A chemodosimetric approach for the selective detection of Pb2+ ions using a cesium based perovskite†
Abstract
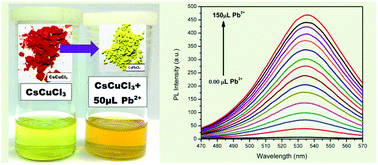
We report the preparation of a lead free inorganic perovskite by a facile wet-chemical method using HCl as a solvent at room temperature. The cesium perovskite CsCuCl3 material was characterized by powdered X-ray diffraction (p-XRD), field emission scanning electron microscopy (FESEM), and energy dispersive X-ray (EDX) spectroscopy. Ultraviolet/Visible (UV-Vis) and steady state photoluminescence (PL) spectroscopy were also carried out on the material. CsCuCl3 showed promising optical properties with a band gap of 2.6 eV. The material was tested as a fluorescent sensor for the detection of metal ions and as a selective fluorescent chemodosimeter for Pb2+ ions. The substitution of Cu2+ ions by Pb2+ in CsCuCl3 proves the success of the chemodosimetry approach for the detection of Pb2+ ions.

 Please wait while we load your content...
Please wait while we load your content...