With an aim to discover bioactive secondary metabolites from the fruits of Schisandra chinensis (Turcz.) Baill, Sun and coworkers isolated seven new C18-dibenzocyclooctadiene lignans, schinlignans A–G 66–72.37 Schinlignan A is a C18-dibenzocyclooctadiene lignan with an ester linkage structurally related to gomisin D that was previously isolated from the same species and whose absolute structure had been determined by X-ray diffraction analysis.38 Schinlignan G exhibited potent anti-hepatitis B viral activity against HBV DNA replication with IC50 values of 5 μg mL−1. Schisandra propinqua is used in Chinese folk medicine for the treatment of arthritis, traumatic injury, gastralgia, angeitis, and related diseases.
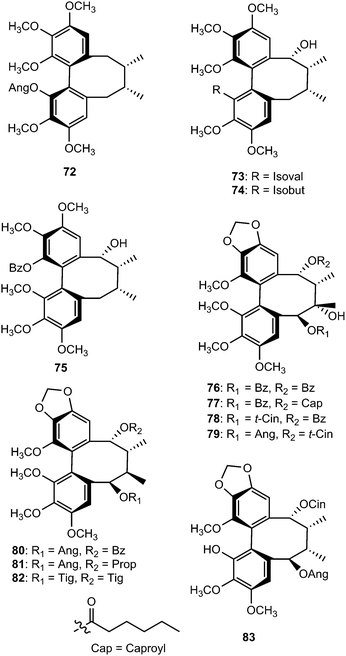
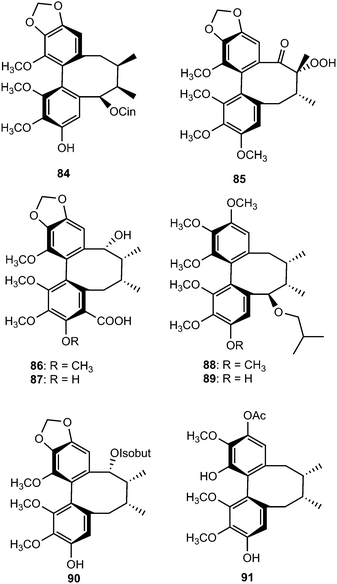
Tiegusanins A–M (73–85) are constituents of the aerial parts of this plant.39 The isolated compounds were tested for their ability to prevent the cytopathic effects of HIV-1 in C8166 cells, and their cytotoxicity was measured in parallel with the determination of antiviral activity, using AZT as a positive control. Tiegusanin G showed anti-HIV-1 activity with an EC50 value of 8 μM and a therapeutic index (TI) of more than 25.
Neglignans A (86), B (87), and E–G (88–90) were isolated from the acetone extract of the stem of Schisandra neglecta. Neglignans A and B are the first dibenzocyclooctadiene lignans bearing a carboxyl group at C-4.40 Neglignans B and F showed anti-HIV-1 activities with therapeutic index values greater than 50. Further phytochemical investigation of S. neglecta using RP-HPLC led to the isolation and characterization of neglectalignans A–D (91–94), which showed moderate anti-HIV-1 activities with therapeutic index values above 62, 23, 58, and 28, respectively.41 Neglschisandrins E (95) and F (96) were reported by Chen et al.18 as constituents of S. neglecta. Compound 96, which possesses an endocyclic double bond rather than the exocyclic bond found in neglschisandrin B,42 exhibited marginal cytotoxicity against the human lung carcinoma A549 cell line with EC50 values of 12 μg mL−1.
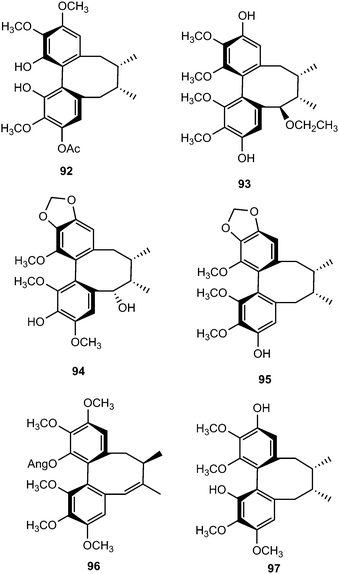
Arisanschinins F–L (97–103), characterized from the fruits of the Taiwanese medicinal plant Schisandra arisanensis, showed moderate in vitro inhibition of proliferation of peripheral blood mononuclear cells (PBMC) induced by phytohemaglutinin (PHA).43 The EtOAc extract of fruits of Schisandra chinensis (Turcz.) Baill yielded four new dibenzocyclooctadiene lignans, namely schisanchinins A–D (104–107). The stereochemistry of the chiral centers and the biphenyl configuration were determined using NOESY, as well as analysis of CD spectra. Compounds 104 and 105 inhibited NO release by LPS-activated microglia cells.44 Schisandrosides A–D (108–111) were also isolated from S. chinensis. Although they demonstrated no cytotoxicity against endometrial cancer cells using 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) assays, they represent the first examples of natural dibenzocyclooctadiene lignan glucosides.45
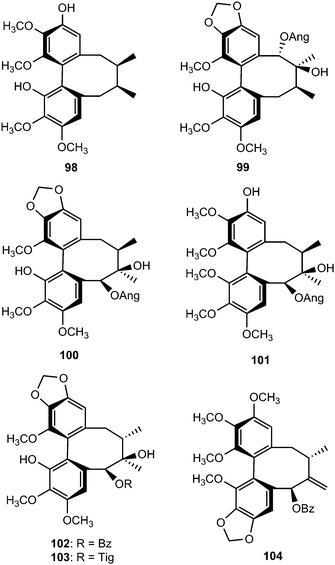
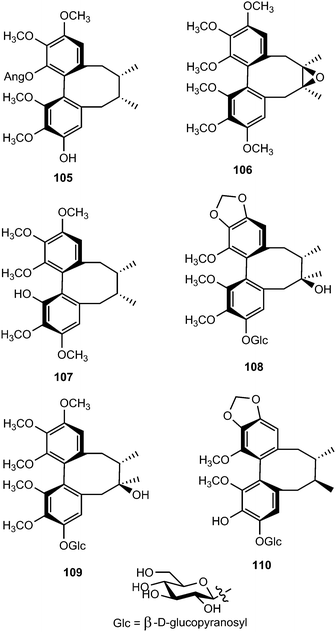
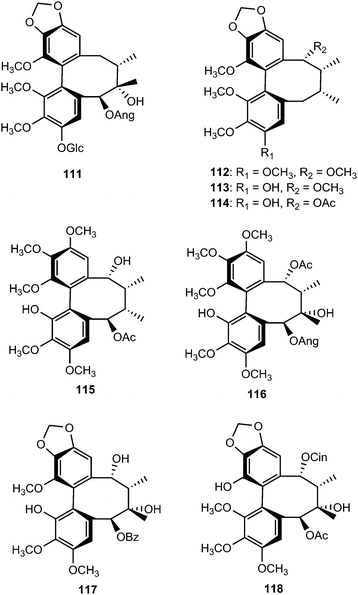
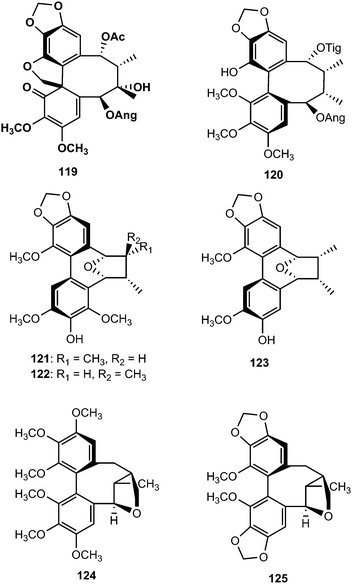
Phytochemical examination of the leaves and stems of Schisandra lancifolia led to the isolation of three new dibenzocyclooctadiene lignans, schilancifolignans A–C (112–114),46 which exhibited weak anti-HIV-1 activity with therapeutic index values of 8, 12 and 7, respectively. Kadsuphilols I–M (115–119) are among the lignans obtained from the aerial parts of Kadsura philippinensis Elmer by column chromatography using normal and reversed phase HPLC. Their structures were determined by analysis of spectroscopic data, CD spectra, as well as by X-ray analysis.17 Kadsuphilol M is a C19-homolignan with a spirobenzofuranoid skeleton. These compounds exhibited moderate in vitro radical scavenging activities on DPPH.
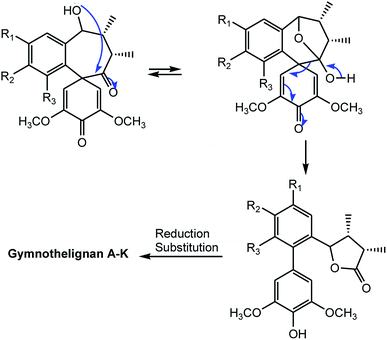
14-O-Demethyl polysperlignan D (120) was obtained from the EtOAc soluble fraction of the methanol extract of the roots of Kadsura coccinea.47 Gymnothelignans L (121) and M (122) are epimers containing a 7,7′-epoxide that were isolated from Gymnotheca chinensis (Saururaceae).48 A related compound, manglisin B (123), recently isolated from the mature carpels of Manglietiastrum sinicum (Magnoliaceae), was shown to exhibit antimicrobial activities against Staphylococcus aureus, MRSA 82#, MRSA 92#, MRSA 98#, and MRSA 331# with MIC of 0.07, 0.14, 0.07, 0.03 and 0.14 μM, respectively.49
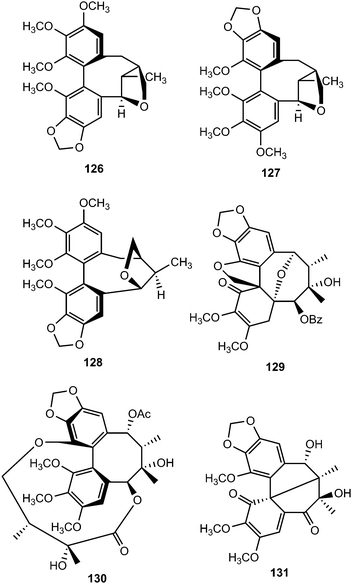
From the dichloromethane extracts of aerial parts of Talauma gloriensis and Magnolia fraseri (Magnoliaceae), five new dibenzocyclooctadiene-type lignans containing a 7,9′-epoxy-2,2′-cyclolignane skeleton, were isolated. By using NMR spectroscopy, mass spectrometry, experimental and calculated circular dichroism (CD) and as well as by X-ray crystallography analysis, their structures were elucidated as (Ra)-3,3′,4,4′,5,5′-hexamethoxypyramidatin (124), (Ra)-3,3′-dimethoxy-4,5,4′,5′-bis(methylenedioxy)pyramidatin (125), (Ra)-3,3′,4,5′-tetramethoxy-4,5-methylenedioxypyramidatin (126), and (Ra)-3,3′,4,5-tetramethoxy-4′,5′-methylenedioxypyramidatin (127) and (Sa)-3,3′,4,5′-tetramethoxy-4,5-methylenedioxypyramidatin (128).50 Taiwankadsurin F (129), kadsuphilins N (130) and O (131) were also isolated from K. philippinensis. The structure of compound 131 was further confirmed by X-ray crystallographic analysis.51
In the search of new inhibitors of prostaglandin E2 and leukotriene B4, four derivatives of schisandrin, a major dibenzocyclooctadiene lignan of Schisandra chinensis (Turcz.) Baillon52 were synthesized and structurally characterized by means of NMR, mass spectrometry and the axial chirality of the biphenyl system was determined by comparison of calculated with measured CD spectra.53 Selective demethylation with 1.1 equivalent of BBr3 (reaction a) turned out to be unsuccessful, because a complex mixture of products was formed, out of which only compounds 133 and 134 could be isolated in pure form. An excess of demethylation reagent (6.5 equiv. BBr3) led to a more uniform product distribution (reaction b) and compound 132 was obtained at 75% yield. By reaction of 9-iodo-9-bora-bicyclononane (9-I-9-BBN) with schisandrin, a procedure proposed by Fürstner and Seidel54 (reaction c), mainly three products were obtained (Scheme 3). Compound 135, in which the ring contraction occurred without any demethylation, alone could be structurally elucidated by NMR. While the original lignan was inactive, compounds 133 and 135 showing the cycloheptadiene skeleton revealed remarkable inhibitory activity on prostaglandin E2 and leukotriene B4 formation in vitro with IC50 values of 4.2 and 4.5 μM, respectively.53
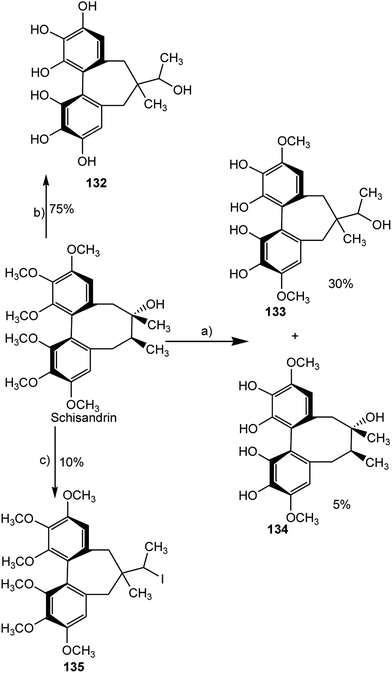 | ||
| Scheme 3 Chemical modification of schisandrin. Reaction conditions: (a) 1.1 equiv. BBr3; (b) 6.5 equiv. BBr3; (c) 5.3 equiv. 9-iodo-9-BBN, CH2OHCH2NH2. | ||
2.2 Arylnaphthalene and aryltetralin derivatives
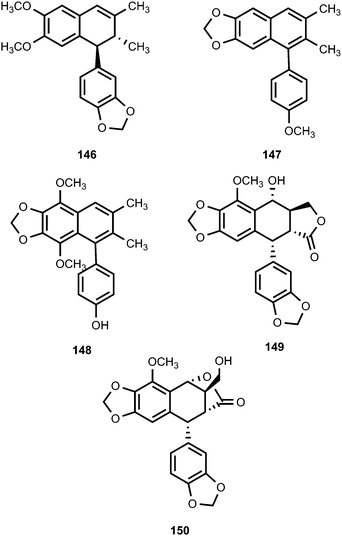
These important classes of lignans continue to attract the attention of the pharmaceutical industries owing to their remarkable biological activities. The structures of ovafolinins A–E (136–140), isolated from the wood of Lyonia ovalifolia (Ericaceae), were elucidated based on 2D NMR spectroscopy, X-ray crystallography, and chemical methods. Although the isolated compounds did not show cytotoxic activity, ovafolinins A–C (136–138) were determined to be unique aryltetralin lignans containing 7-membered ring formed by the C9–C2′ ether linkage. Furthermore, ovafolinin D (139) is the first example of a naturally occurring aryltetralin-lignan bearing a methyleneoxy-bridge.55 More recently, Kuo et al.56 reported (+)-ovafolinin B-9′-O-β-D-glucopyranoside (141), (−)-ovafolinin B-9′-O-β-D-glucopyranoside (142), (+)-ovafolinin E-9′-O-β-D-glucopyranoside (143), and (−)-ovafolinin E-9′-O-β-D-glucopyranoside (144) the stems of Eurya japonica (Theaceae). Compounds 141 and 142 demonstrated potent antioxidant activities (ED50 23 and 25, respectively) compared to the positive control α-tocopherol (ED50 27 μM).The roots of Pycnanthus angolensis (Myristicaceae) afforded four new cyclolignene derivatives, named pycnanthulignenes A–D (145–148).57 Pycnanthulignenes A and C showed moderate antimicrobial activities against a panel of drug-resistant pathogens with an MIC of 29 μM for pycnanthulignene A against Staphylococcus aureus and 64 μM for pycnanthulignene C against S. aureus, Escherichia coli, and Candida albicans. Bioassay-guided purification of the CH2Cl2 extract from the fruits of Cleistanthus indochinensis (Euphorbiaceae) afforded two aryltetralin lignans, cleistantoxin (149) and neocleistantoxin (150).58 These compounds were evaluated for their cytotoxicity. Cleistantoxin had strong activity against KB (IC50 0.02 μM), MCF-7 (IC50 0.04 μM), MCF-7R (IC50 0.01 μM), and HT29 (IC50 0.03 μM) cancer cell lines while neocleistantoxin had only moderate cytotoxicity. The structure of cleistantoxin is similar to that of podophyllotoxin, which is well-known for its antiviral and antitumor properties.59
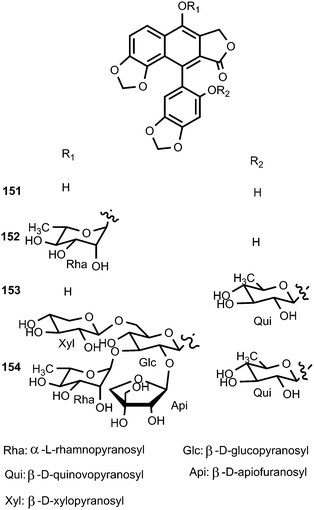
Four new arylnaphthalene lignans (151–154) were isolated from the aerial parts of Acanthus mollis (Acanthaceae) during its flowering season. Owing to the axial chirality, their structures, including absolute configurations, were determined by means of extensive spectroscopic data and computational chiroptical methods. Compounds 152–154 showed significant inhibition of the growth of crown gall tumors on potato disks. Furthermore, they exhibited significant antiproliferative activity against Paracentrotus lividus.60
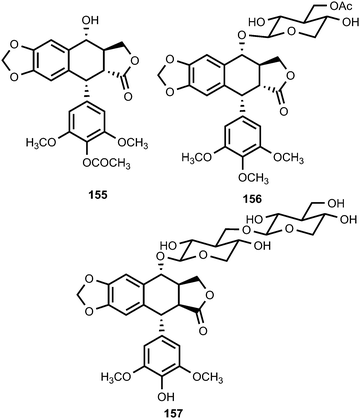
Sun et al.61 obtained the aryltetralin lignans, 4-acetyl-4-demethyl-podophyllotoxin (155), sinolignan A (156) and sinolignan B (157) from the roots and rhizomes of Sinopodophyllum emodi (Berberidaceae).61 Compound 155 showed potent cytotoxicity against Hela and KB cell lines with IC50 values of 0.08 and 0.05 μM, respectively. Sargentodosides A–C (158–160) were isolated from ethanol extracts (60%) of Sargentodoxa cuneata (Lardizabalaceae) together with a couple of racemic mixtures constituting sargentodognans F (161) and G (162) bearing tyramine moieties.62 The aryltetralin-type lignan glycosides 163 and 164, isolated from the branches of Tabebuia chrysotricha (Martius ex De Candolle) Standley (Bignoniaceae), showed moderate DPPH radical-scavenging activity (IC50 of 44 and 35 μM, respectively) compared to Trolox used as a positive control (IC50 of 17 μM).63
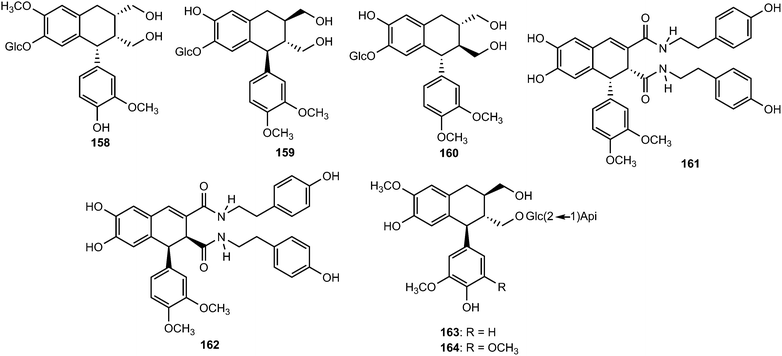
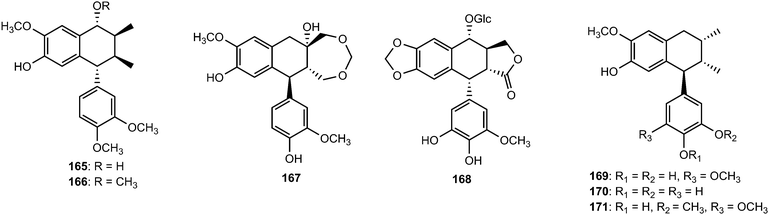
Other aryltetralin lignans include compound 165 and its methyl ether analog 166 isolated from Holostylis reniformis (Aristolochiaceae)64 and the aryltetrahydronaphthalene (+)-cycloolivil formaldehyde condensate (167) obtained from the whole plants of Epimedium brevicornum (Berberidaceae).65 4,5-Didemethylpodophyllotoxin 7′-O-β-D-glucopyranoside (168), one of the constituents of the n-butanol extract of roots and rhizomes of Sinopodophyllum emodi, showed in vitro cytotoxicity against four human cancer cell lines (Hela, K562, SH-SY5Y and CNE).66 The bark of Machilus robusta (Lauraceae) yielded four new lignans (169–172).25 At a concentration of 10 μM, 169 and 171 reduced DL-galactosamine-induced hepatocyte (WBF344 cells) damage with 77.2 ± 4.1% and 64.0 ± 5.4% inhibition, respectively, while the positive control bicyclol gave a 67.0 ± 4.6% inhibition.
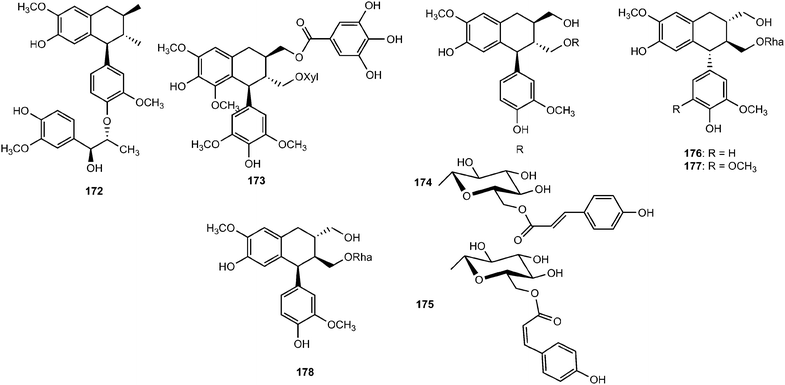
Omar et al.67 published the first example of a lignan galloyl ester (+)-2α-O-galloyl lyoniresinol 3α-O-β-D-xylopyranoside (173) from sapwood of Quercus mongolica var. crispula (Fagaceae). The structures and absolute configurations of lignan glycosides 174–178, recently isolated from the aerial parts of Lespedeza cuneata (Leguminosae), were elucidated on the basis of spectroscopic analyses. These compounds were tested for their anti-ulcerative colitis activity by dual luciferase report gene assay targeting xbp1. Compounds 174, 176 and 178 could activate the transcription of xbp1 to varying degrees with EC50 values of 0.17 ± 0.06, 0.25 ± 0.05, and 0.12 ± 0.03 μM, respectively.68
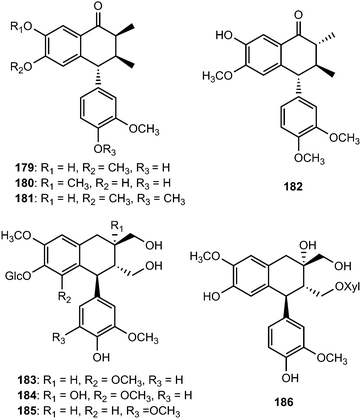
Arisantetralones A–D (179–182) are among the lignans isolated from the fruits of Schisandra arisanensis.43 The structure and stereochemistry of Arisantetralone A were confirmed by X-ray crystallographic analysis. Arisantetralone D exhibited significant inhibition of proliferation of peripheral blood mononuclear cells (PBMC) induced by phytohemaglutinin (PHA). The aryltetralin type lignan glycosides 5′-methoxy-(+)-isolariciresinol-4′-O-β-D-glucopyranoside (183), 5′-methoxy-8′-hydroxyl-(+)-isolariciresinol-4′-O-β-D-glucopyranoside (184), 5-methoxy-(+)-isolariciresinol-4′-O-β-D-glucopyranoside (185), and 8′-hydroxy-(+)-isolariciresinol-9-O-β-D-xylopyranoside (186) were isolated from the roots of Rhodiola crenulata (Crassulaceae).69
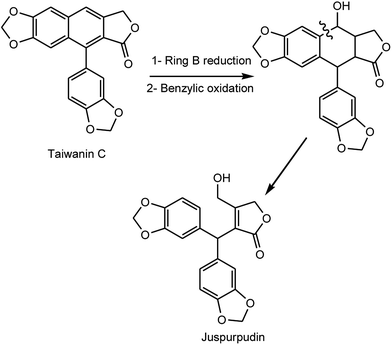
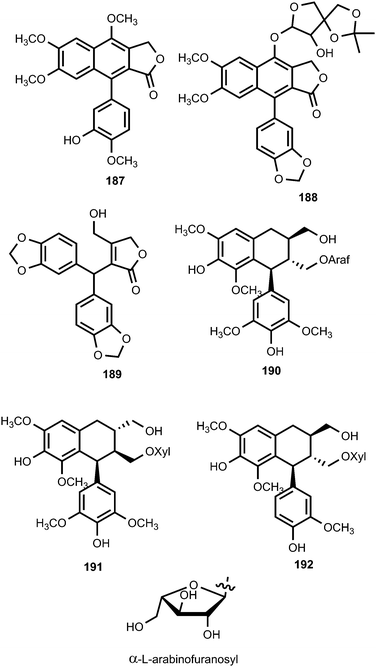
Pronaphthalide A (187), procumbenoside J (188) and juspurpudin (189) are two lignans and a secolignan from Justicia procumbens L. (Acanthaceae).70 Procumbenoside contains an apiofuranosyl acetonide residue in its structure. In its 1H NMR spectrum, some signals existed as pairs due to the equilibrium between two conformational isomers resulting from the slow rotation of the sugar unit at room temperature around the glucosidic linkage with the aglycone. Juspurpudin is a rare type of secolignan, being part of a group of only a few other similar compounds reported so far.71 Its biosynthesis was suggested to occur via a typical lignan derivative taiwanin C, also isolated from the same plant (Scheme 4). It was proposed that taiwanin C could be transformed to an intermediate which shared a structural similarity with podophyllotoxin by the process of ring B reduction and benzylic oxidation. The following C–C bond cleavage in the intermediate and formation of α,β-unsaturated-γ-lactone would lead to juspurpudin. Compounds 187 and 188 exhibited potent cytotoxic activities in vitro against sensitive human LoVo and BGC-823 cell lines with IC50 values in the range of 0.11 to 0.53 μM.70
Linderanosides A (190) and B (191), isolated from the trunk of Lindera glauca (Lauraceae), had selective cytotoxicity against A498 cells, with IC50 values of 21 and 22 μM, respectively.72 A bioassay-guided fractionation and chemical investigation of the methanolic extract of Tilia amurensis Rupr. (Tiliaceae) trunk resulted in the isolation and identification two new lignan glycosides named tiliamurosides A (192) and B (193). Tiliamuroside B showed significant cytotoxicity against A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines with inhibitory concentration (IC50) of 7.3, 8.9, 7.8 and 6.2 μM, respectively.73
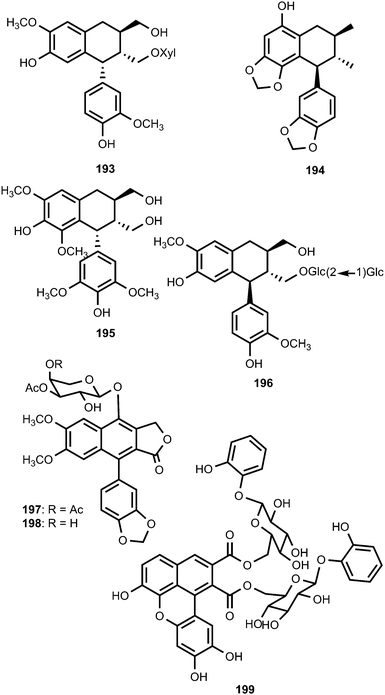
Saurunarin (194) is one of the previously unreported lignans from the aerial parts of Saururus chinensis (Saururaceae).74 Phytochemical investigation of the extracts from the leaves and twigs of Euonymus alatus (Celastraceae) having antihyperglycaemic, hyperlipidaemic and immune-stimulating activities75 led to the isolation and structure elucidation of (7R,8R,7′R)-(+)-lyoniresinol (195).76 Saposide A (196), isolated from the maple sap of Acer saccharum (Aceraceae), showed moderate antioxidant activity on superoxide dismutase.77 The structure of the arylnaphthalene lignan lactone glycoside 7-O-[(3,4-di-O-acetyl)-α-L-arabinopyranosyl]diphyllin (197) obtained from Phyllanthus poilanei (Phyllanthaceae), together with 7-O-[(3-O-acetyl)-α-L-arabinopyranosyl]-diphyllin (198), was confirmed by analysis of its single-crystal X-ray diffraction data. These compounds exhibited cytotoxic activities against the HT-29 cells with IC50 values of 0.17 and 1.8 μM, respectively.78 The lignan ester 199 was isolated from the leaves of Dodecadenia grandiflora (Lauraceae) and is believed to be derived from dimerization of two phenylpropanoyl esters of catechol glycoside units.79 It showed significant antihyperglycemic activity in streptozotocin-induced (STZ) diabetic rats, which is comparable to the standard drug metformin.79
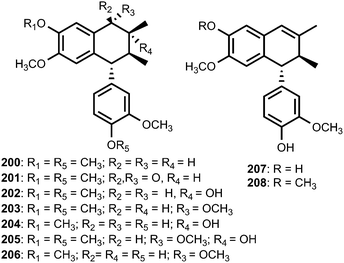
The well-known ability of Cunninghamella echinulata to hydroxylate benzylic positions of aromatic substrates80 prompted Messiano et al.81 to investigate the metabolism of aryltetralin lignans by this fungus. To this end, the aryltetralin lignan (−)-isogalbulin (200) was obtained in high overall yield by the treatment of (−)-8′-epi-aristoligone (201) with NaBH4/methanol followed by hydrogenolysis (H2/10% Pd on C/EtOH). When 200 was incubated with C. echinulata for 20 days, the EtOAc extract of the resulting culture broth afforded seven new metabolites: (−)-8-hydroxyisogalbulin (202), (−)-7-methoxyisogalbulin (203), (−)-4′-O-demethyl-8-hydroxyisogalbulin (204), (−)-7-methoxy-8-hydroxyisogalbulin (205), (−)-4′-O-demethyl-7-methoxyisogalbulin (206), (−)-4′,5-O-didemethylcyclogalgravin (207), and (−)-4′-O-demethylcyclogalgravin (208).
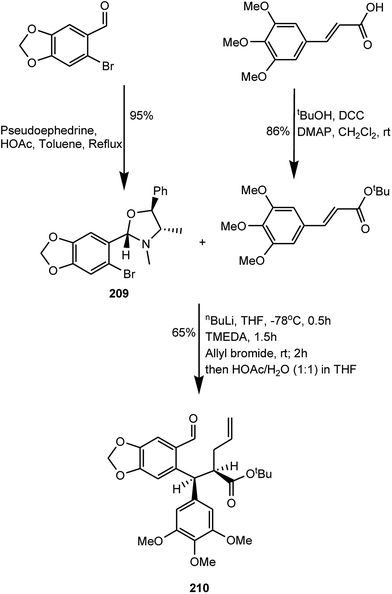
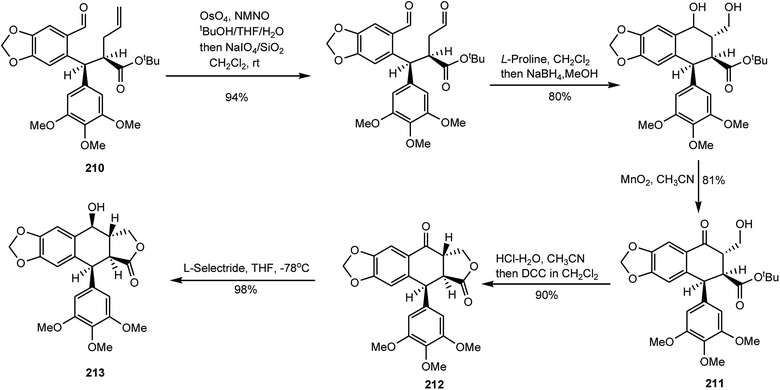
Podophyllotoxin has been in use for a while for treating venereal warts and also serves as an important precursor for the preparation of clinical antitumor drugs etoposide and teniposide.82,83 A growing worldwide demand for podophyllotoxin has exerted severe pressure on the plant resource. Wu and coworkers have reported a highly flexible and concise total synthesis of (+)-podophyllotoxin featured with an enantioselective sequential conjugate addition–allylation reaction. Starting from commercially available 3,4,5-trimethoxycinnamic acid, this new route leads to (+)-podophyllotoxin in only eight steps with 29% overall yield.84 First, 3,4,5-trimethoxycinnamic acid was converted to its n-butylester, and 6-bromopiperonal was treated with (+)-pseudoephedrine and directly converted to oxazolidine 209 as a single diastereoisomer. For Michael addition–alkylation,85N,N,N′,N′-tetramethylethylenediamine (TMEDA), a frequently used solvating agent in organolithium chemistry with observed beneficial effects on stereoselectivity, was added in order to optimize the stereoselectivity and aldehyde 210 was obtained with 65% yield (Scheme 5). Aldehyde 210 was then submitted to an oxidative cleavage followed by an L-proline-mediated aldol cyclization and a selective oxidation with MnO2 to afford intermediate 211. After the tert-butyl ester group was hydrolyzed by treatment with hydrochloric acid in acetonitrile, an esterification with DCC provided (+)-podophyllotoxone (212) with around 90% yield. Selective reduction with L-selectride provided ent-podophyllotoxin (213) with around 98% yield as a single isomer (Scheme 6).
Many arylnaphthalene lignans are components of traditional herbal remedies and have received increased attention over the past several decades owing to their cytotoxic,86 antiviral,87 fungicidal, antiprotozoal, and antiplatelet activities in cell-based assays.88,89 A silver-catalyzed one-pot synthesis of the arylnaphthalene lactone core using carbon dioxide, phenylpropargyl chloride, and phenylacetylene, previously demonstrated by Foley et al.,90 was applied to the synthesis of the naturally occurring arylnaphthalene lignans retrochinensin (216), justicidin B (217), retrojusticidin B (218), chinensin (219), justicidin E (220), and taiwanin C (221).91 By treating 3,4-methylenedioxyphenylpropargyl alcohol and 3,4-dimethoxyphenylpropargyl alcohol with thionyl chloride in N,N-dimethylacetamide (DMA), the desired phenylpropargyl chlorides 214a and 214b were generated with 96% and 84% yields, respectively. These substituted phenylpropargyl chlorides, together with phenylacetylenes 215a and 215b, could then be taken on to the multicomponent tandem coupling reaction as shown in Scheme 7.
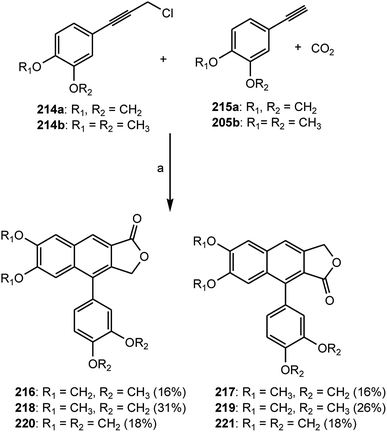 | ||
| Scheme 7 Preparation of arylnaphthalene lignans 216–221; areagents: SOCl2, DMA; K2CO3, 18-crown-6, 4 Å molecular sieves, DMA, 100 °C. | ||
2.3 Dibenzylbutane, dibenzylbutyrolactol and dibenzylbutyrolactone derivatives
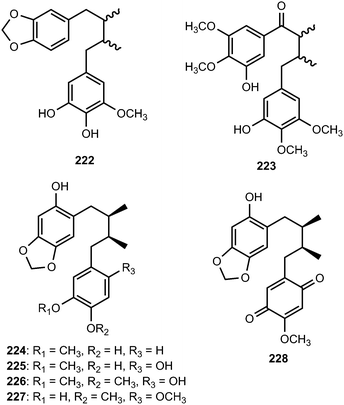
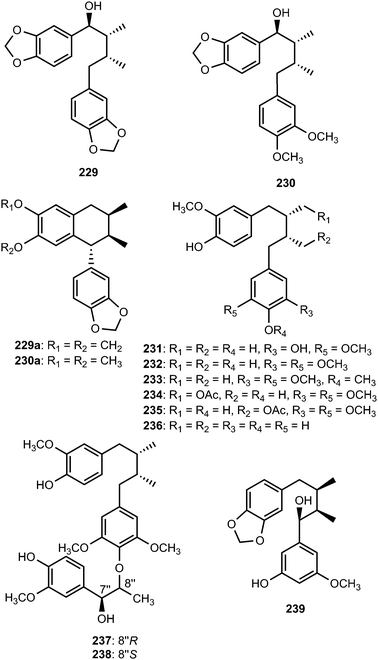
The EtOAc soluble fraction of methanol extract of the roots of Kadsura coccinea yielded two dibenzylbutane lignans kadsurindutin E (222) and coccilignan A (223), even though their absolute configurations remain to be determined. Compound 222 inhibited NO production in BV-2 cells with IC50 value of 24 μM.47 Saurulignans A–E (224–228) were reported from Saururus chinensis, a Chinese medicinal plant used to cure hepatitis, pneumonia, edema, jaundice, and gonorrhea.74 Saurulignan E, characterized by a 2-methoxy-1,4-benzoquinone moiety in its structure, showed significant inhibition of adenosine diphosphate-induced aggregation with an IC50 of 10 μM and arachidonic acid-induced aggregation with an IC50 of 14 μM.Magnovatins A (229) and B (230) are secondary metabolites from the leaves of Magnolia ovata (Magnoliaceae).92 In order to determine the absolute configuration of these compounds, they were submitted to an acid-catalyzed cyclization, yielding the new lignans 229a and 230a, respectively. CD analysis of the products allowed deduction of the absolute configurations of the substrates given that C-7 undergoes inversion of configuration during the cyclization process (SN2 mechanism).92 Magnovatin A was tested for free-radical scavenging activity using DPPH as well as for antimicrobial activity, but was inactive under the tested conditions. Phytochemical investigation of ethanol extract of bark of Machilus robusta (Lauraceae) led to the isolation of eight new lignans (231–238).25 Compounds 237 and 238 are uncommon 4′,8′′-oxy-8,8′-sesquilignans that exhibited in vitro activity against HIV-1 replication with IC50 of 2.5 and 2 μM, respectively. Furthermore, 231 reduced DL-galactosamine-induced hepatocyte (WBF344 cells) damage at 10 μM and could additionally attenuate rotenone-induced PC12 cell damage by increasing the cell viability from 73.0 ± 9.6% to 96.2 ± 15.6%. The dibenzylbutane-type lignan 239 and saururin B 240 was identified from the roots of Schisandra sphenanthera93 and Saururus chinensis Bail (Saururaceae),94 respectively.
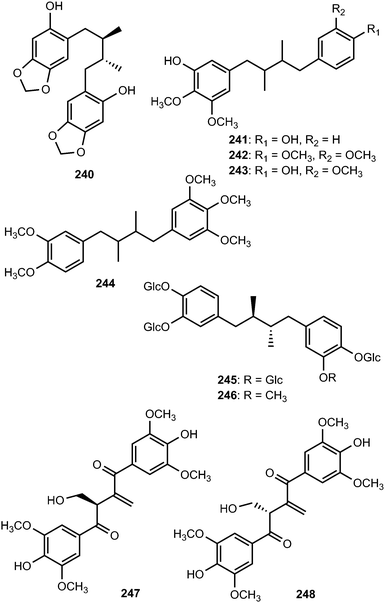
Schineolignins A–C (241–243) were isolated from the fruit of Schisandra chinensis and their relative stereochemistry could not be determined on the basis of ROESY spectrum because their C–C bonds can rotate randomly.95 The substituted 1,4-biphenyl-2,3-dimethylbutane-type lignan tiegusanin N (244) was reported from Schisandra propinqua as a racemate.39 The two meso-nordihydroguaiaretic acid glycoside derivatives, larrealignans A (245) and B (246), isolated from Larrea tridentata (Zygophyllaceae), are believed to be the first representatives of lignan tetra- and tri-desmosides, respectively. They did not exhibit cytotoxicity even at a sample concentration of 20 μM.96 During the investigation of bioactive components of Liriodendron hybrid (Magnoliaceae), a pair of unprecedented enantiomeric lignans, (+)- and (−)-liriodenols bearing a 1,1-disubstituted olefinic group was discovered. The above enantiomers were separated by HPLC to yield 8R-liriodenol (247) and 8S-liriodenol (248).97 The cytotoxicity of these three lignans ((±)-, (+)-, and (−)-liriodenols) was evaluated in vitro against four selected human tumor cell lines. It was noticed that (+)-liriodenol showed more significant cytotoxic effects (IC50 of 31, 45, 34 and 29 μg mL−1 against NIC-H460, BGC-823, SGC-7901 and MDA-MB-231, respectively) than the (±)- and (−)-liriodenol. The spatial configuration of lignans in 8-positions might induce different cytotoxic activities on tumor cells.
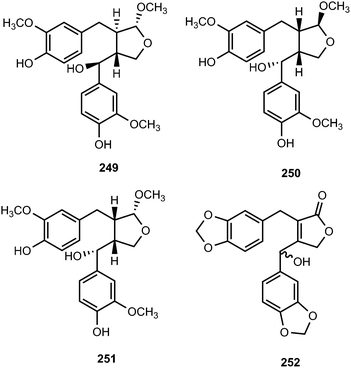
The EtOAc phase obtained from a methanol extract of the trunk of Abies holophylla (Pinaceae) exhibited inhibitory effects on NO production in murine microglia BV-2 cells. Successive chromatography through silica gel, Sephadex LH-20, and preparative HPLC of this extract resulted in isolation of three new 9,9′-epoxylignans, holophyllol A–C (249–251). Their structures were determined by spectroscopic methods, CD experiments, acid hydrolysis, and the use of a modified Mosher's method. The anti-neuroinflammatory activity of the isolates was evaluated via measurement of NO levels using bacterial endotoxin, LPS, in the murine microglia BV-2 cell line. Compounds 249 moderately inhibited NO production with IC50 of 36 μM in LPS-stimulated BV-2 cells without showing cell toxicity.98 The α,β-unsaturated-γ-lactone lignan procumbiene 252 was isolated from Justicia procumbens L.70
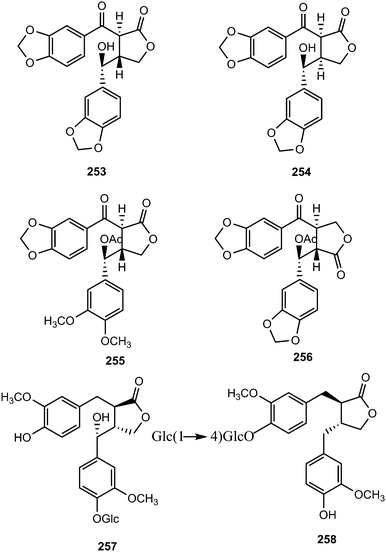
The leaves of Piper sanguineispicum Trel. (Piperaceae), used in Peru (Loreto Department) as an anti-inflammatory remedy, displayed one of the most promising in vitro leishmanicidal activity against axenic amastigote forms of Leishmania amazonensis.99 A bioassay-guided fractionation of a 90% EtOH leaf extract of this plant led to the isolation of sanguinolignans A–D (253–256). Their structures were determined by spectroscopic methods, HRCIMS, CD experiments, and chemical methods. Sanguinolignan A showed a moderate antileishmanial activity, but interestingly it had a very low cytotoxicity on macrophage and cancer or even normal cell lines. 4-O-β-D-Glucopyranosyl-7-hydroxymatairesinol (257) and matairesinol 4-O-β-D-cellobioside (258) were isolated from Thellungiella salsuginea (Brassicaceae)100 and Trachelospermum jasminoides (Lindl.) (Apocynaceae),101 respectively.
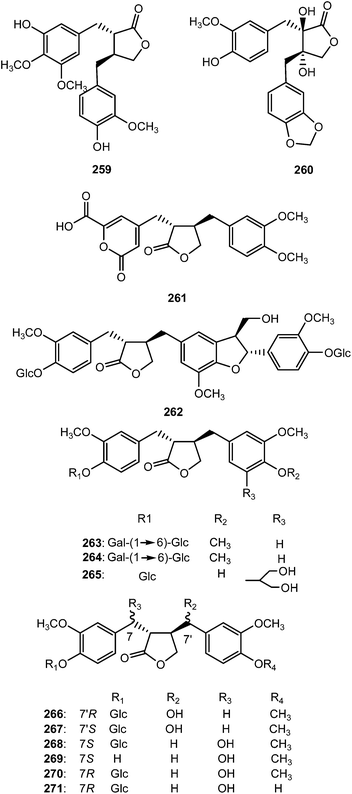
The previously unreported lignans (8S,8′-S)-4,3′-dihydroxy-3,4′,5′-trimethoxylignan-9′,9-olide (259) and trans-4′,8,8′-trihydroxy-3′-methoxy-3,4-methylenedioxylignan-9′,9-olide (260) were reported from the wood of Cunninghamia konishii (Taxodiaceae).102 Eleven butyrolactone type lignans (261–271) were isolated from the fruits of Arctium lappa L. (Asteraceae). Compound 261 is a rare butyrolactone lignan with a 6-carboxyl-2-pyrone moiety. Compounds 261, 266, 268, and 270 exhibited stronger anti-inflammatory effects than the positive control curcumin, particularly 261, which showed 75, 71, and 61% inhibition at 10, 1, and 0.1 μM, respectively.103
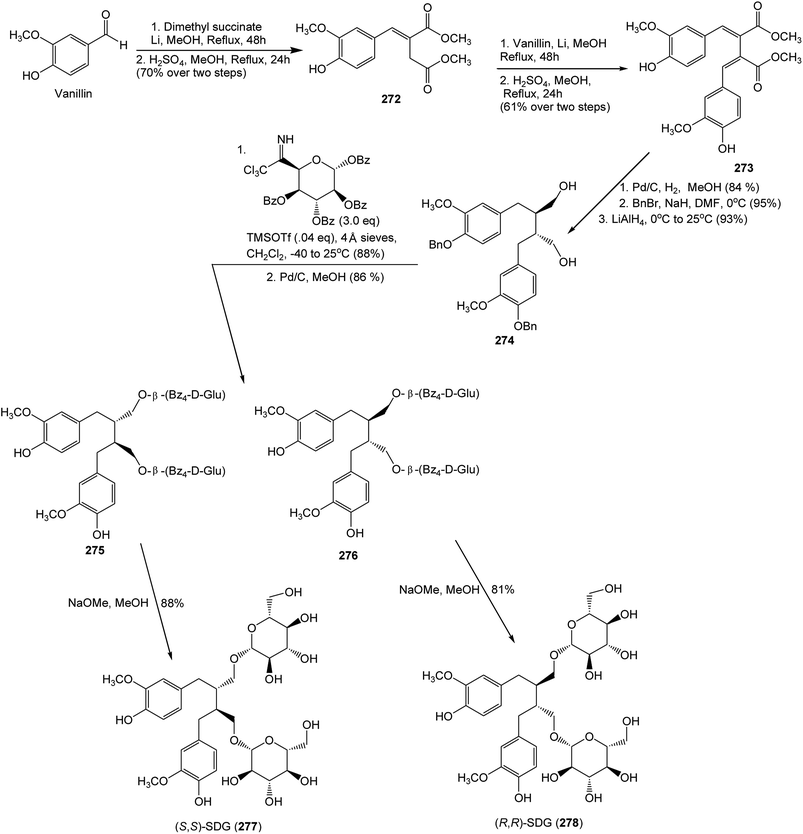
Secoisolariciresinol diglucosides (SDGs) (S,S)-SDG (277) (major isomer in flaxseed (Linum Usitatissimum) (Linaceae)) and (R,R)-SDG (278) (minor isomer in flaxseed) were synthesized from vanillin via secoisolariciresinol and a glucosyl donor through a concise route that involved chromatographic separation of diastereomeric diglucoside derivatives.104 (S,S)-SDG-1 is a potent antioxidant both in vitro and in vivo105,106 and a powerful in vitro scavenging agent against hydroxyl free radicals.107 Vanillin was subjected to Stobbe condensation108 with dimethyl succinate in the presence of lithium wire in refluxing methanol, and the resulting mixture of carboxylic acids was esterified with methanol under acidic conditions to furnish dimethyl ester 272 in 70% overall yield (single isomer, unassigned olefin geometry). A second Stobbe condensation involving product with vanillin under the same lithium-mediated conditions, followed by esterification with methanol under the same acidic conditions, led to diene 273 in 61% overall yield (single isomer, unassigned olefin geometry) (Scheme 8). The latter compound underwent diastereoselective hydrogenation (Pd/C, H2, 84% yield), phenolic benzylation (NaH, BnBr, 95% yield), and ester reduction (LiAlH4, 93% yield) to provide bis-benzyl secoisolariciresinol (274) whose relative stereochemistry was confirmed by X-ray crystallography. The glycosidation of 274 was achieved through the use of perbenzoyl-protected trichloroacetimidate under the influence of trimethylsilyl trifluoromethane sulfonate (TMSOTf), furnishing a diastereomeric mixture of inseparable bis-β-glucosides (88%). Cleavage of the benzyl ethers by hydrogenolysis provided a mixture of diastereomeric bis-phenols (86%), which proved separable by preparative thin layer chromatography (multiple elusions) to afford pure 275 and 276. Each perbenzoylated bis-glucoside was treated with NaOMe in methanol to give the corresponding secoisolariciresinol diglucoside (S,S)-SDG (277) (88% yield) and (R,R)-SDG (278) (81% yield)103 (Scheme 8). The synthetic compounds (S,S)-SDG (277) and (R,R)-SDG (278) demonstrated powerful scavenging activities against hydroxyl (277: EC50 = 2.09 ± 0.16 μM; 278: EC50 = 1.96 ± 0.27 μM), peroxyl (277: EC50 = 2.20 ± 0.10 μM; 278: EC50 = 3.03 ± 0.04 μM) and DPPH (277: EC50 = 157.54 ± 21.30 μM; 278: EC50 = 123.63 ± 8.67 μM) radicals.104
2.4 Furofuran derivatives
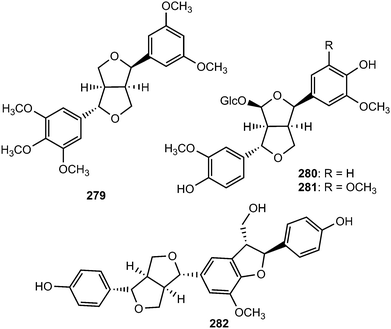
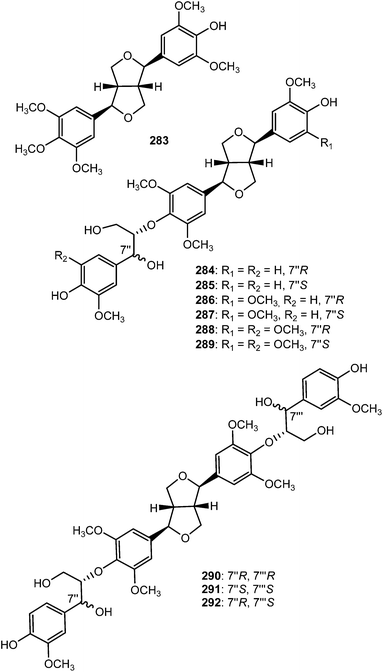
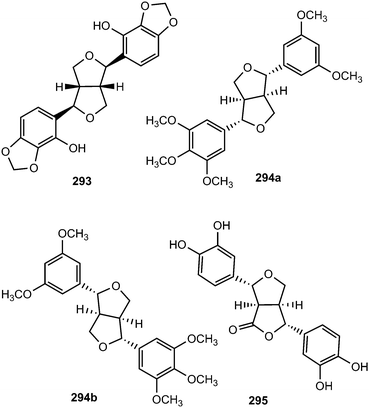
Epimagnolin B (279) is a furofuran lignan isolated from the flower buds of the medicinal plant Magnolia fargesii (Magnoliaceae). It was shown to inhibit the production of NO and PGE2 and the expression of respective enzyme iNOS and COX-2 through the suppression of I-κB-α degradation and nuclear translocation of p65 subunit of NF-κB.109 Lactuberins A (280) and B (281) are constituents of Lactuca tuberosa, a wild edible plant species in Poland. Although furofuran lignans were previously reported from Lactuca indica and Lactuca sibirica, the two new natural products are the first reported ones from Lactuca species possessing axial/equatorial aryl groups in their 2,6 diaryl-3,7-dioxabi-cyclo[3.3.0]octane skeleton.110 Chushizisin I (282), a sesquilignan with partial pinoresinol- and dehydrodiconiferyl alcohol-type constituent units is among the lignans obtained from the fruits of Broussonetia papyrifera (Moraceae).111Ten new furofuran lignans 283–292 were isolated from the ethanolic extract of the stem of Sinocalamus affinis (Poaceae).112 The configurations of compounds 284–292 were elucidated by NMR and CD data analyses in combination with chemical transformations. The new sesamin type furofuran lignan, (−)-sesamin-2,2′-diol (293) was obtained from the aerial parts of Isodon japonicus (Lamiaceae), which are used for the treatment of gastrointestinal disorders, tumors, and inflammatory diseases.113 It showed relatively weaker inhibition of LPS-induced NO production in RAW264.7 cells than the positive control aminoguanidine. Acortatarinowin F (294a/294b) constitutes one of the pairs of lignan enantiomers isolated from the rhizomes of Acorus tatarinowii Schott (Araceae) through separation by chiral HPLC using a Daicel IC column.114 (−)-3,4,3′,4′-Tetrahydroxy-9,7′-β-epoxylignano-7β,9′-lactone (295) is among the lignans isolated from the methanol extract from Morinda citrifolia.115
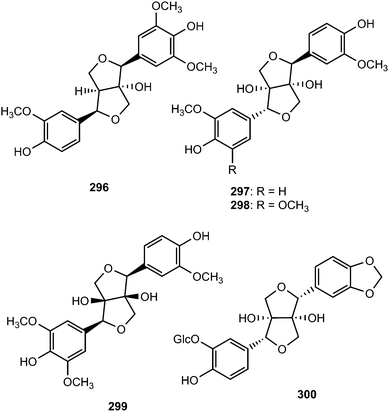
Dipsalignan A–D (296–299), were reported from the roots of Dipsacus asper Wall (Caprifoliaceae). Compound 299 displayed weak activity against HIV-1 integrase.116 An investigation into the leaves of Vitex glabrata (Verbenaceae) led to the isolation khainaoside A (300), which showed potent inhibitory effects on estrogen-enhanced cell proliferation. The absolute configuration of the dioxabicyclooctyl ring was determined to be 7R,8S,7′R,8′S from the negative Cotton effect at 227 nm in the CD spectrum.117
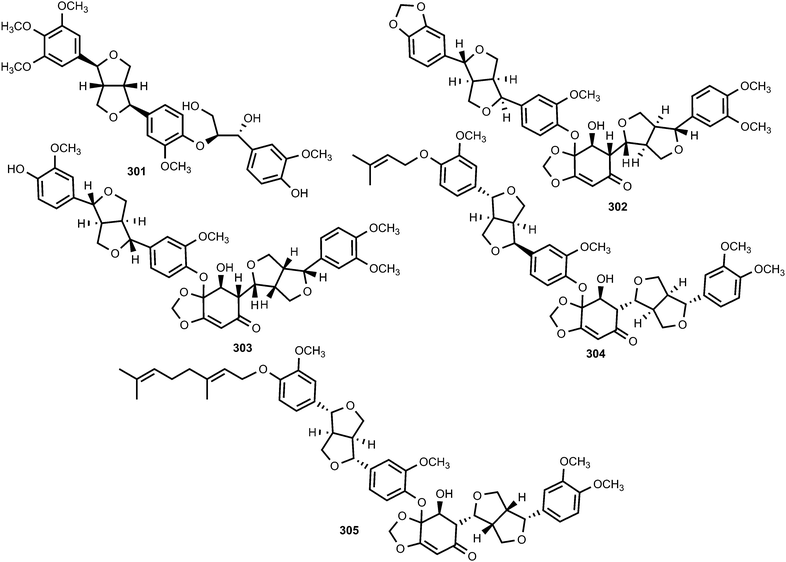
The furofuran lignan named colocasinol A (301) was reported from Colocasia antiquorum var. esculenta (Araceae), an edible vegetable in many tropical and subtropical regions of the world.118Zanthoxylum podocarpum belongs to the genus Zanthoxylum of Rutaceae family, and its bark has been widely used as a folk medicine in PR China mainly for the treatment of rheumatoid arthritis and swelling.119 Zanthpodocarpins A (302) and B (303), isolated from its bark, are rare dilignans bearing an unusual α,β-unsaturated ketone group from a natural resource. These compounds inhibited nitric oxide (NO) production in LPS stimulated RAW264.7 cells with IC50 of 5.3 and 12 μM, respectively.119,120 Two previously unreported dilignans bearing the same α,β-unsaturated ketone group, bizanthplanispines A (304) and B (305), were recently isolated from the roots of Zanthoxylum planispinum Sieb. et Zucc (Rutaceae),121 a deciduous shrub widely used as folk medicine for preventing toothache, stomachache and snake bites, treating colds and expelling round worms. The above compounds moderately reduced the proliferation of Hela cells with IC50 of 22 and 26 μg mL−1, respectively.
2.5 Furan derivatives
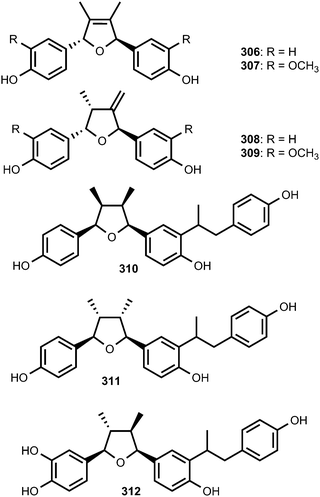
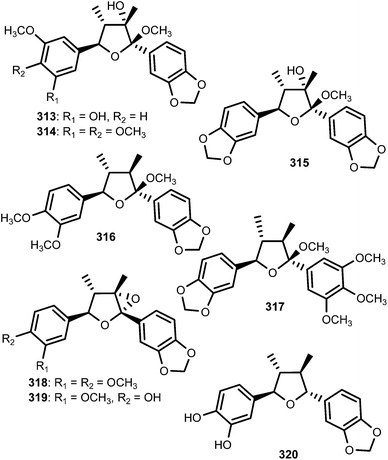
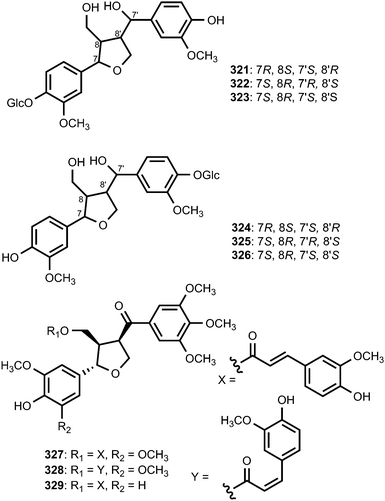
The 7,7′-epoxylignans, ribesins A–D (306–309) and the tetrahydrofuran-type sesquilignans, ribesins E–G (310–312), were obtained from the leaves of Ribes nigrum (Grossulariaceae). Ribesins D and G demonstrated potent superoxide anion scavenging activities with EC50 of 1.2 and 1.1 μM, respectively.122 A systematic bioactivity-guided isolation of the 70% aqueous acetone extract of Schisandra sphenanthera (Schisandraceae) roots led to the identification of eight new tetrahydrofuran lignans 313–320. Their absolute configuration were established on the basis of circular dichroism. Compound 320 showed the anti-oxidative haemolysis of human red blood cells (RBCs) activity with the IC50 of 35 μg mL−1, which is similar to that of vitamin C (33 μg mL−1).93The absolute configurations of tetrahydrofuranoid lignans 321–326 isolated from the fruit of Arctium lappa L. (Asteraceae) were confirmed using ROESY, the circular dichroic exciton chirality method, and Rh2(OCOCF3) 4-induced CD spectrum analysis. They showed significantly stronger hepatoprotective activity against D-galactosamine-induced cytotoxicity in HL-7702 hepatic cells than even the positive control (bicyclol) at a concentration of 1 × 10−5 M.123 A bioassay-guided fractionation and chemical investigation of methanol extract of twigs of Lindera glauca (Sieb et Zucc.) Blume (Lauraceae) resulted in the isolation of three new lignan derivatives, named linderuca A (327), B (328), and C (329). The isolated compounds significantly inhibited NO production with IC50 of 12, 9.4, and 9.9 μM, respectively.124
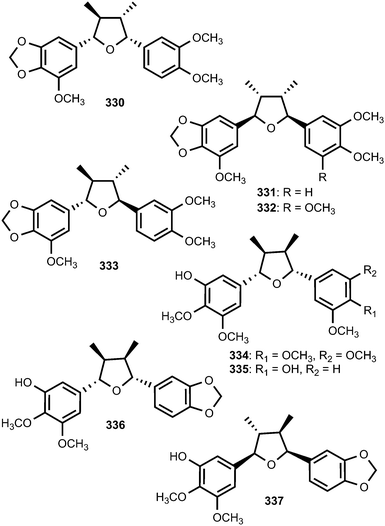
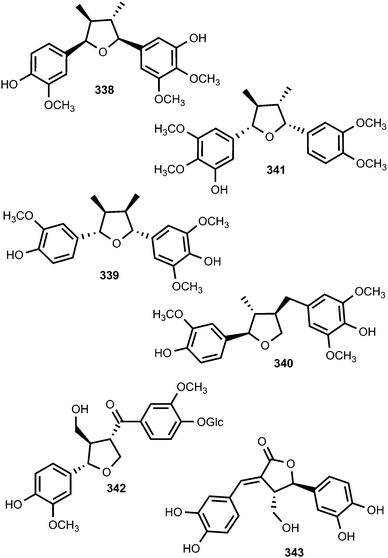
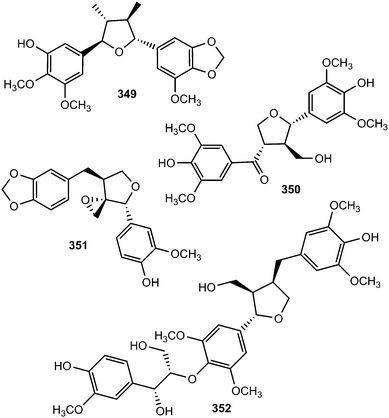
Saurufurins A–D (330–333) are secondary metabolites of Saururus chinensis.74 Furthermore, schinlignins A (334) and B (335) have also been isolated from S. chinensis.95 In addition to manglisin B, manglisins C–E (336–338) were isolated from the mature carpels of Manglietiastrum sinicum (Magnoliaceae).49 Compounds 336 and 337 showed moderate antimicrobial activities (MIC 0.03–0.13 μM) against Staphylococcus aureus, MRSA 82#, MRSA 92#, MRSA 98#, and MRSA 331#. Additional previously unreported furan-type lignans include compounds 339 and 340, both isolated from the bark of Machilus robusta (Lauraceae),25 (+)-3-hydroxyveraguensin (341) from the leaves and the stem of Miliusa fragrans (Annonaceae),125 compound 342 from the fruits of Arctium lappa L. (Asteraceae),126 and 343 from the methanol extract of Morinda citrifolia.115
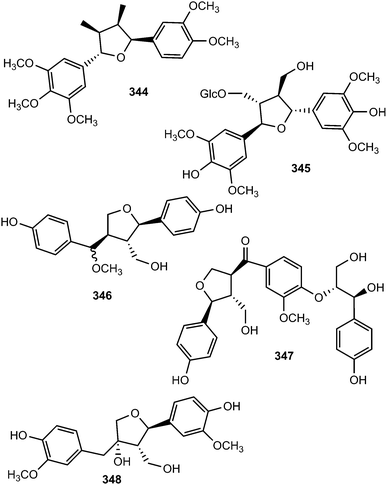
The investigation of methanol extract of the rhizomes of Acorus gramineus (Araceae) led to the isolation of two new tetrahydrofuran lignans, ligraminols A (344) and B (345) whose structures were determined by a combination of 1D and 2D NMR, HRMS, CD, and enzymatic hydrolysis.127 These compounds were evaluated for their antiproliferative activities against three human cancer cell lines including A549, SK-OV-3, and SK-MEL-2 using the SRB bioassay. Ligraminol A was the most active against the SK-MEL-2 cell line with an IC50 value of 4.5 μM. Chushizisins G (346) and H (347) are secondary metabolites reported from the fruits of Broussonetia papyrifera (Moraceae).111 The new tetrahydrofuran lignan, (−)-diepiolivil (348), was isolated from a stem methanol-soluble extract of Cameraria latifolia (Apocynaceae); the absolute configuration of the 1,3-diol moiety was proposed from the CD spectrum induced with dimolybdenum tetraacetate in DMSO solution.128 Beilschminol A (349) and the epoxylignan 350 were reported from Beilschmiedia tsangii (Lauraceae)129 and Sinocalamus affinis,112 respectively. The lignan epoxide (351) was isolated from the whole plant of Phillyrea angustifolia L. (Oleaceae)130 while the sesquilignan (352) has been reported to be a constituent of fruits of Melia toosendan (Meliaceae)131
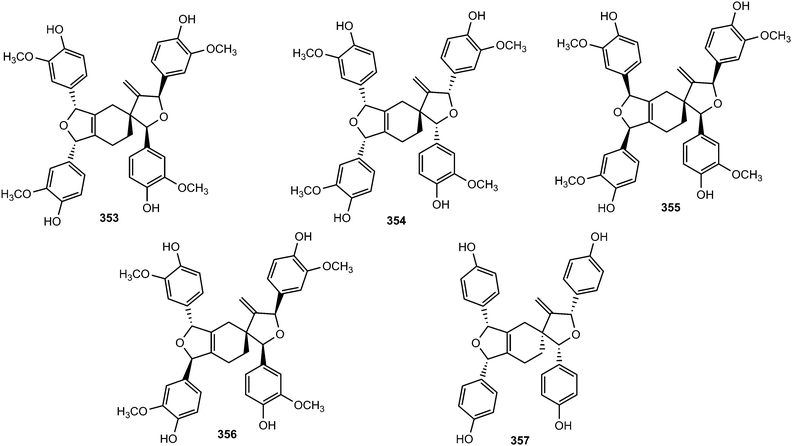
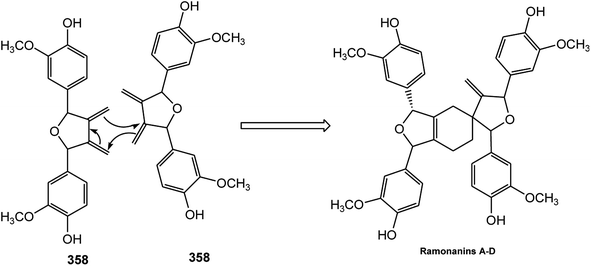
Activity-guided fractionation of Guaiacum officinale (Zygophyllaceae) heartwood extracts led to the isolation of four new spirocyclic lignans, named ramonanins A–D (353–356).132 These metabolites exhibited moderate in vitro cytotoxic activities against human breast cancer cell lines and induced cell death via apoptosis. More recently, ribesin H (357), bearing the same spirocyclic skeleton, was reported from the leaves of Ribes nigrum.122 The furano-2-oxaspiro[4,5]decane core of the ramonanins is unprecedented among known natural products; nonetheless, these compounds appear to be derived from a fairly straightforward oligomerization of four units of coniferyl alcohol-like precursors. Intriguingly, the ramonanins could be considered as dimers of two identical 1,3-di-(4-hydroxy-3-methoxyphenyl)-substituted furans such as 358 (Scheme 9).132 The dimerization of 358, via a Diels–Alder-like mechanism, would directly lead to ramonanins A–D.
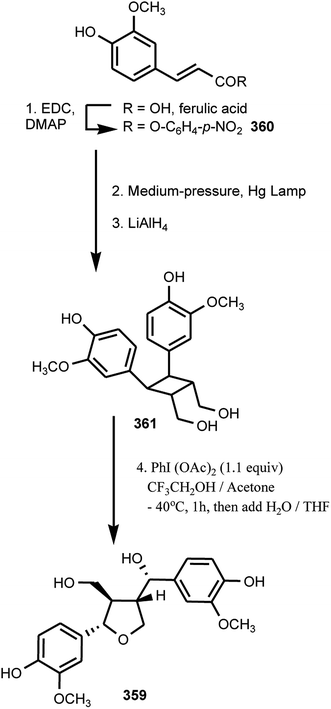
The bio-inspired total synthesis of the furan-type lignan tanegool (359) in four steps starting from ferulic acid was recently developed by Albertson and Lumb133 based on the fact that lignan biosynthesis involves regio- and chemoselective oxidative coupling of propenyl phenols. They began their investigation by optimizing the [2 + 2] photodimerization of p-nitro-ferulate ester 360 through irradiation of a suspension in hexane with a medium-pressure mercury lamp (Scheme 10). Reduction of the crude material with lithium aluminum hydride completed a 3-step synthesis of diarylcyclobutanediol 361, which proceeded with an overall yield of 74%. The oxidative ring opening of 361 with phenyliodo diacetate led to natural product (±)-tanegool as a single diastereomer with a yield of up to 40%.
3 Neolignans
3.1 Benzofurans
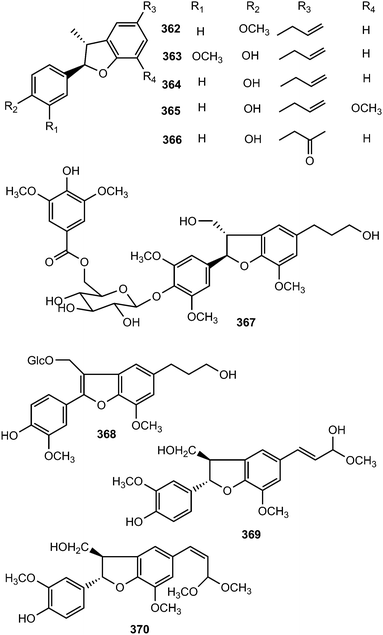
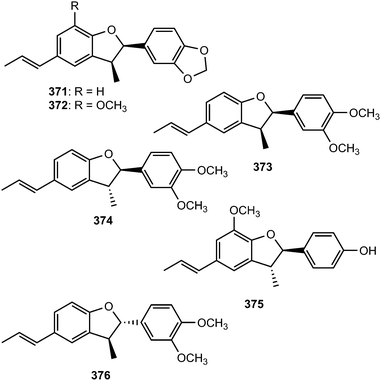
From the leaves of Miliusa mollis Pierre (Annonaceae), five new dihydrobenzofuran neolignans, namely 4′-O-methylmiliumollin, (362), 3′-methoxymiliumollin (363), miliumollin (364), 7-methoxymiliumollin (365) and miliumollinone (366), were isolated. Miliumollin, 3′-methoxymiliumollin and miliumollinone exhibited weak cytotoxicity against KB, MCF7 and NCI-H187 cells.134 Roots of Pittosporum glabratum Lindl. (Pittosporaceae) have been known for analgesic and antidotal properties in Chinese folk medicine. The structures and absolute stereochemistry of pittogosides A (367) and B (368) obtained from this plant were determined on the basis of spectroscopic analysis and chemical evidence, with a combination of circular dichroism. They were not active in a DPPH radical scavenging assay even at the concentration of 100 μM.135 Methanol extract of the fruits of Vitex rotundifolia showed inhibitory effect on the nitric oxide (NO) production in RAW264.7 cells. The bioassay-guided fractionation of the CH2Cl2-soluble fraction led to the isolation of two new neolignans 369 and 370. Compound 369 was found to inhibit nitric oxide production with an IC50 of 21 μM.136Bioassay-guided fractionation of the ethanolic extract of the stem of Aristolochia fordiana (Aristolochiaceae) led to the isolation of six new dihydrobenzofuran neolignans (371–376).137 The structures were established by spectroscopic methods, and their absolute configurations determined by analyses of the specific rotation and electronic circular dichroism data.
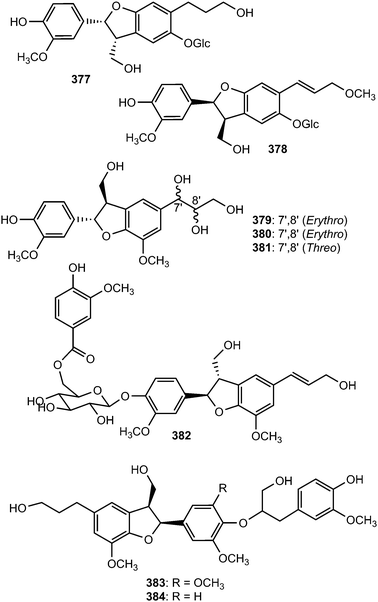
Phytochemical investigation of the methanol extract of Akebia quinata (Lardizabalaceae) afforded the neolignan glycosides akequintosides A (377) and B (378). The latter showed moderate inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells.138 Meliasendanins B–D (379–381), exhibiting moderate ABTS radical-scavenging activity, are metabolites of Melia toosendan (Meliaceae) fruits.139 Saccharumoside A (382) is one of the glycosides isolated from the bark of Acer saccharum (Aceraceae). It showed no cytotoxicity against two human colon tumorigenic (HCT-116 and Caco-2) cell lines and a nontumorigenic (CCD-18Co) cell line.140 Phytochemical investigation of seeds of Euryale ferox (Nymphaeaceae) led to the isolation of two new sesquilignans, named euryalins A (383) and B (384).141 Euryalin B exhibited strong effect against DPPH assay with SC50 values of 6.8 μM, comparable to that of the positive control (gallic acid or ascorbic acid). Strikingly, even though the structures of euryalins A and B are similar, except that compound A contains an additional methoxy group, their antioxidant activities are very different. These data suggested that the presence of OMe-5′ might decrease the inhibitory activity.
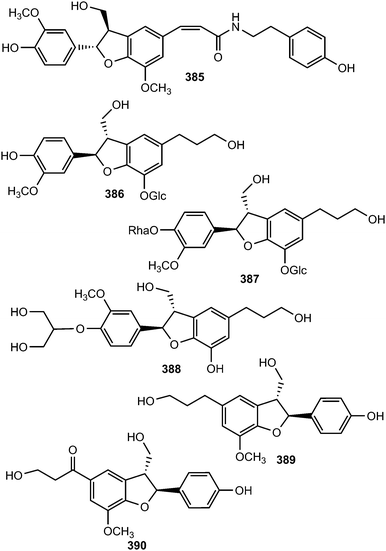
The new acyclic phenylpropane lignanamide, named cis-grossamide K (385), was isolated from Colocasia antiquorum var. esculenta, (Araceae). This compound exerted moderate antimelanogenic activity on the cells without having high cell toxicity (IC50 = 54 μM and LD50 = 164 μM).118 Two neolignan glycosides 386 and 387 were isolated from the roots of Rhodiola crenulata69 while compound 388, characterized by a 1,2,3-propantriol moiety, was obtained from the root bark of Illicium henryi (Illiciaceae).142 The fruits of Broussonetia papyrifera yielded chushizisins E (389) and F (390), which exhibited antioxidant activities against H2O2-induced impairment in PC12 cells with concentrations ranging from 0.16 to 100 μM.111
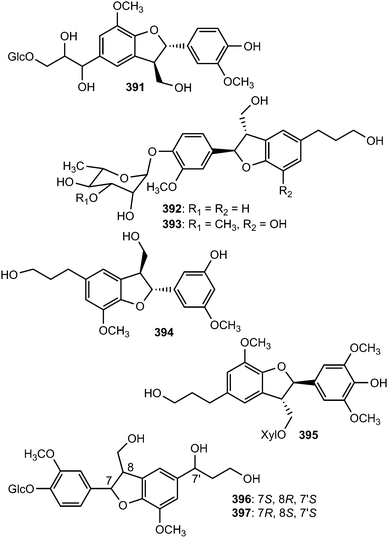
Radulignan (391), a neolignan glycoside was obtained from polar extract of the roots of Lantana radula (Verbenaceae) growing in Brazil.143 The strong positive Cotton effect at 290 nm on its CD spectrum was indicative of a 7S,8R configuration. It was tested for cytotoxicity against several cell lines (HL-60, K562, U937, CEM, KG-1, Jurkat, U266, and NCI-H929) and did not inhibit cell viability at 10 μM. (7R,8S)-Cedrusinin 4-O-α-L-rhamnoside (392) and (7R,8S)-cedrusin 4-O-(3-O-methyl-α-L-rhamnoside) (393) were isolated from the trunk of Abies holophylla98 while (7R,8S)-3,5′-dimethoxy-4′,7-epoxy-8,3′-neolignane-5,9,9′-triol (394) inhibiting NO production in lipopolysaccharide-activated RAW264.7 macrophages (IC50 value of 36 μM) was reported from the aerial parts of Leontopodium leontopodioides.144 Linderanoside C (395) was characterized from the trunk of Lindera glauca.72 It was evaluated for its cytotoxicity against four human cell lines using sulforhodamine B assays in vitro and was shown to exhibit selective cytotoxicity against A498 cells with IC50 value of 23 μM. Benzofuran-type neolignans 396 and 397 are among the lignans obtained from the fruit of Arctium lappa L.123
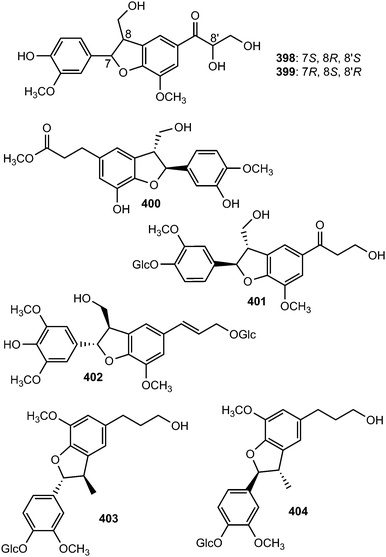
The absolute configuration of C-8′ in (−)-7′-dehydrosismbrifolin (398) and (+)-7′-dehydrosismbrifolin (399) isolated from the fruits of Xanthium sibiricum (Asteraceae) was established using the Mo2(OAc)4-induced circular dichroism (ICD).145 Trogopterin A (400) was isolated from the feces of Trogopterus xanthipes Milne-Edwars (Petauristidae) which have been reported to promote blood circulation and resolve stasis. It exhibited moderate cytotoxic activities against HL-60 cells with IC50 of 46 μM.146 The lignan glycosides 401 and (+)-aquilignanoside A (402) were obtained from the fruits of Arctium lappa L.126 and Aquilaria sinensis (Thymelaeaceae),147 respectively. Compounds 403 and 404 are two neolignan glycoside enantiomers having extremely similar 1H and 13C NMR spectra isolated from the roots of Paeonia lactiflora (Ranunculaceae) after separation by chiral HPLC.148
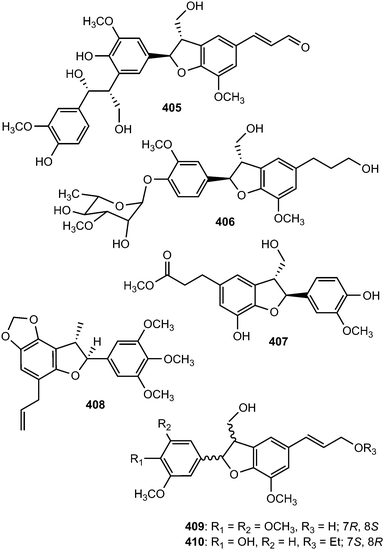
The sesquineolignan prepicrasmalignan (405), was detected in the fruit wall of Cirsium eriophorum (L.) Scop (Asteraceae) using a combination of optimized acid treatments and complementary analytical methods (HPLC-MS, GC-MS, CD and NMR).149 Juniperigiside (406) was reported from the CHCl3 soluble fraction of the methanol extract of stem and leaves of the medicinal plant Juniperus rigida (Cupressaceae)150 while compound 407 was obtained from Abies fabri (Pinaceae).151 The dihydrobenzofuranoid neolignan bearing a 1,3,4,5-tetrasubstituted benzene ring ococymosin (408) was characterized from the stem or bark of the Madagascan plant Ocotea cymosa (Lauraceae).152 It exhibited antiparasitic activity against the Dd2 strain of Plasmodium falciparum with an IC50 of 0.45 μM. Compounds 409 and 410 are constituents of the stem of Sinocalamus affinis (Poaceae).112
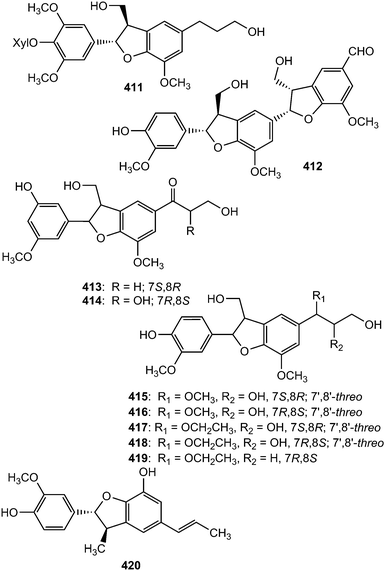
The absolute configuration of the neolignan glycoside 411, isolated from the stem of Eurya japonica, was solved by ECD analysis which showed a positive Cotton effect at 232 nm and a negative effect at 217 nm, indicating a 7S,8R configuration.24 The sesquineolignan 412, possessing two dihydrobenzofuran moieties in its structure, is one of the secondary metabolites described from the fruits of Melia toosendan (Meliaceae).131 In traditional Chinese medicine, the hawthorn fruits of Crataegus pinnatifida (Rosaceae) are primarily used to improve circulation, remove blood stasis and treat indigestion, hyperlipidemia and hypertension. Seven new dihydrobenzofuran neolignans, pinnatifidanin CI–VII (413–419) exhibiting moderate cytotoxic and antioxidant activities were isolated from the seeds of this plant.153 Callislignan A (420) was reported from the leaves of Callistemon lanceolatus (Myrtaceae). It showed moderate antibacterial activity against S. aureus ATCC25923 and MRSA SK1.154
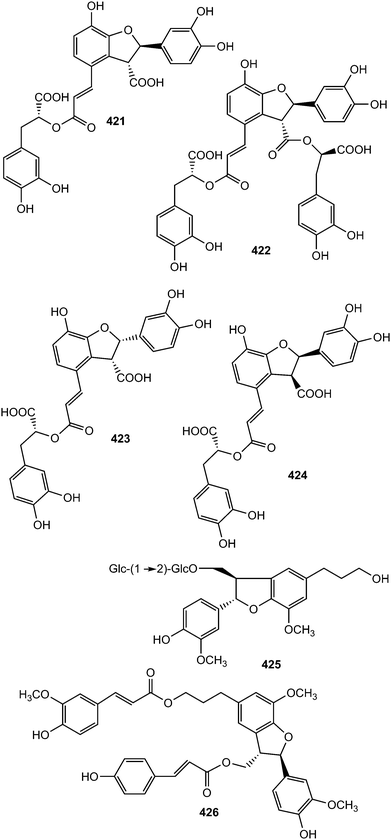
Monardic acids A (421), B (422) and C (423) were isolated from Monarda fistulosa L. (Lamiaceae) while lithospermic acid C (424) was reported from the roots of Lithospermum erythrorhizon (Boraginaceae).155 The absolute configuration of 421 was confirmed by analysis of its hydrolysates, 7-epiblechnic acid and 2R-3-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid. The configuration in the dihydrobenzofuran moieties of 422–424 was extrapolated by using the phenylglycine methyl ester method and a Cotton effect at approximately 250–260 nm in their electronic circular dichroism spectra.155 The isolated compounds displayed moderate hyaluronidase inhibitory and histamine release inhibitory activities. Saposide B (425) is one of the lignan glycosides exhibiting moderate antioxidant activity on superoxide dismutase isolated from the maple sap of Acer saccharum77 and Boehmenan X (426) was obtained from the bark of Durio carinatus.156
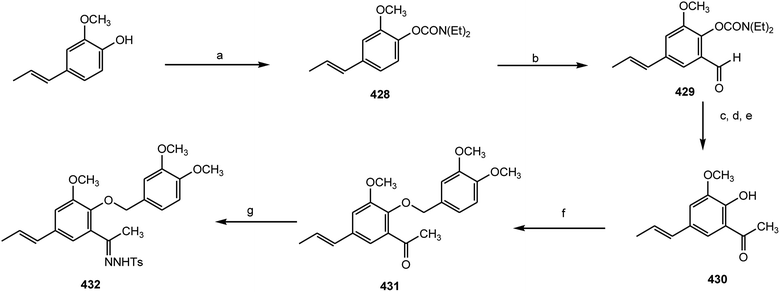
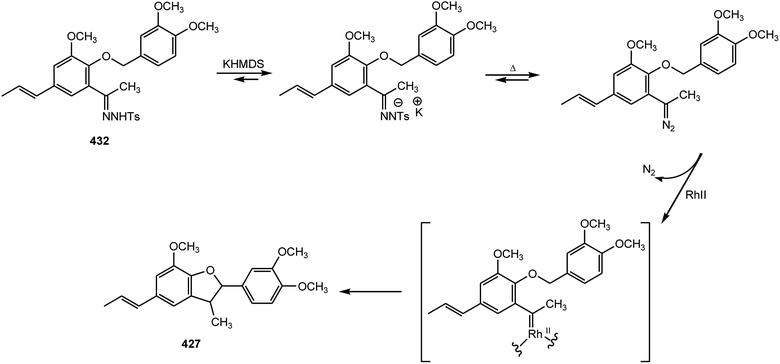
An efficient and practical asymmetric synthesis of the 2,3-dihydrobenzo[b]furan neolignan acuminatin (427) was achieved by using trans-isoeugenol as the starting material by Lopez-Sanchez et al.157 The key step is an intramolecular C–H insertion through a non-stabilized carbenoid, prepared by decomposition of a tosylhydrazone in the presence of an anthracenyl-derived cinchonidine quaternary ammonium salt as a chiral phase-transfer catalyst. First, the phenolic OH was protected with the ortho-directing group N,N′-diethylcarbamate to form compound 428. Formilation in ortho position to give 429 was optimal when s-BuLi was used as a metallating agent and DMF as an electrophile. Aldehyde 429 was transformed into ketone 430 by a three-step process involving Grignard addition, Dess–Martin oxidation and hydrolysis of the carbamate group in alkaline media. Williamson etherification with 4-(iodomethyl)-1,2-dimethoxybenzene and treatment of 431 with tosylhydrazine yielded the desired tosylhydrazone 432 (Scheme 11).158 When the tosylhydrazone 432 is treated with potassium hexamethyldisilazane KHMDS in THF at −80 °C, cloudiness appears due to the formation of the potassium salt, which on heating, is unstable and decomposes in situ to form the diazo compound derivative (Scheme 12).157,158 In the presence of a metal catalyst, the diazo compound reacts with an activated C–H bond through a carbenoid intermediate. The process inserts a 1,5-intramolecular carbon–hydrogen bond to form a five-member heterocycle through a well-established mechanism (Scheme 12). The reaction can be highly improved by the presence of phase-transfer catalysts, like quaternary ammonium salts, because they favor the transformation of the tosylhydrazone salt into the diazo compound and promote the solubility of rhodium catalysts. A series of rhodium salts were used but mixtures of cis/trans diastereoisomers were formed except for Rh2(OCOCF3)4, which shows complete cis diastereoselectivity and 90% yield.157
3.2 Alkyl aryl ethers
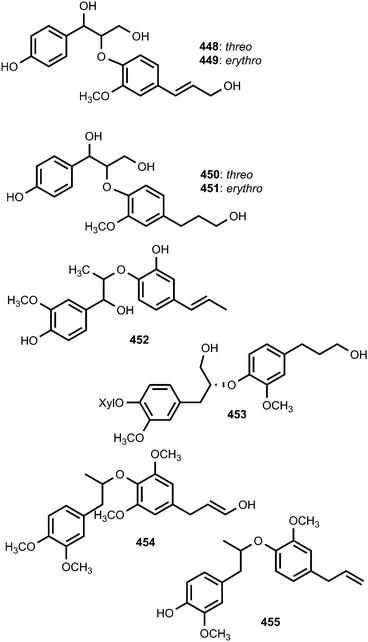
Hispidacine (433) is an 8,4′-oxyneolignan featuring incorporation of an unusual 2-hydroxyethylamine moiety at C-7 which induces moderate vasorelaxant activity in rat aorta isolated from the stem-bark and leaves of Malaysian Ficus hispida Linn (Moraceae).159 Myrifralignans A–E (434–438) were reported from the seeds of Myristica fragrans Houtt (Myristicaceae).160 Compounds 436–438 exhibited potent inhibitory activity against the production of nitric oxide (NO) in the RAW264.7 cell line stimulated by lipopolysaccharide. Repeated column chromatography of the 70% ethanol extract of the seeds of Crataegus pinnatifida (Rosaceae) resulted in the isolation of nine new 8-O-4′ neolignans, pinnatifidanin BI–IX (439–447). Pinnatifidanin BVIII displayed potent cytotoxic activity against the cancer cell line U937 with an IC50 of 2.7 μM.161Fruits of Broussonetia papyrifera (Moraceae) have been used in traditional Chinese medicine for the treatment of age-related disorders. In order to find compounds that are responsible for the antioxidant effects of this plant, Mei et al.111 isolated chushizisins A–D (448–451). Chushizisins A, B and D showed DPPH radical-scavenging activities with IC50 of 237, 156, and 274 μM, respectively. Callislignan B (452) is a lignan reported from the leaves of Callistemon lanceolatus. It exhibited antibacterial activity against S. aureus ATCC25923 and MRSA SK1 with a MIC of 8 mg mL−1.154 Ligraminol E 4-O-β-D-xyloside (453) is a secondary metabolite isolated from the trunk of Abies holophylla.98 Phytochemical investigation of the methanol extracts prepared from leaves and stem of Miliusa fragrans led to the isolation of (−)-miliusfragranol A (454) and (−)-miliusfragranol B (455).125 Since no CD data of 8-O-4′-neolignans with established absolute configurations were available at that time, the absolute configurations of compounds 454 and 455 were not determined during this investigation.125
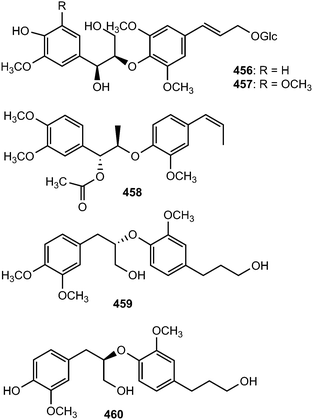
Syringa velutina Kom. (Oleaceae) has been widely cultivated in the northern parts of China and Korea for its medicinal applications to treat infectious fevers, counteract inflammations, dampness and acute icteric hepatitis. The neolignan glycosides, (7S,8R)-guaiacylglycerol-8-O-4′-sinapyl ether 9′-O-β-D-glucopyranoside (456) and (7S,8R)-syringylglycerol-8-O-4′-sinapyl ether 9′-O-β-D-glucopyranoside (457) were reported from this medicinal plant.162 Ligraminols C–E (458–460) are three previously unreported neolignans from the rhizomes of Acorus gramineus (Araceae). Ligraminols C and D showed weak inhibitory activity against the proliferation of the human cancer A549 cell lines with IC50 of 9.5 and 8.3 μM, respectively.127
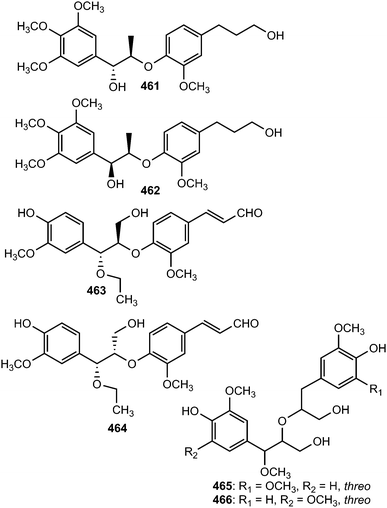
Acorus gramineus Soland (Araceae) is an aquatic perennial herbaceous plant widely distributed in Korea, Japan, and China. The plant's rhizomes have been used as a traditional medicine in China for the treatment of various disorders including cognitive problems, sedation, stomach ache, and oedema.163 A bioassay-guided fractionation and chemical investigation of the methanol extract of rhizomes of this plant resulted in the isolation and identification of thirteen phenolic derivatives including two new 8-O-4′-neolignans, surinamensinols A (461) and B (462). The newly isolated compounds showed antiproliferative activities against A549, SKOV-3, SK-MEL-2, and HCT-15 cell lines with IC50 values in the range of 4.2 to 26 μM.164 The neolignans threo-guaiacylethoxyglycerol-β-O-4′-coniferyl aldehyde ether (463) and erythro-guaiacylethoxyglycerol-β-O-4′-coniferyl aldehyde ether (464) are secondary metabolites of Melia toosendan.131 Compound 464 exhibited moderate agonistic activity on MT1 with agonistic rate of 21% at about 1 mM.147 Jeong et al.76 reported the isolation of neolignans 465 and 466 from the leaves and twigs of the medicinal plant Euonymus alatus. Their absolute configurations have not been fully determined because the amounts obtained were too small.
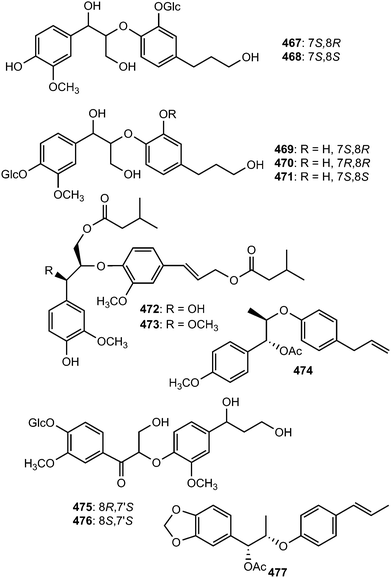
The structures of compounds 467–471 isolated from the roots of Rhodiola crenulata were elucidated by spectroscopic data and chemical evidence supporting that they are optical isomers of two 8-O-4′ neolignan glycosides.69 Two neolignans 472 and 473 related to guaiacylglycerol-β-coniferyl ether with two isovaleroyloxy groups were identified from the roots of Nannoglottis carpesioides (Asteraceae).165 They showed moderate radical-scavenging activity with IC50 of 22 and 17 μM, respectively. Miliusamollin (474) was among the lignans isolated from the leaves of Miliusa mollis Pierre134 while compounds 475 and 476 were obtained from the fruit of Arctium lappa L.123 The 8-O-4′ neolignan (477) was reported from the ethanolic extract of the stem of Aristolochia fordiana.137
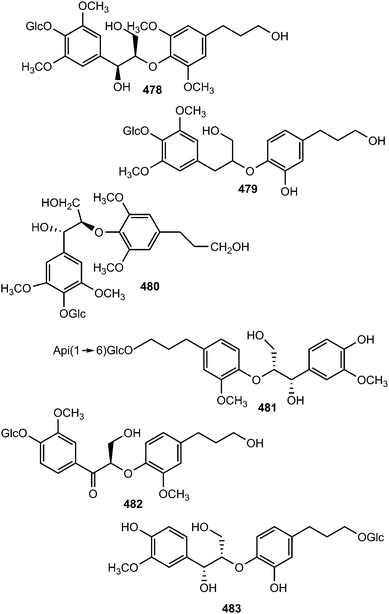
Systematic investigation of the rhizomes of Smilax trinervula (Smilacaceae) led to the isolation of compounds 478 and 479. The absolute configuration of 478 was deduced from CD data analysis while the stereochemistry of C-8 in 479 was not confirmed. These compounds showed no cytotoxic activity against five cancer cell lines (SH-SY5Y, SGC-7901 and HCT-116).166 Symplocosneolignan 480 was characterized from the n-butanol soluble fraction of the methanol extract of Symplocos cochinchinensis var. philippinensis (Symplocaceae).167 Ethanol extract of the fruits of Arctium lappa L yielded 481 and 482125 while camellioside A (483) has been isolated from the leaves and branches of Camellia amplexicaulis (Theaceae).168
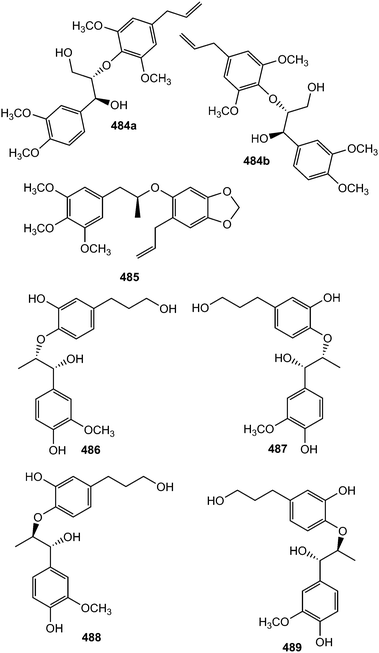
The pair of new 8-O-4′-type neolignan enantiomers (±)-acortatarinowin D (484a/484b) was separated from the ethyl acetate soluble fraction of 95% methanol extract of Acorus tatarinowii Schott by chiral HPLC using a Daicel IC column.114 Compound 485 was reported from the stem or bark of Ocotea cymosa.152 Its configuration was tentatively assigned as S based on its positive optical rotation, as for synthetic (S)-virolongin B, a closely related compound even though the magnitude of the rotations differed significantly. Compounds 486–489 are stereoisomers sharing a common planar structure isolated from the roots of Paeonia lactiflora by chiral HPLC. The latter was found to show the optimal Aβ1–42 aggregation inhibition potency (81% at 20 μM).148
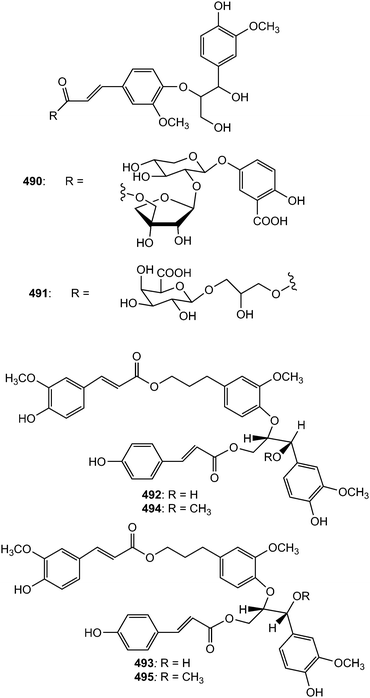
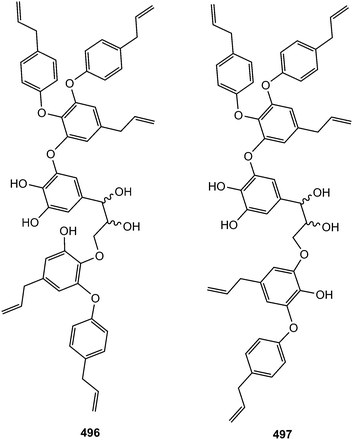
The new guaiacylglycerol-feruloyl derivatives 490 and 491 were obtained from the roots of Medicago truncatula (Fabaceae). The guaiacylglycerol-feruloyl moiety of these compounds is a neolignan derivative obtained from the oxidative dimerization of two coniferyl alcohol units.169 Investigation of chemical constituents from the bark of Durio carinatus (Bombacaceae) led to the isolation of threo-carolignan X (492), and erythro-carolignan X (493) while bark of Durio oxleyanus yielded threo-carolignan Y (494) and erythro-carolignan Y (495).156 The structures and stereochemistry of these compounds were solved by MS and NMR methods including NOESY and HSQC-HECADE and by interpretation of CD data. The dichloromethane extract from the leaves of Magnolia garrettii (Magnoliaceae) collected in Austria afforded two dimeric lignans, garrettilignans A (496) and B (497), each substituted with two additional p-allylphenolic moieties.170 Although the relative configuration of C-7 and C-8 of the propyl chain was not assigned, the occurrence of dineolignans bearing additional 4-allylphenyl moieties as described herein for garrettilignans A and B has not been reported previously.
3.3 Benzodioxanes
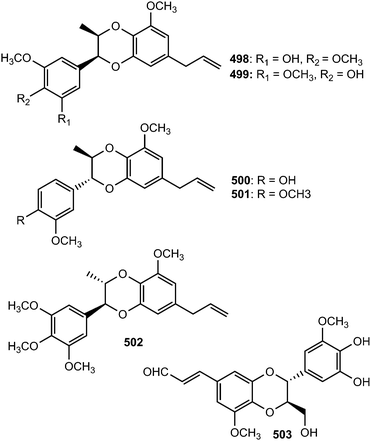
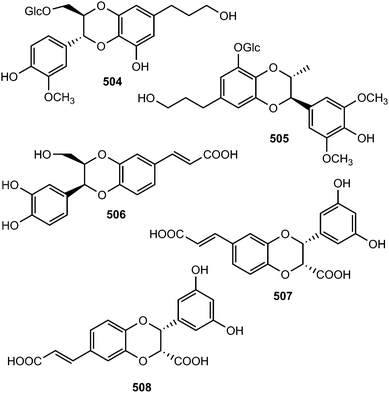
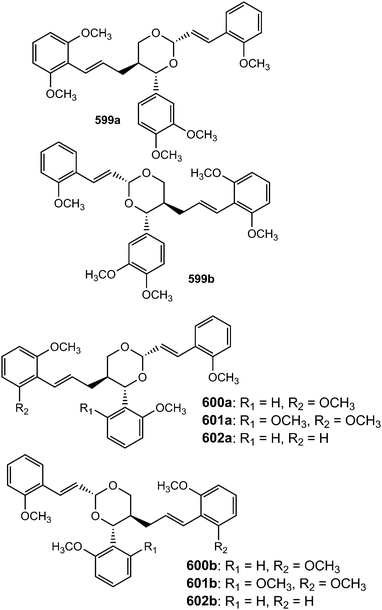
Because of the wide range of interesting biological activities exhibiting by this class of secondary metabolites, Pilkington and Barker171 have published recently a review on the synthesis and biology of 1,4-benzodioxane lignan natural products. The methanol extracts prepared from the leaves and stem of Miliusa fragrans were subjected to biological activity evaluation and were found to possess inhibitory activity against both herpes simplex virus and cancer cells. Chemical examination of these extracts resulted in the isolation of five benzodioxane neolignans namely (+)-3-O-demethyleusiderin (498) C, (+)-4-O-demethyleusiderin C (499), (−)-miliusfragrin (500), (−)-4-O-methylmiliusfragrin (501) and (+)-eusiderin A (502).125 Compound 499 showed recognizable anti-herpetic activity whereas 498, 499 and 500 exhibited appreciable cytotoxicity against some cancer cells. (7R,8R)-5-O-Demethylbilagrewin (503) is one of the aromatic compounds isolated from the heartwood of the medicinal plant Santalum album (L.) (Santalaceae). It exhibited strong cytotoxic activity against HL-60 cells with IC50 of 1.5 ± 0.02 μM.172 Other benzodioxanes include the glycoside 504 from the root bark of Illicium henryi,142 eusiderin N (505) from the stem of Eurya japonica,24 and compound 506 from the methanol extract of Morinda citrifolia.115Bae and Kim173 undertook a convenient enzymatic transformation of caffeic acid with polyphenol oxidase originating from pear which afforded two 1,4-benzodioxane-type neolignans caffeicinic acid (507), and isocaffeicinic acid (508). Compound 507 significantly enhanced inhibitory activity against cyclooxygenase-2 (COX-2) when compared to parent caffeic acid with an IC50 of 7.5 μM.
3.4 Biphenyl neolignans
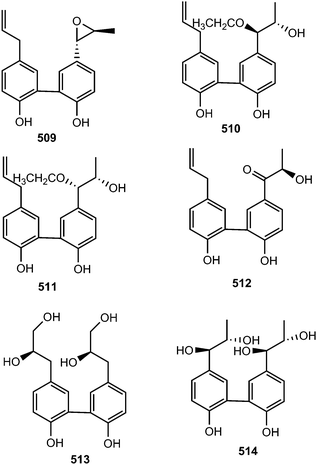
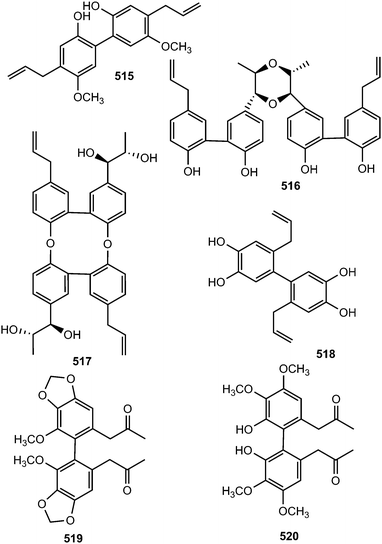
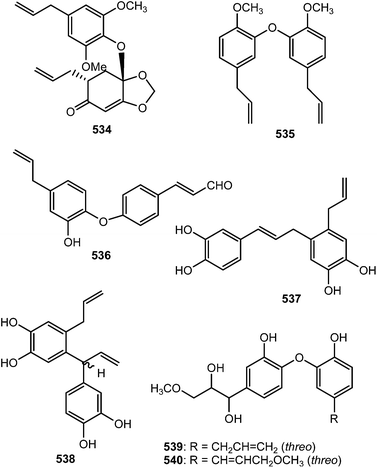
Streblus asper (Moraceae) is used by traditional healers as a remedy for hepatitis B virus in South China; notably, a plethora of biological activities have been reported with its extracts against dysentery, toothache, gingivitis, as well as having antibacterial and anti-inflammatory efficacies. The n-butanol soluble portion of a methanol extract of the stem bark of this plant was repeatedly purified by chromatography to afford 22 compounds including five neolignans: (7′S,8′S)-trans-streblusol A (509), (7′R,8′S)-erythro-streblusol B (510), (7′S,8′S)-threo-streblusol B (511), 8′R-streblusol C (512), and (8R,8′R)-streblusol D (513). The stereochemistry at the chiral centers was determined using CD spectra, as well as analyses of coupling constants and optical rotation data. The new compounds exhibited anti-hepatitis B virus activity with IC50 ranging from 38 to 396 μM.174 Chen et al.175 reported (7R,8S,7′R,8′S)-erythro-strebluslignanol H (514) from the same plant species while biseugenol A (515) was isolated from the bark of Litsea costalis (Lauraceae).176Strebluslignanol F (516) and (7′R,8′S,7′′R,8′′S)-erythro strebluslignanol G (517) are two biphenyl neolignan dimers obtained from the ethyl acetate soluble part of methanol extract from the roots of Streblus asper.177 The configuration was determined using CD spectra, as well as analyses of coupling constants and optical rotation data. They could be derived biogenetically via dehydration of 7′,8′-dihydroxyl and 4,4′-dihydroxyl groups of two strebluslignanols, respectively to form a dimer. Compound 517 exhibited significant anti-hepatitis B virus activity with an IC50 of 1.6 μM.177 Diallylcatechol (518), exhibiting moderate inhibitory effects on the aggregation of human platelet induced by collagen (IC50 of 64 μM), is among the important secondary metabolites reported from Piper taiwanense (Piperaceae).178 Neglignans C (519) and D (520), obtained from the acetone extract of the stems of Schisandra neglecta, are biphenyl neolignans characterized by the presence of 2-oxopropyl groups40 considered as 7,8-secodibenzocyclooctadienes. Neglignan D exhibited cytotoxicity against the NB4 and SHSY5Y cancer cell lines with IC50 of 3 and 3.3 μM.
3.5 Other neolignans
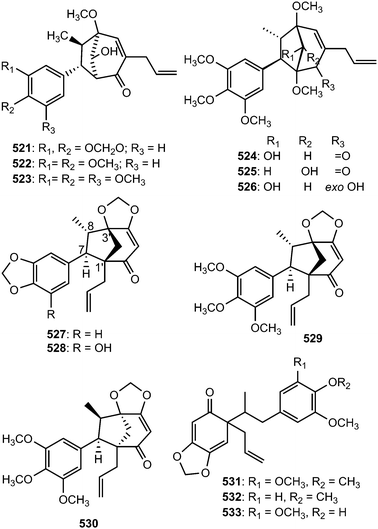
The macrophyllin-type bicyclo[3.2.1]octanoid neolignans ocophyllols A–C (521–523) were isolated from the leaves of Ocotea macrophylla Kunth (Lauraceae). They exhibited inhibition of platelet activating factor (PAF)-induced aggregation of rabbit platelets.179 Phytochemical exploration carried out on the leaves of Nectandra amazonum (Lauraceae) also afforded three new macrophyllin-type bicyclo[3.2.1]octanoid neolignans, nectamazins A–C (524–526) whose absolute configuration was assigned on the basis of CD spectra supported by NOESY correlations.180 The new compounds showed inhibition of PAF-induced aggregation of rabbit platelets; nectamazin A (524) was found to be the most potent PAF-antagonist, even though the activity was slightly lower (IC50 of 1.4 μM) than the positive control ginkgolide B, a known PAF-antagonist obtained from Ginkgo biloba. The bark of the Madagascan plant Ocotea cymosa afforded compounds 527–530 together with the 6′-oxo-8.1′-lignans cymosalignans A–C (531–533) and didymochlaenone C (534).152 Compounds 527–530 belong to a rare group of bicyclo[3.2.1]octanoid neolignans possessing a 7.1′.8.3′ coupling with unique features such as the deoxygenated C-2′ between the bridge heads and the methylenedioxy group on the cyclohexenone ring. The relative and absolute configurations of 531–533 were not assigned.142Biseugenol B (535) and isostrebluslignanaldehyde (536) are constituents of Litsea costalis176 and Streblus asper,177 respectively. Bioassay-guided fractionation of roots of Piper taiwanense led to isolation of four previously unreported neolignans namely neotaiwanensol A (537), neotaiwanensol B (538), taiwandimerol A (539) and taiwandimerol B (540).178 Compounds 539 and 540 showed moderate inhibitory effects on the aggregation of human platelet induced by collagen with IC50 of 56 and 72 μM, respectively.
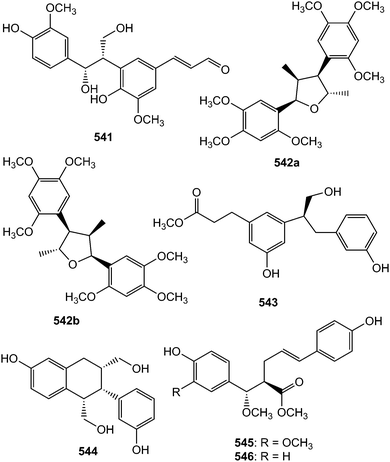
Prebalanophonin (541) is one of the main neolignans detected in Cirsium eriophorum using a combination of optimized acid treatments and complementary spectroscopic (HPLC-MS, GC-MS, CD and NMR) methods.149 A pair of rare C7–C8-type neolignan enantiomers trivially named (+)-acortatarinowin E (542a) and (−)-acortatarinowin E (542b) was obtained from the rhizomes of Acorus tatarinowii Schott after separation using a chiral HPLC column.114 Trogopterins B (543) and C (544) are among the lignans obtained from the feces of Trogopterus xanthipes Milne-Edwars.146 They exhibited moderate cytotoxic activities against HL-60 cells with IC50 of 35 and 42 μM, respectively.
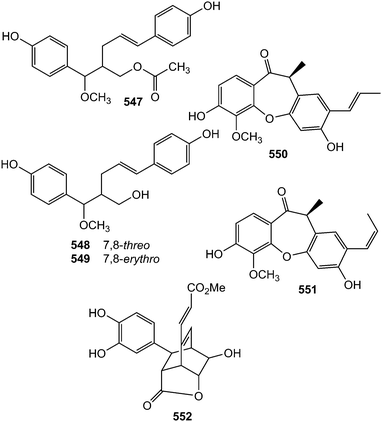
Five new 8-9′ linked neolignans, conchigeranals A–E (545–549) were isolated from the whole plant of Alpinia conchigera (Zingiberaceae).181 However, the relative stereostructures of 547–549 were not determined. Compounds 548 and 549 exhibited cytotoxicity against A549 cell lines with IC50 of 12 and 10 μg mL−1, respectively. Two 2,4′-epoxy-8,5′-neolignans, penthorins A (550) and B (551), were isolated from an ethyl acetate soluble portion of a hepatoprotective water decoction of Penthorum chinese (Saxifragaceae). Their structures and absolute configurations were elucidated by extensive spectroscopic analyses and electronic circular dichroism (ECD) calculations. Interestingly, this was the second report on occurrence of 2,4′-epoxy-8,5′-neolignans in plants. The latter showed in vitro protective activities against acetaminophen-induced hepatocyte injury at 5 μM.182 Do Vale et al.183 reported the unusual caffeic acid derived bicyclic[2.2.2]octane neolignan, rufescenolide (552) from the stem of Cordia rufescens (Boraginaceae).
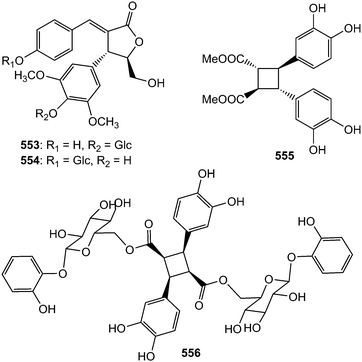
The neolignan glucosides saccharnans A (553) and B (554) possessing a rare skeleton were isolated from the desugared sugar cane (Saccharum officinarum) extract.184 Phytochemical investigation of the chloroform and ethyl acetate-soluble extracts of the leaves of Vitex quinata (Verbenaceae) afforded a new δ-truxinate derivative, dimethyl 3,4,3′,4′-tetrahydroxy-δ-truxinate (555), which did not show any efficacy against a panel of three selected human cancer cells.185 Compound 556, also believed to be derived from dimerization of two phenylpropanoyl esters of catechol glycoside units, was obtained from the leaves of Dodecadenia grandiflora.79
4 Miscellaneous
Tsangin C (557) is a secolignan reported from the roots of Beilschmiedia tsangii.129 Closely related compounds 558 and 559 were also characterized from the mature carpels of Manglietiastrum sinicum.49 They were derived from tetrahydrofuran lignans via oxidative cleavage of the C7–C8 bond. Secondary metabolites 560 and 561 are norbenzodioxane neolignans resulting from a phytochemical study of Morinda citrifolia.115 The dihydrobenzofuran type dinorneolignans, myrisfrageals A (562) and B (563), were isolated from the dried ripe seeds of Myristica fragrans Houtt. (Myristicaceae) and their absolute configurations determined by optical rotations and CD data. They showed inhibition of NO production in lipopolysaccharide activated murine monocyte macrophage RAW264.7 with IC50 of 76 and 45 μM, respectively.186 Ocophyllals A (564) and B (565) are dinor-benzofuran neolignan aldehydes isolated from the leaves of Ocotea macrophylla Kunth.179 The 7′,8′,9′-trinor- and 8′,9′-dinor-8,4-oxyneolignans 566 and 567, respectively and the 8′,9′-dinor-4,8′′-oxy-8,3′-sesquineolignan 568 are some of compounds isolated from stem of Sinocalamus affinis.112 Schischinone 569 is a 6,7-seco-homolignan, obtained from Schisandra chinensis (Turcz.) Baill.37Gymnothelignans A–K (570–580), N (581) and O (582), bearing tetrahydrofuran with variable conformations, were isolated from Gymnotheca chinensis.48 The absolute configurations of these compounds were assigned using X-ray single crystal diffraction and chemical transformations. Gymnothelignans N and O are eupodienone lignans, a class of secondary metabolites previously isolated only from the Australian shrubs Eupomatia bennettii and Eupomatia laurina.187,188 The unusual tetrahydrofuran patterns in gymnothelignan A–K was believed to derive biosynthetically from the eupadienone-type lignans as represented in Scheme 13.48 The isolated compounds were tested for their cytotoxic against HepG2 and Bcl7404 cell lines using an MTT assay. Gymnothelignan C exhibited moderate cytotoxicity against HepG2 and Bcl7404 cells with IC50 of 15 and 17 μg mL−1, respectively.
Two new eupodienone lignans, named gymnothelignans T (583) and gymnothelignan U (584), were also obtained from the endemic medicinal plant G. chinensis. Compound 583 showed moderate cytotoxic activity when tested against HCT15, HCT116, A549, MCF-7 and HepG2 cells with IC50 values above 50 μM.188 The absolute configuration of manglisin A (585) from the mature carpels of Manglietiastrum sinicum was assigned as 7R,8R on the basis of X-ray diffraction using the Flack parameter [0.06(9)] and Cu Kα radiation.49
Systematic investigation on the EtOAc extract from Schisandra sphenanthera fruit was performed during a search for HSV-2 and adenovirus inhibitors leading to the rare lignan 586 which exhibited moderate inhibition.189 In addition to dibenzocyclooctadienes, norlignans named marphenols C–F (587–590) were isolated from the fruits of Schisandra wilsoniana.36 5′α-Hydroxy-4′-methoxy-sauchinone (591) and 5′β-hydroxy-4′-methoxy-sauchinone (592) are from Saururus chinensis (Lour.) Baill. (Saururaceae), a perennial herb widely cultivated in China and southern Korea where it is used as folk medicine for the treatment of inflammation, jaundice and gonorrhea. The latter exhibited selective inhibitory activity on (diacylglycerol acyltransferase) DGAT1 with IC50 (87.5 ± 1.3 μM), an enzyme which catalyzes the final step of the triacylglycerol synthesis pathway in mammalian cells by using diacylglycerol and fatty acyl CoA as substrates.190 The unusual cyclolignan (593) was also isolated from the stem of Sinocalamus affinis.112
Two new sesqui-neolignans with novel conjugation patterns, simonols A (594) and B (595) were isolated from the ethanol extract of the fruits of Illicium simonii (Illiciaceae). Their structures were elucidated by spectroscopic methods including 1D and 2D NMR, HRESIMS, and calculation of ECD using density functional theory (DFT). Simonol A has a unique motif of a 5,5-dihydropyran with a hemiketal carbon, while simonol B possesses two dihydrofuran rings in the same direction.191 Compound 594 showed moderate inhibitory activities comparable to 5-fluorouracil against the cancer cell lines NCI-H460, SMMC-7721, MCF-7, and BGC-823 (IC50 of 18, 17, 46 and 36 μM, respectively). The ethyl acetate fraction of Acorus tatarinowii Schott (Araceae) exhibited antihyperglycemic activity. Activity-guided isolation furnished three novel sesquinlignans, tatanans A–C (596–598) with the unprecedented carbon skeleton as characterized by a unique C8–C7′ linkage pattern.192 Their structures were established by spectroscopic techniques and single-crystal X-ray analysis. Tatanans A–C were evaluated for their in vitro antidiabetic activities, including GK activity, dipeptidyl-peptidase-4 (DPP-4) antagonist activity, α-glycosidase inhibitory activity, Na+–glucose cotransporter (SGLT) antagonist activity, and aldose reductase inhibitory activity. Their activities were more potent than that of GKA22, one of the most potent GK activators reported in vitro to date, serving as a positive control. In addition, all metabolites were inactive against DPP-4, R-glycosidase, SGLT, and aldose reductase.192
(±)-Torreyunlignans A–D (599a/b–602a/b) are four pairs of new 8–9′ linked neolignan enantiomers featuring a rare (E)-2-styryl-1,3-dioxane moiety isolated from the trunk of Torreya yunnanensis (Taxaceae).193 The structures were determined by combined spectroscopic and chemical methods, and the absolute configurations elucidated by ECD calculations. Lignans featuring a C-8–C-9′ linked skeleton are rarely found in nature, as only 18 examples of this chemotype have been reported. All of the enantiomers exhibited inhibition against phosphodiesterase-9A with IC50 values ranging from 5.6 to 15 μM.193
The neolignan chimarrhinin 603 with an unprecedented squeleton was obtained from the leaves of Chimarrhis turbinata, a Rubiaceae plant species. The antioxidant activity of the new derivative was investigated using DPPH and an IC50 of 7.5 ± 0.5 μM was obtained. This result may be related to the presence of two catechol groups in the molecule.194 Hovenins A–D (604–607) constitute two pairs of diastereomeric flavonolignans (a class of lignans biosynthetically produced by the oxidative coupling of a flavonoid and a phenylpropanoid) from the seeds of Hovenia acerba (Rhamnaceae).195 Their absolute configurations were determined with the aid of 2D NMR, and CD spectroscopy. These compounds showed better activity than silybin (SLB), and exhibited moderate inhibition on the production of NO and IL-6 in LPS-stimulated RAW264.7 cells with IC50 values ranging from 45 to 63 μM.195
Other previously unreported flavonolignans include compounds 608–611 isolated from the ethanolic extract of the stem of Sinocalamus affinis112 and anthelminthicol A (612) from the EtOAc extract of the seeds of Hydnocarpus anthelminthica (Flacourtiaceae).196 The coumarinolignan cleomiscosin A methyl ether (613) was isolated from the methanolic extract of seeds of Hyoscyamus niger (Solanaceae).197 Uragogin (614), the first example of a triterpene-neolignan ester hetero-Diels–Alder adduct built with a dioxane bridge, was isolated from the EtOH extract of the stem of Crossopetalum uragoga (Celastraceae). Since the biosynthesis of dimeric and trimeric triterpenes containing a 1,4-dioxane bridge, which are chemotaxanomic markers of the Celastraceae species, has been hypothesized via Diels–Alderase systems, uragogin could be formed by the reaction of trans-coniferyl acetate with caffeoyl-olean-12-ene as ortho-quinone (Scheme 14).198
5 Outlook
This review presents 564 examples of lignans and neolignans, including 131 dibenzocyclooctadienes from plants, reported between 2009 and 2015. Their relevant bioactivities are also indicated. In terms of structural diversity, the lignan and neolignan class of secondary metabolites has been enriched with some unprecedented carbon skeletons. For instance, ramonanins A–D (353–356) isolated from Guaiacum officinale,132 or tatanans A–C (596–598) isolated from Acorus tatarinowii Schott192 have inimitable skeletons, while uragogin (614) represents the first example of a triterpene-neolignan ester hetero-Diels–Alder adduct built with a dioxane bridge.198 Furthermore, four rare furofuran dilignans, bearing an unusual α,β-unsaturated ketone group, were isolated from Zanthoxylum podocarpum119,120 and Zanthoxylum planispinum.121 In addition to the new popophyllotoxin derivatives cleistantoxin (149) and 4-acetyl-4-demethyl-podophyllotoxin (155), the benzodioxane-type lignan (7R,8R)-5-O-demethylbilagrewin (503) exhibited strong cytotoxic activity while (7′R,8′S,7′′R,8′′S)-erythro strebluslignanol G (517) showed significant anti-hepatitis B virus activity. These demonstrate the remarkable variety of lignans not only encompassing different and unique chemical scaffolds, but also exhibiting various functionalities worth exploitation by the pharmaceutical industries. For example, many of these compounds could be used as lead pro-drugs and further developed as new anticancer or antimicrobial agents, particularly at a time when microbial resistance is emergently causing global problems with few options of tackling the problem.Despite the isolation and characterization of numerous lignans and neolignans in the last decade, a lot of work remains to be accomplished on this class of secondary metabolites particularly because they represent a huge resource of bioactive compounds. Since these compounds could mostly be obtained in small amounts from plants, one of the main challenges that need to be addressed in the future is their total synthesis in order to allow further bioactivity studies. However, lignans and neolignans are characterized by the stereoselective oxidative coupling of two phenylpropane units; the presence of chiral centres is an interesting challenge that needs to be overcome on a case-by-case basis for synthesizing these remarkable classes of compounds. Notably, during the last few years, total synthesis of several biologically active lignans and neolignans has already been achieved. This includes, for instance, a highly flexible and concise total synthesis of (+)-podophyllotoxin featuring an enantioselective sequential conjugate addition–allylation reaction,84 a silver-catalyzed one-pot synthesis of a series of naturally occurring arylnaphthalene lignans,91 and an efficient and practical asymmetric synthesis of the 2,3-dihydrobenzo[b]furan neolignan acuminatin.157 Admittedly however, much remains to be learned about the biosynthetic pathway(s) leading to production of some of the recently-reported lignans/neolignans, which could be used in devising their total synthesis.
Another alternative source of lignans and neolignans could be their production from plant-associated microflora, in particular a diverse group of fungi and bacteria, called endophytes, which colonize living, internal plant tissues without causing abrupt, visible symptoms of disease within the hosts. In fact, the pharmacologically active arylnaphthalene lignan podophyllotoxin is already shown to be produced by several endophytic fungi, including Alternaria tenuissima isolated from Sinopodophyllum emodi (Wall.) Ying,199 and Mucor fragilis isolated from Sinopodophyllum hexandrum (Royle) Ying.200 Furthermore, some of us isolated and characterized the endophytic fungus Aspergillus fumigatus from the medicinal plant Juniperus communis, which is capable of producing the analogue of podophyllotoxin, called deoxypodophyllotoxin.201 Although this approach of using endophytes has been exploited by various researchers for sourcing known as well as novel lignans, commercial success is still limited due to low content of desired metabolites produced in vitro, recalcitrant nature and slow growth rate of many endophytes, genotypic variations, chemical instability, and complicated bioprocess design and downstream processing, among others.202
6 Acknowledgements
R. B. T. gratefully acknowledges the Alexander von Humboldt Foundation for granting a Georg Forster Research Fellowship for a research stay at the Institute of Environmental Research (INFU), Department of Chemistry and Chemical Biology, TU Dortmund. We are grateful to Prof. Dr Gail M. Preston (Department of Plant Sciences, University of Oxford, United Kingdom) for critically reviewing our manuscript and providing invaluable comments. We thank the anonymous reviewers for their critical comments and valuable advises. Notably, we have rounded off the bioactivities we report herein to two significant digits (wherever applicable) as recommended by one of the anonymous reviewers, which still convey the accuracy and comparability of the activities taking into consideration the variable nature and sensitivity of the assays.7 Notes and references
- C. Tringali, Bioactive Compounds from Natural Sources Isolation, Characterisation and Biological Properties, Corrado Tringali, first published 2001, Taylor & Francis, New York, 2001 Search PubMed.
- J.-Y. Pan, S.-L. Chen, M.-H. Yang, J. Wu, J. Sinkkonen and K. Zoud, Nat. Prod. Rep., 2009, 26, 1251–1292 RSC.
- R. Bernini, G. Gualandi, C. Crestini, M. Barontini, M. C. Belfiore, S. Willför, P. Eklund and R. Saladino, Bioorg. Med. Chem., 2009, 17, 5676–5682 CrossRef CAS PubMed.
- G. P. Moss, Pure Appl. Chem., 2000, 72, 1493–1523 CrossRef CAS.
- H. Satake, E. Ono and J. Murata, J. Agric. Food Chem., 2013, 61, 11721–11729 CrossRef CAS.
- H. Satake, T. Koyama, S. E. Bahabadi, E. Matsumoto, E. Ono and J. Murata, Metabolites, 2015, 5, 270–290 CrossRef CAS PubMed.
- A. Tsopmo, F. M. Awah and V. Kuete, in Medicinal Plant Research in Africa, ed. V. Kuete, Elsevier, Amsterdam, 2013, vol. 1, ch. 12, pp. 435–478 Search PubMed.
- T. Vogt, Mol. Plant, 2010, 3, 2–20 CrossRef CAS PubMed.
- A. A. Dar and N. Arumugam, Bioorg. Chem., 2013, 50, 1–10 CrossRef CAS PubMed.
- H. J. Kim, E. Ono, K. Morimoto, T. Yamagaki, A. Okazawa, A. Kobayashi and H. Satake, Plant Cell Physiol., 2009, 50, 2200–2209 CrossRef CAS PubMed.
- T. Umezawa, S. K. Ragamustari, T. Nakatsubo, S. Wada, L. Li, M. Yamamura, N. Sakakibara, T. Hattori, S. Suzuki and V. L. Chiang, Plant Biotechnol., 2013, 30, 97–109 CrossRef CAS.
- S. K. Ragamustari, M. Yamamura, E. Ono, T. Hattori, S. Suzuki, H. Suzuki, D. Shibata and T. Umezawa, Plant Biotechnol., 2014, 31, 257–267 CrossRef CAS.
- K. Morimoto and H. Satake, Biol. Pharm. Bull., 2013, 36, 1519–1523 CAS.
- S. K. Ragamustari, T. Nakatsubo, T. Hattori, E. Ono, Y. Kitamura, S. Suzuki, M. Yamamura and T. Umezawa, Plant Biotechnol., 2013, 30, 375–384 CrossRef CAS.
- J. V. Marques, K.-W. Kim, C. Lee, M. A. Costa, G. D. May, J. A. Crow, L. B. Davin and N. G. Lewis, J. Biol. Chem., 2013, 288, 466–479 CrossRef CAS PubMed.
- X. M. Gao, J. X. Pu, S. X. Huang, L. M. Yang, H. Huang, W. L. Xiao, Y. T. Zheng and H. D. Sun, J. Nat. Prod., 2008, 71, 558–563 CrossRef CAS.
- Y. C. Shen, Y. C. Lin, Y. B. Cheng, M. Y. Chiang, S. S. Liou and A. T. Khalil, Phytochemistry, 2009, 70, 114–120 CrossRef CAS PubMed.
- M. Chen, X. Xu, B. Xu, P. Yang, Z. Liao, S. L. Morris-Natschke, K.-H. Lee and D. Chen, Molecules, 2013, 18, 2297–2306 CrossRef CAS PubMed.
- Y. H. Kuo, H. C. Huang, L. M. Y. Kuo and C. F. Chen, J. Org. Chem., 1999, 64, 7023–7027 CrossRef CAS.
- D. E. Sok, H. S. Cui and M. R. Kim, Recent Pat. Food, Nutr. Agric., 2009, 1, 87–95 CrossRef CAS.
- A. Sibylle, S. Tatsuo, S. Marlen, R. Dagmar-Ulrike, P. Birgit and B. Volker, Arch. Gynecol. Obstet., 2012, 285, 1145–1151 CrossRef PubMed.
- M. DellaGreca, S. Zuppolini and A. Zarrelli, Phytochem. Rev., 2013, 12, 717–731 CrossRef CAS.
- A. Khoddami, M. A. Wilkes and T. H. Roberts, Molecules, 2013, 18, 2328–2373 CrossRef CAS PubMed.
- L.-M. Y. Kuo, L.-J. Zhang, H.-T. Huang, Z.-H. Lin, C.-C. Liaw, H.-L. Cheng, K.-H. Lee, S. L. Morris-Natschke, Y.-H. Kuo and H.-O. Ho, J. Nat. Prod., 2013, 76, 580–587 CrossRef CAS PubMed.
- Y. Li, W. Cheng, C. Zhu, C. Yao, L. Xiong, Y. Tian, S. Wang, S. Lin, J. Hu, Y. Yang, Y. Guo, Y. Yang, Y. Li, Y. Yuan, N. Chen and J. Shi, J. Nat. Prod., 2011, 74, 1444–1452 CrossRef CAS PubMed.
- H. Liu, J. Zhang, X. Li, Y. Qi, Y. Peng, B. Zhang and P. Xiao, Phytomedicine, 2012, 19, 1234–1241 CrossRef CAS.
- T. J. Schmidt, S. Hemmati, M. Klaes, B. Konuklugil, A. Mohagheghzadeh, I. Ionkova, E. Fuss and A. W. Alfermann, Phytochemistry, 2010, 71, 1714–1728 CrossRef CAS.
- N. Cukelj, I. Jakasa, H. Sarajlija, D. Novotni and D. Curic, Talanta, 2011, 84, 127–132 CrossRef CAS PubMed.
- F. Bonzanini, R. Bruni, G. Palla, N. Serlataite and A. Caligiani, Food Chem., 2009, 117, 745–749 CrossRef CAS.
- G. Yuan, Y. Liu, T. Li, Y. Wang, Y. Sheng and G. Ming, Molecules, 2011, 16, 3713–3722 CrossRef CAS.
- J.-H. Yang, H.-Y. Zhang, J. Wen, X. Du, J.-H. Chen, H.-B. Zhang, W.-L. Xiao, J.-X. Pu, X.-C. Tang and H.-D. Sun, J. Nat. Prod., 2011, 74, 1028–1035 CrossRef CAS PubMed.
- J.-H. Yang, H.-Y. Zhang, J. Wen, X. Du, W. Wang, W.-L. Xiao, J. Wen, J.-X. Pu, X.-C. Tang and H.-D. Sun, Tetrahedron, 2011, 64, 4498–4504 CrossRef.
- K. Dong, J.-X. Pu, H.-Y. Zhang, X. Du, X.-N. Li, J. Zou, J.-H. Yang, W. Zhao, X.-C. Tang and H.-D. Sun, J. Nat. Prod., 2012, 75, 249–256 CrossRef CAS PubMed.
- G.-Y. Yang, Y.-K. Li, R.-R. Wang, X.-N. Li, W.-L. Xiao, L.-M. Yang, J.-X. Pu, Y.-T. Zheng and H.-D. Sun, J. Nat. Prod., 2010, 73, 915–919 CrossRef CAS.
- W.-H. Ma, Y. Lu, H. Huang, P. Zhou and D.-F. Chen, Bioorg. Med. Chem. Lett., 2009, 19, 4958–4962 CrossRef CAS PubMed.
- G.-Y. Yang, R.-R. Wang, H.-X. Mu, Y.-K. Li, X.-N. Li, L.-M. Yang, Y.-T. Zheng, W.-L. Xiao and H.-D. Sun, J. Nat. Prod., 2013, 76, 250–255 CrossRef CAS PubMed.
- Y. Xue, X. Li, X. Du, X. Li, W. Wang, J. Yang, J. Chen, J. Pu and H. Sun, Phytochemistry, 2015, 116, 253–261 CrossRef CAS.
- Y. Ikeya, H. Taguchi, I. Yosioka, Y. Iitaka and H. Kobayashi, Chem. Pharm. Bull., 1979, 27, 1395–1401 CrossRef CAS PubMed.
- X.-N. Li, J.-X. Pu, X. Du, L.-M. Yang, H.-M. An, C. Lei, F. He, X. Luo, Y.-T. Zheng, Y. Lu, W.-L. Xiao and H.-D. Sun, J. Nat. Prod., 2009, 72, 1133–1141 CrossRef CAS.
- X.-M. Gao, R.-R. Wang, D.-Y. Niu, C.-Y. Meng, L.-M. Yang, Y.-T. Zheng, G.-Y. Yang, Q.-F. Hu, H.-D. Sun and W.-L. Xiao, J. Nat. Prod., 2013, 76, 1052–1057 CrossRef CAS PubMed.
- X.-M. Gao, D.-Y. Niu, C.-Y. Meng, F.-Q. Yao, B. Zhou, R.-R. Wang, L.-M. Yang, Y.-T. Zheng, Q.-F. Hu, H.-D. Sun and W.-L. Xiao, J. Braz. Chem. Soc., 2013, 24, 2021–2027 CAS.
- M. Chen, X. M. Xu, Z. H. Liao, L. Dong, L. Li and C. Z. Huang, Molecules, 2008, 13, 548–555 CrossRef CAS.
- Y.-B. Cheng, M.-T. Chang, Y.-W. Lo, C.-J. Ho, Y.-C. Kuo, C.-T. Chien, S.-Y. Chen, S.-S. Liou, Y.-H. Kuo and Y.-C. Shen, J. Nat. Prod., 2009, 72, 1663–1668 CrossRef CAS PubMed.
- D. Hu, Z. Yang, X. Yao, H. Wang, N. Han, Z. Liu, Y. Wang, J. Yang and J. Yin, Phytochemistry, 2014, 104, 72–78 CrossRef CAS.
- H. M. Kim, B. Ryu, J. S. Lee, J. H. Choi and D. S. Jang, Chem. Pharm. Bull., 2015, 63, 746–751 CrossRef CAS PubMed.
- G.-Y. Yang, P. Fan, R.-R. Wang, J.-L. Cao, W.-L. Xiao, L.-M. Yang, J.-X. Pu, Y.-T. Zheng and H.-D. Sun, Chem. Pharm. Bull., 2010, 58, 734–737 CrossRef CAS.
- L. Fang, C. Xie, H. Wang, D.-Q. Jin, J. Xu and Y. Guo, Phytochem. Lett., 2014, 9, 158–162 CrossRef CAS.
- D. He, L. Ding, H. Xu, X. Lei, H. Xiao and Y. Zhou, J. Org. Chem., 2012, 77, 8435–8443 CrossRef CAS.
- J.-Y. Ding, C.-M. Yuan, M.-M. Cao, W.-W. Liu, C. Yu, H.-Y. Zhang, Y. Zhang, Y.-T. Di, H.-P. He, S.-L. Li and X.-J. Hao, J. Nat. Prod., 2014, 77, 1800–1805 CrossRef CAS PubMed.
- W. Schühly, B. Gröblacher, J. Neyer, W. M. F. Fabian, F. R. Fronczek and O. Kunert, Phytochemistry, 2010, 71, 1787–1795 CrossRef PubMed.
- Y.-C. Lin, Y.-B. Cheng, C.-C. Liaw, I.-W. Lo, Y.-H. Kuo, M. Y. Chiang, C. H. Chou and Y.-C. Shen, Molecules, 2013, 18, 6573–6583 CrossRef CAS PubMed.
- Y. Ikeya, H. Taguchi, I. Yosioka and H. Kobayashi, Chem. Pharm. Bull., 1979, 27, 1383–1394 CrossRef CAS PubMed.
- M. Blunder, E. M. Pferschy-Wenzig, W. M. F. Fabian, A. Hüfner, O. Kunert, R. Saf, W. Schühly and R. Bauer, Bioorg. Med. Chem., 2010, 18, 2809–2815 CrossRef CAS PubMed.
- A. Fürstner and G. Seidel, J. Org. Chem., 1997, 62, 2332–2336 CrossRef.
- K. Kashima, K. Sano, Y. S. Yun, H. Ina, A. Kunugi and H. Inoue, Chem. Pharm. Bull., 2010, 58, 191–194 CrossRef CAS PubMed.
- L.-M. Y. Kuo, L.-J. Zhang, H.-T. Huang, Z.-H. Lin, C.-C. Liaw, H.-L. Cheng, K.-H. Lee, S. L. Morris-Natschke, Y.-H. Kuo and H.-O. Ho, J. Nat. Prod., 2013, 76, 580–587 CrossRef CAS.
- E. C. N. Nono, P. Mkounga, V. Kuete, K. Marat, P. G. Hultin and A. E. Nkengfack, J. Nat. Prod., 2010, 73, 213–216 CrossRef CAS PubMed.
- V. T. T. Thanh, V. C. Pham, H. D. T. Mai, M. Litaudon, F. Gueritte, P. Retailleau, V. H. Nguyen and V. M. Chau, J. Nat. Prod., 2012, 75, 1578–1583 CrossRef CAS.
- Q. L. Ying, Y. Liu and T. Xuan, Curr. Bioact. Compd., 2007, 3, 37–66 CrossRef.
- T. Rezanka, P. Rezanka and K. Sigler, Phytochemistry, 2009, 70, 1049–1054 CrossRef CAS.
- Y.-J. Sun, Z.-L. Li, H. Chen, X.-Q. Liu, W. Zhou and H.-M. Hua, Bioorg. Med. Chem. Lett., 2011, 21, 3794–3797 CrossRef CAS.
- X. Zeng, H. Wang, Z. Gong, J. Huang, W. Pei, X. Wang, J. Zhang and X. Tang, Fitoterapia, 2015, 101, 153–161 CrossRef CAS.
- S. Takahashi, S. Kawakami, S. Sugimoto, K. Matsunami and H. Otsuka, Am. J. Plant Sci., 2015, 6, 676–684 CrossRef CAS.
- M. D. P. Pereira, M. R. Ferreira, G. B. Messiano, I. P. Ceravolo, L. M. X. Lopes and A. U. Krettli, Phytochem. Lett., 2015, 13, 200–205 CrossRef CAS.
- H. Z. Li, G. J. Luo, H. M. Li, X. L. Li and R. T. Li, Chin. Chem. Lett., 2011, 22, 85–87 CrossRef CAS.
- C. Q. Zhao, Y. Y. Zhu, S. Y. Chen and Y. Ogihara, Chin. Chem. Lett., 2011, 22, 181–184 CrossRef CAS.
- M. Omar, Y. Matuo, H. Maeda, Y. Saito and T. Tanaka, Phytochem. Lett., 2013, 6, 486–490 CrossRef CAS.
- J. Zhou, C.-J. Li, J.-Z. Yang, J. Ma, L.-Q. Wu, W.-J. Wang and D.-M. Zhang, Phytochemistry, 2016, 121, 58–64 CrossRef CAS.
- Y.-N. Yang, Z.-Z. Liu, Z.-M. Feng, J.-S. Jiang and P.-C. Zhang, J. Agric. Food Chem., 2012, 60, 964–972 CrossRef CAS.
- H. Jin, H.-L. Yin, S.-J. Liu, L. Chen, Y. Tian, B. Li, Q. Wang and J.-X. Dong, Fitoterapia, 2014, 94, 70–76 CrossRef CAS PubMed.
- J.-L. Wu, S. Jun-ichi and A. Masayoshi, J. Nat. Prod., 2006, 69, 790–794 CrossRef CAS PubMed.
- W. S. Suh, K. H. Kim, H. K. Kim, S. U. Choi and K. R. Lee, Helv. Chim. Acta, 2015, 98, 1087–1094 CrossRef CAS.
- K. H. Kim, E. Moon, S. Y. Kim, S. U. Choi and K. R. Lee, Food Chem. Toxicol., 2012, 50, 3680–3686 CrossRef CAS PubMed.
- W.-J. Tsai, C.-C. Shen, T.-H. Tsai and L.-C. Lin, J. Nat. Prod., 2014, 77, 125–131 CrossRef CAS PubMed.
- S. H. Park, S. K. Ko and S. H. Chung, J. Ethnopharmacol., 2005, 102, 326–335 CrossRef PubMed.
- E. J. Jeong, J. H. Cho, S. H. Sung, S. Y. Kim and Y. C. Kim, Bioorg. Med. Chem. Lett., 2011, 21, 2283–2286 CrossRef CAS PubMed.
- K. Yoshikawa, S. Tani, C. Baba and T. Hashimoto, Molecules, 2013, 18, 9641–9649 CrossRef CAS PubMed.
- Y. Ren, D. D. Lantvit, Y. Deng, R. Kanagasabai, J. C. Gallucci, T. N. Ninh, H.-B. Chai, D. D. Soejarto, J. R. Fuchs, J. C. Yalowich, J. Yu, S. M. Swanson and A. D. Kinghorn, J. Nat. Prod., 2014, 77, 1494–1504 CrossRef CAS.
- M. Kumar, P. Rawat, N. Rahuja, A. K. Srivastava and R. Maurya, Phytochemistry, 2009, 70, 1448–1455 CrossRef CAS PubMed.
- H. L. Holland, E. J. Bergen, P. C. Chenchaiah, S. H. Khan, B. Munoz, R. W. Ninniss and D. Richards, Can. J. Chem., 1987, 65, 502–507 CrossRef CAS.
- G. B. Messiano, E. M. K. Wijeratne, L. M. X. Lopes and A. A. L. Gunatilaka, J. Nat. Prod., 2010, 73, 1933–1937 CrossRef CAS.
- Y.-Q. Liu, L. Yang and X. Tian, Curr. Bioact. Compd., 2007, 3, 37–66 CrossRef CAS.
- A. M. Rojas-Sepúlveda, M. Mendieta-Serrano, M. Y. Antúnez Mojica, E. Salas-Vidal, S. Marquina, M. L. Villarreal, A. M. Puebla, J. I. Delgado and L. Alvarez, Molecules, 2012, 17, 9506–9519 CrossRef PubMed.
- Y. Wu, J. Zhao, J. Chen, C. Pan, L. Li and H. Zhang, Org. Lett., 2009, 11, 597–600 CrossRef CAS PubMed.
- C. Agami, S. Comesse and C. Kadouri-Puchot, J. Org. Chem., 2002, 67, 1496–1500 CrossRef CAS PubMed.
- H. Joseph, J. Gleye, C. Moulis, L. J. Mensah, C. Roussakis and C. Gratas, J. Nat. Prod., 1988, 51, 599–600 CrossRef CAS.
- C. W. Chang, M. T. Lin, S. S. Lee, K. C. S. C. Liu, F. L. Hsu and J. Y. Lin, Antiviral Res., 1995, 27, 367–374 CrossRef CAS PubMed.
- J. Gertsch, R. T. Tobler, R. Brun, O. Sticher and J. Heilmann, Planta Med., 2003, 69, 420–424 CrossRef CAS PubMed.
- J. R. Weng, H. H. Ko, T. L. Yeh, C. H. Lin and C. N. Lin, Arch. Pharm. Pharm. Med. Chem., 2004, 337, 207–212 CrossRef CAS PubMed.
- P. Foley, N. Eghbali and P. T. Anastas, Green Chem., 2010, 12, 888–892 RSC.
- P. Foley, N. Eghbali and P. T. Anastas, J. Nat. Prod., 2010, 73, 811–813 CrossRef CAS PubMed.
- L. F. L. Barros, A. Barison, M. J. Salvador, R. De Mello-Silva, E. C. Cabral, M. N. Eberlin and M. A. Stefanello, J. Nat. Prod., 2009, 72, 1529–1532 CrossRef CAS PubMed.
- K. Jiang, Q.-Y. Song, S.-J. Peng, Q.-Q. Zhao, G.-D. Li, Y. Li and K. Gao, Fitoterapia, 2015, 103, 63–70 CrossRef CAS PubMed.
- J. S. Lee, M. S. Huh, Y. C. Kim, M. Hattori and T. Otake, Antiviral Res., 2010, 85, 425–428 CrossRef CAS PubMed.
- Y.-B. Xue, Y.-L. Zhang, J.-H. Yang, X. Du, J.-X. Pu, W. Zhao, X.-N. Li, W.-L. Xiao and H.-D. Sun, Chem. Pharm. Bull., 2010, 58, 1606–1611 CrossRef CAS PubMed.
- A. Yokosuka, Y. Matsuo, M. Jitsuno, K. Adachi and Y. Mimaki, Chem. Pharm. Bull., 2011, 59, 1467–1470 CrossRef CAS PubMed.
- D.-T. Yang, S.-S. Lin, J.-H. Chen, S.-T. Yuan, J.-S. Shi, J.-S. Wang and A.-Q. Jia, Bioorg. Med. Chem. Lett., 2015, 25, 1976–1978 CrossRef CAS PubMed.
- C. S. Kim, O. W. Kwon, S. Y. Kim and K. R. Lee, J. Nat. Prod., 2013, 76, 2131–2135 CrossRef CAS PubMed.
- B. J. Cabanillas, A.-C. Le Lamer, D. Castillo, J. Arevalo, R. Rojas, G. Odonne, G. Bourdy, B. Moukarzel, M. Sauvain and N. Fabre, J. Nat. Prod., 2010, 73, 1884–1890 CrossRef CAS PubMed.
- M. S. C. Pedras and Q.-A. Zheng, Phytochemistry, 2010, 71, 581–589 CrossRef CAS PubMed.
- C. Zhu, L. Jing, N. Yu, X. Yang and Y. Zhao, Acta Pharm. Sin. B, 2013, 3, 109–112 CrossRef.
- C.-I. Chang, Y.-C. Li, C.-C. Kuo, C.-Y. Chao, H.-S. Chang, J.-H. Wu, S.-Y. Wang and Y.-H. Kuo, Nat. Prod. Commun., 2013, 8, 1–2 Search PubMed.
- Y.-Y. Yang, X.-Y. Huang, Z.-M. Feng, J.-S. Jiang and P.-C. Zhang, J. Agric. Food Chem., 2015, 63, 7958–7966 CrossRef CAS PubMed.
- O. P. Mishra, N. Simmons, S. Tyagi, R. Pietrofesa, V. V. Shuvaev, R. A. Valiulin, P. Heretsch, K. C. Nicolaou and M. Christofidou-Solomidou, Bioorg. Med. Chem. Lett., 2013, 23, 5325–5328 CrossRef CAS PubMed.
- J. Rajesha, K. N. Murthy, M. K. Kumar, B. Madhusudhan and G. A. Ravishankar, J. Agric. Food Chem., 2006, 54, 3794–3799 CrossRef CAS PubMed.
- S. S. Moree and J. Rajesha, Mol. Cell. Biochem., 2013, 373, 179–187 CrossRef CAS PubMed.
- K. Prasad, Mol. Cell. Biochem., 1997, 168, 117–123 CrossRef CAS PubMed.
- E. Lee, V. S. J. Ahamed, M. S. Kumar, S. W. Rhee, S.-S. Moon and I. S. Hong, Bioorg. Med. Chem. Lett., 2011, 21, 6245–6249 CrossRef CAS PubMed.
- J. Y. Kim, H. J. Lim, D. Y. Lee, J. S. Kim, D. H. Kim, H. J. Lee, H. D. Kim, R. Jeon and J.-H. Ryu, Bioorg. Med. Chem. Lett., 2009, 19, 937–940 CrossRef CAS PubMed.
- A. Stojakowska, K. Michalska, J. Malarz, A. Beharav and W. Kisiel, Food Chem., 2013, 138, 1250–1255 CrossRef CAS PubMed.
- R.-O. Mei, Y.-H. Wang, G.-H. Du, G.-M. Liu, L. Zhang and Y.-X. Cheng, J. Nat. Prod., 2009, 72, 621–625 CrossRef CAS PubMed.
- L. Xiong, C. Zhu, Y. Li, Y. Tian, S. Lin, S. Yuan, J. Hu, Q. Hou, N. Chen, Y. Yang and J. Shi, J. Nat. Prod., 2011, 74, 1188–1200 CrossRef CAS PubMed.
- S. S. Hong, C. Lee, C. H. Lee, M. Park, M. S. Lee, J. T. Hong, H. Lee, M. K. Lee and B. Y. Hwang, Arch. Pharmacal Res., 2009, 32, 501–504 CrossRef CAS PubMed.
- Y. Lu, Y. Xue, J. Liu, G. Yao, D. Li, B. Sun, J. Zhang, Y. Liu, C. Qi, M. Xiang, Z. Luo, G. Du and Y. Zhang, J. Nat. Prod., 2015, 78, 2205–2214 CrossRef CAS PubMed.
- P.-H. Nguyen, J.-L. Yang, M. N. Uddin, S.-L. Park, S.-Il Lim, D.-W. Jung, D. R. Williams and W.-K. Oh, J. Nat. Prod., 2013, 76, 2080–2087 CrossRef CAS PubMed.
- X. Sun, G. Ma, D. Zhang, W. Huang, G. Ding, H. Hu, G. Tu and B. Guo, Molecules, 2015, 20, 2165–2175 CrossRef PubMed.
- P. Luecha, K. Umehara, T. Miyase and H. Noguchi, J. Nat. Prod., 2009, 72, 1954–1959 CrossRef CAS PubMed.
- K. H. Kim, E. Moon, S. Y. Kim and K. R. Lee, J. Agric. Food Chem., 2010, 58, 4779–4785 CrossRef CAS PubMed.
- X.-J. Zhou, X.-L. Chen, X.-S. Li, J. Su, J.-B. He, Y.-H. Wang, Y. Li and Y.-X. Cheng, Bioorg. Med. Chem. Lett., 2011, 21, 373–376 CrossRef CAS PubMed.
- X.-J. Zhou, X.-L. Chen, X.-S. Li, J. Su, J.-B. He, Y.-H. Wang, Y. Li and Y.-X. Cheng, Bioorg. Med. Chem. Lett., 2013, 23, 5452 CrossRef CAS.
- G.-Y. Su, K.-W. Wang, X.-Y. Wang and B. Wu, Phytochem. Lett., 2015, 11, 120–126 CrossRef CAS.
- T. Sasakia, W. Li, a. S. Zaike, Y. Asada, Q. Li, F. Ma, Q. Zhang and K. Koike, Phytochemistry, 2013, 95, 333–340 CrossRef PubMed.
- Y.-N. Yang, X.-Y. Huang, W.-M. Feng, J.-S. Jiang and P.-C. Zhang, J. Agric. Food Chem., 2014, 62, 9095–9102 CrossRef CAS PubMed.
- K. H. Kim, E. Moon, S. K. Ha, W. S. Suh, H. K. Kim, S. Y. Kim, S. U. Choi and K. R. Lee, Chem. Pharm. Bull., 2014, 62, 1136–1140 CrossRef CAS PubMed.
- K. Sawasdee, T. Chaowasku, V. Lipipun, T.-H. Dufat, S. Michel and K. Likhitwitayawuid, Tetrahedron Lett., 2013, 54, 4259–4263 CrossRef CAS.
- X.-Y. Huang, Z.-M. Feng, Y.-N. Yang, J.-S. Jiang and P.-C. Zhang, J. Asian Nat. Prod. Res., 2015, 17, 504–511 CrossRef CAS PubMed.
- K. H. Kim, H. K. Kim, S. U. Choi, E. Moon, S. Y. Kim and K. R. Lee, J. Nat. Prod., 2011, 74, 2187–2192 CrossRef CAS PubMed.
- Y. Ren, H. Chai, M. Goetz and A. D. Kinghorn, Tetrahedron Lett., 2013, 54, 4854–4858 CrossRef CAS PubMed.
- Y.-T. Huang, H.-S. Chang, G.-J. Wang, M.-J. Cheng, C.-H. Chen, Y.-J. Yang and I.-S. Chen, J. Nat. Prod., 2011, 74, 1875–1880 CrossRef CAS PubMed.
- M. DellaGreca, A. Mancino, L. Previtera, A. Zarrelli and S. Zuppolini, Phytochem. Lett., 2011, 4, 118–121 CrossRef CAS.
- H. Wang, C.-A. Geng, H.-B. Xu, X.-Y. Huang, Y.-B. Ma, C.-Y. Yang, X.-M. Zhang and J.-J. Chen, Planta Med., 2015, 81, 847–854 CrossRef CAS PubMed.
- K. J. Chavez, X. Feng, J. A. Flanders, E. Rodriguez and F. C. Schroeder, J. Nat. Prod., 2011, 74, 1293–1297 CrossRef CAS PubMed.
- A. K. F. Albertson and J.-P. Lumb, Angew. Chem., Int. Ed., 2015, 54, 2204–2208 CrossRef CAS PubMed.
- K. Sawasdee, T. Chaowasku, V. Lipipun, T.-H. Dufat, S. Michel and K. Likhitwitayawuid, Fitoterapia, 2013, 85, 49–56 CrossRef CAS PubMed.
- H. Zhao, T. Nie, H. Guo, J. Li and H. Bai, Phytochem. Lett., 2012, 5, 240–243 CrossRef CAS.
- C. Lee, J. W. Lee, Q. Jin, H. J. Lee, S.-J. Lee, D. Lee, M. K. Lee, C. K. Lee, J. T. Hong, M. K. Lee and B. Y. Hwang, Bioorg. Med. Chem. Lett., 2013, 23, 6010–6014 CrossRef CAS PubMed.
- G.-H. Tang, Z.-W. Chen, T.-T. Lin, M. Tan, X.-Y. Gao, J.-M. Bao, Z.-B. Cheng, Z.-H. Sun, G. Huang and S. Yin, J. Nat. Prod., 2015, 78, 1894–1903 CrossRef CAS PubMed.
- H.-G. Jin, A. R. Kim, H. J. Ko, S. K. Lee and E.-R. Woo, Chem. Pharm. Bull., 2014, 62, 288–293 CrossRef CAS PubMed.
- L. Wang, F. Li, C.-Y. Yang, A.-A. Khan, X. Liu and M.-K. Wang, Fitoterapia, 2014, 99, 92–98 CrossRef CAS PubMed.
- T. Yuan, C. Wan, A. G. Ez-Sarria, V. Kandhi, N. B. Cech and N. P. Seeram, J. Nat. Prod., 2011, 74, 2472–2476 CrossRef CAS PubMed.
- C.-W. Song, S.-M. Wang, L.-L. Zhou, F.-F. Hou, K.-J. Wang, Q.-B. Han, N. Li and Y.-X. Cheng, J. Agric. Food Chem., 2011, 59, 1199–1204 CrossRef CAS PubMed.
- W.-J. Xiang, L. Ma and L.-H. Hu, Fitoterapia, 2010, 81, 1228–1231 CrossRef CAS PubMed.
- J. G. S. Filho, S. L. Nimmo, H. S. Xavier, J. M. Barbosa-Filho and R. H. Cichewicz, J. Nat. Prod., 2009, 72, 1344–1347 CrossRef PubMed.
- X. Li, J.-G. Luo, X.-B. Wang, J. Luo, J.-S. Wang and L.-Y. Kong, Fitoterapia, 2012, 83, 883–887 CrossRef CAS PubMed.
- Y.-S. Shi, Y.-B. Liu, S.-G. Ma, Y. Li, J. Qu, L. Li, S.-P. Yuan, Q. Hou, Y.-H. Li, J.-D. Jiang and S.-S. Yu, J. Nat. Prod., 2015, 78, 1526–1535 CrossRef CAS PubMed.
- S. Baek, X. Xia, B. S. Min, C. Park and S. H. Shim, Beilstein J. Org. Chem., 2014, 10, 2955–2962 CrossRef PubMed.
- Y. Wu, C. Liu, H.-F. Li, J.-B. Sun, Y.-Y. Li, W. Gu, D.-Y. Wang, J.-G. Liu and Y.-L. Hu, Biochem. Syst. Ecol., 2014, 55, 41–45 CrossRef CAS.
- X. Liu, M.-H. Yang, X.-B. Wang, S.-S. Xie, Z.-R. Li, D.-H. Kim, J.-S. Park and L.-Y. Kong, Fitoterapia, 2015, 103, 136–142 CrossRef CAS PubMed.
- A. Solyomvary, G. Toth, B. Komjati, P. Horvath, M. Kraszni, B. Noszal, I. Molnar-Perl and I. Boldizsar, Proc. Biochem., 2015, 50, 853–858 CrossRef CAS.
- K. W. Woo, S. U. Choi, J. C. Park and K. R. Lee, Arch. Pharmacal Res., 2011, 34, 2043–2049 CrossRef CAS PubMed.
- Y.-L. Li, Y.-X. Gao, H.-Z. Jin, L. Shan, W.-L. Chang, X.-W. Yang, H.-W. Zeng, N. Wang, A. Steinmetz and W.-D. Zhang, Phytochemistry, 2015, 117, 135–143 CrossRef CAS PubMed.
- L. H. Rakotondraide, P. R. Graupner, Q. Xiong, M. Olson, J. D. Wiley, P. Krai, P. J. Brodie, M. W. Callmander, E. Rakotobe, F. Ratovoson, V. E. Rasamison, M. B. Cassera, D. R. Hahn, D. G. I. Kingston and S. Fotso, J. Nat. Prod., 2015, 78, 431–440 CrossRef PubMed.
- X.-X. Huang, C.-C. Zhou, L.-Z. Li, Y. Peng, L.-L. Lou, S. Liu, D.-M. Li, T. Ikejima and S.-J. Song, Fitoterapia, 2013, 91, 217–223 CrossRef CAS PubMed.
- S. Rattanaburi, W. Mahabusarakam, S. Phongpaichit and A. R. Carroll, Phytochem. Lett., 2012, 5, 18–21 CrossRef CAS.
- T. Murata, K. Oyama, M. Fujiyama, B. Oobayashi, K. Umehara, T. Miyase and F. Yoshizaki, Fitoterapia, 2013, 91, 51–59 CrossRef CAS PubMed.
- Rudiyansyah, L. K. Lambert and M. J. Garson, J. Nat. Prod., 2010, 73, 1649–1654 CrossRef CAS PubMed.
- C. Lopez-Sanchez, M. Alvarez-Corral, L. Jimenez-Gonzalez, M. Munoz-Dorado and I. Rodríguez-García, Tetrahedron, 2013, 69, 5511–5516 CrossRef CAS.
- J. R. Fulton, V. K. Aggarwal and J. de Vicente, Eur. J. Org. Chem., 2005, 2005, 1479–1492 CrossRef.
- V. A. Yap, B.-J. Loong, K.-N. Ting, S. H.-S. Loh, K.-T. Yong, Y.-Y. Low, T.-S. Kam and K.-H. Lim, Phytochemistry, 2015, 109, 96–102 CrossRef CAS PubMed.
- G.-Y. Cao, W. Xu, X.-W. Yang, F. J. Gonzalez and F. Li, Food Chem., 2015, 173, 231–237 CrossRef CAS PubMed.
- X.-X. Huang, C.-C. Zhou, L.-Z. Li, F.-F. Li, L.-L. Lou, D.-M. Li, T. Ikejima, Y. Peng and S.-J. Song, Bioorg. Med. Chem. Lett., 2013, 23, 5599–5604 CrossRef CAS PubMed.
- X.-S. Feng, Y. Qu, L. Xu, D.-C. Wang, L.-J. Wu, F.-H. Meng and Y.-R. Liu, Molecules, 2009, 14, 953–958 CrossRef CAS PubMed.
- J. F. Liao, S. Y. Huang, Y. M. Jan, L. L. Yu and C. F. Chen, J. Ethnopharmacol., 1998, 61, 185–193 CrossRef CAS PubMed.
- K. H. Kim, E. Moon, H. K. Kim, J. Y. Oh, S. Y. Kim, S. U. Choi and K. R. Lee, Bioorg. Med. Chem. Lett., 2012, 22, 6155–6159 CrossRef CAS PubMed.
- C.-B. Xue, D.-W. Chai, X.-J. Jin, Y.-R. Bi, X.-J. Yao, W.-S. Wu and Y. Zhu, Phytochemistry, 2011, 72, 1804–1813 CrossRef CAS PubMed.
- J. Shu, F. Liang, J. Liang, Y. Liang, F. Li, F. Shao, R. Liu and H. Huang, Fitoterapia, 2015, 104, 64–68 CrossRef CAS PubMed.
- W.-H. Cai, K. Matsunami, H. Otsuka and Y. Takeda, Am. J. Plant Sci., 2011, 2, 609–618 CrossRef CAS.
- N. H. Tung, Y. Ding, E. M. Choi, C. V. Minh and Y. H. Kim, Chem. Pharm. Bull., 2009, 57, 65–68 CrossRef CAS PubMed.
- A. Stochmal, I. Kowalska, B. Janda, A. Perrone, S. Piacente and W. Oleszek, Phytochemistry, 2009, 70, 1272–1276 CrossRef CAS PubMed.
- W. Schuehly, W. Voith, H. Teppner and O. Kunert, J. Nat. Prod., 2010, 73, 1381–1384 CrossRef CAS PubMed.
- L. I. Pilkington and D. Barker, Nat. Prod. Rep., 2015, 32, 1369–1388 RSC.
- Y. Matsuo and Y. Mimaki, Chem. Pharm. Bull., 2010, 58, 587–590 CrossRef CAS PubMed.
- J.-S. Bae and T. H. Kim, Bioorg. Med. Chem. Lett., 2012, 22, 793–796 CrossRef CAS PubMed.
- J. Li, Y. Huang, X.-L. Guan, J. Li, S.-P. Deng, Q. Wu, Y.-J. Zhang, X.-J. Su and R.-Y. Yang, Phytochemistry, 2012, 82, 100–109 CrossRef CAS PubMed.
- H. Chen, J. Li, Q. Wu, X.-T. Niu, M.-T. Tang, X.-L. Guan, J. Li, R.-Y. Yang, S.-P. Deng and X.-J. Su, Fitoterapia, 2012, 83, 643–649 CrossRef CAS PubMed.
- M. Hosseinzadeh, J. Mohamad, M. A. Khalilzadeh, M. R. Zardoost, J. Haak and M. Rajabi, J. Photochem. Photobiol., B, 2013, 128, 85–91 CrossRef CAS PubMed.
- J. Li, A.-P. Meng, X.-L. Guan, J. Li, Q. Wu, S.-P. Deng, X.-J. Su and R.-Y. Yang, Bioorg. Med. Chem. Lett., 2013, 23, 2238–2244 CrossRef CAS PubMed.
- S. Chen, H.-Y. Huang, M.-J. Cheng, C.-C. Wu, T. Ishikawa, C.-F. Peng, H.-S. Chang, C.-J. Wang, S.-L. Wong and I.-S. Chen, Phytochemistry, 2013, 93, 203–309 CrossRef CAS PubMed.
- E. D. Coy-Barrera, L. E. Cuca-Suárez and M. Sefkow, Phytochemistry, 2009, 70, 1309–1314 CrossRef CAS PubMed.
- E. D. C. Barrera and L. E. C. Suarez, Chem. Pharm. Bull., 2009, 57, 639–642 CrossRef.
- J.-J. Xu, G.-Z. Zeng, S.-C. Yang, Y. Shen and N.-H. Tan, Fitoterapia, 2013, 91, 82–86 CrossRef CAS PubMed.
- Y.-C. He, Y. Zou, C. Peng, J.-L. Liu, C.-J. He, L. Guo, X.-F. Xie and L. Xiong, Fitoterapia, 2015, 100, 7–10 CrossRef CAS PubMed.
- A. E. Do Vale, J. M. David, E. O. Dos Santos, J. P. David, L. C. R. C. Silva, M. V. Bahia and H. N. Brandão, Phytochemistry, 2012, 76, 158–161 CrossRef CAS PubMed.
- Y.-M. Chung, H.-C. Wang, M. El-Shazly, Y.-L. Leu, M.-C. Cheng, C.-L. Lee, F.-R. Chang and Y.-C. Wu, J. Agric. Food Chem., 2011, 69, 9219–9225 CrossRef PubMed.
- Y. Deng, Y.-W. Chin, H.-B. Chai, E. C. De Blanco, L. B. S. Kardono, S. Riswan, D. D. Soejarto, N. R. Farnsworth and A. D. Kinghorn, Phytochem. Lett., 2011, 4, 213–217 CrossRef CAS PubMed.
- G.-Y. Cao, X.-W. Yang, W. Xu and F. Li, Food Chem. Toxicol., 2013, 62, 167–171 CrossRef CAS PubMed.
- A. R. Carroll and W. C. Taylor, Aust. J. Chem., 1991, 44, 1627–1633 CrossRef CAS.
- S.-J. Xiao, F. Chen, L.-S. Ding and Y. Zhou, Chin. Chem. Lett., 2014, 25, 463–464 CrossRef CAS.
- Q.-Y. Song, C.-J. Zhang, Y. Li, J. Wen, X.-W. Zhao, Z.-L. Liu and K. Gao, Phytochem. Lett., 2013, 6, 174–178 CrossRef CAS.
- N. Li, Z.-D. Tuon, S.-Z. Qi, S.-S. Xing, H.-S. Lee, J.-G. Chen and L. Cui, Fitoterapia, 2015, 101, 46–50 CrossRef CAS PubMed.
- P.-J. Yin, J.-S. Wang, D.-D. Wei, Y. Zhang, P.-R. Wang, X.-B. Wang and L.-Y. Kong, Fitoterapia, 2013, 88, 31–37 CrossRef CAS PubMed.
- G. Ni, Z.-F. Shen, Y. Lu, Y.-H. Wang, Y.-B. Tang, R.-Y. Chen, Z.-Y. Hao and D.-Q. Yu, J. Org. Chem., 2011, 76, 2056–2061 CrossRef CAS PubMed.
- Z.-B. Cheng, X. Lu, J.-M. Bao, Q.-H. Han, Z. Dong, G.-H. Tang, L.-S. Gan, H.-B. Luo and S. Yin, J. Nat. Prod., 2014, 77, 2651–2657 CrossRef CAS PubMed.
- C. L. Cardoso, I. Castro-Gamboa, G. M. Bergamini, A. J. Cavalheiro, D. H. S. Silva, M. N. Lopes, A. R. Araujo, M. Furlan, H. Verli and V. S. Bolzani, J. Nat. Prod., 2011, 74, 487–491 CrossRef CAS PubMed.
- X.-Q. Zhang, F.-F. Xu, L. Wang, M.-Y. Huang, Z. Liu, D.-M. Zhang, G.-C. Wang, Y.-L. Li and W.-C. Ye, Phytochem. Lett., 2012, 5, 292–293 CrossRef CAS.
- J.-F. Wang, G.-F. Yin, X.-J. Zhou, J. Su, Y. Li, H.-M. Zhong, G. Duan and Y.-X. Cheng, J. Asian Nat. Prod. Res., 2011, 13, 80–83 CrossRef CAS PubMed.
- S. Begum, B. Saxena, M. Goyal, R. Ranjan, V. B. Joshi, C. V. Rao, S. Krishnamurthy and M. Sahai, Fitoterapia, 2010, 81, 178–184 CrossRef CAS PubMed.
- M. J. Nunez, M. L. Kennedy, I. A. Jimenez and I. L. Bazzocchi, Tetrahedron, 2011, 67, 3030–3033 CrossRef CAS.
- Z. Liang, J. Zhang, X. Zhang, J. Li, X. Zhang and C. Zhao, J. Chromatogr. Sci., 2015, 54, 175–178 Search PubMed.
- J. X. Huang, J. Zhang, X. R. Zhang, K. Zhang, X. Zhang and X. R. He, Pharm. Biol., 2014, 52, 1237–1243 CrossRef CAS PubMed.
- S. Kusari, M. Lamshöft and M. Spiteller, J. Appl. Microbiol., 2009, 107, 1019–1030 CrossRef CAS PubMed.
- S. Kusari, C. Hertweck and M. Spiteller, Chem. Biol., 2012, 19, 792–798 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |