Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A BODIPY sensor for water based on a photo-induced electron transfer method with fluorescence enhancement and attenuation systems†
Yousuke
Ooyama
*,
Marin
Hato
,
Toshiaki
Enoki
,
Satoshi
Aoyama
,
Kensuke
Furue
,
Nao
Tsunoji
and
Joji
Ohshita
*
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: yooyama@hiroshima-u.ac.jp; Fax: +81 82-424-5494
First published on 12th July 2016
Abstract
It was found that BODIPY MH-1 can act as a dual function-based fluorescent sensor for trace amounts of water possessing fluorescence intensity and fluorescence quantum yield as a function of water detection based on the PET (photo-induced electron transfer) method with fluorescence enhancement and attenuation systems.
The development of fluorescence sensing methods for the detection and quantification of a trace amount of water in solutions, solids, the atmosphere and products has received considerable attention in recent years from the viewpoint of fundamental study in analytical chemistry and photochemistry, and is eagerly anticipated in industry because of their potential applications in environmental and quality control monitoring systems, and sanitary and medical materials.1–4 Most of fluorescent sensors for a trace amount of water developed so far, including conjugated polymer and organic fluorescent dyes, are based on fluorescence quenching systems, that is, the fluorescence intensity of the sensor decreases as a function of water content in organic solvents.5–8 This feature can be attributed to the solvatochromic properties of sensors,5 the aggregation of sensors6 or the formation of hydrogen bonding7 between the sensor and water molecules with the increase in the water content. However, this fluorescence quenching system makes it difficult to detect trace amounts of water. Therefore, in our previous work, as a new approach for improving the detection limit of water, we have devised the fluorescence enhancement system based on PET (photo-induced electron transfer),9 that is, anthracene-aminomethyl-phenylboronic acid pinacol ester (PhenylBPin) OF-2 was designed and synthesized as a fluorescent PET sensor for a trace amount of water (Scheme 1a).10a In the low water content region below 1.0 wt%, the addition of water to organic solvents containing OF-2 causes an efficient formation of fluorescent ionic structure OF-2a with a protonated tertiary amino group, resulting in the occurrence of fluorescence by the suppression of the PET process from the electron-donating tertiary amino group to the photoexcited anthracene fluorophore due to the lowering of the HOMO level of the tertiary amino group. The detection limits (DLs) were as low as 0.009 wt% for water in acetonitrile. Thus, we demonstrated that the fluorescence enhancement system based on the PET method is useful for the detection and quantification of a trace amount of water in organic solvents.10 On the other hand, as another fluorescence enhancement system for the detection of water, an AIE (aggregation-induced emission)-based fluorescent sensor is attributed to the emission enhancement induced by aggregate formation upon addition of large amounts of water (over 40 wt% in almost every case) into the solution, leading to restricted intramolecular rotation (RIR) in the molecular structure, that is, the aggregation can restrict the intramolecular free rotation, resulting in the elimination of radiationless (non-emissive) relaxation of the photoexcited fluorophore.11–14 Meanwhile, in some cases, the fluorescence attenuation with the increase in the water content was observed in the low water content region below 1.0–2.0 wt% due to the increase in polarity of the solution.13,14 However, the fluorescence enhancement system based on the AIE sensors developed so far is not sufficient for the detection of a trace amount of water because the addition of large amounts of water into the solution is necessary for being AIE active.
 | ||
| Scheme 1 Proposed mechanisms of fluorescent sensors: (a) OF-2 and (b) MH-1 with a PET (photo-induced electron transfer) structure for the detection of water in non-polar solvent and polar solvent. | ||
Through our continuing efforts to gain insight into the molecular design toward developing a high-sensitivity optical sensor for water based on the PET method, we found that boron-dipyrromethene (BODIPY) MH-1 with a PhenylBPin unit can act as a fluorescent sensor for trace amounts of water based on the PET method with both fluorescence enhancement and attenuation systems (Scheme 1b). Herein, we report that MH-1 is a dual function-based fluorescent sensor possessing fluorescence intensity and fluorescence quantum yield (ΦF) as a function of detection of water.
BODIPY MH-1 studied in this work was synthesized according to a stepwise synthetic protocol (Scheme S1, ESI†). We first prepared 2,6-diethyl-8-(bromomethyl)-1,3,5,7-tetramethyl BODIPY 1via the reaction of 3-ethyl-2,4-dimethylpyrrole with bromoacetyl chloride followed by treatment with BF3·OEt2. Subsequently, BODIPY 1 was reacted with methyl amine to give 2,6-diethyl-8-[(N-methylamino)methyl]-1,3,5,7-tetramethyl BODIPY 2. We obtained MH-1 with PhenylBPin via the reaction of 2 with 2-bromomethyl-4-cyanophenylboronic acid.
The absorption and fluorescence spectra of MH-1 in THF and various solvents are shown in Fig. 1a and Fig. S1 (ESI†), respectively, and their optical data in various solvents are summarized in Table 1. In all the solvents, the absorption maximum wavelength (λabs) of MH-1 was observed at around 535 nm, which is assigned to the S0 → S1 transition of the BODIPY core. The molar extinction coefficient (ε) at λabs is 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–78
000–78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. The corresponding fluorescence maximum wavelength (λfl) appeared at around 550 nm, which arises from the BODIPY locally excited (LE) state. It is worth mentioning here that a significant dependence of ΦF value on the solvent polarity was observed (Fig. 1b), that is, the ΦF value significantly decreases with increasing the empirical solvent polarity parameter (ENT) from toluene (ENT = 0.099, ΦF = 62%) to acetonitrile (ENT = 0.460, ΦF = 2%). Thus, on the basis of the fact that there is no change in λfl by changing the solvent polarity, which reveals the decrease in ΦF value with increasing the solvent polarity is not attributed to the intramolecular charge transfer (ICT) properties and the change of photoexcited state,15 this result indicates that in non-polar solvents such as toluene and 1,4-dioxane the formation of strong boron–nitrogen (B–N) interaction16 between BPin and a tertiary amino group in MH-1(BN) can lead to the suppression of the PET process from the electron-donating tertiary amino group to the photoexcited BODIPY core due to the lowering of the HOMO levels of the tertiary amino group, resulting in a high ΦF value (Scheme 1b). Moreover, it is revealed by the fact that 2,6-diethyl-1,3,5,7-tetramethyl-8-[(N,N-dimethylamino)methyl] BODIPY (ΦF = 5% in THF)17 suffers from a drastic fluorescence attenuation due to the PET process from the electron-donating tertiary amino group to the photoexcited BODIPY core when compared to the corresponding 1,3,5,7,8-pentamethyl-2,6-diethyl BODIPY (PM567) without the amino group (ΦF = 75% in THF).18 On the other hand, in polar solvents such as acetone and acetonitrile the weakened B–N interaction accompanied with the promotion of intramolecular rotation of PhenylBPin in MH-1 due to the polar environment can lead to the activation of the PET process, resulting in a low ΦF value.
000 M−1 cm−1. The corresponding fluorescence maximum wavelength (λfl) appeared at around 550 nm, which arises from the BODIPY locally excited (LE) state. It is worth mentioning here that a significant dependence of ΦF value on the solvent polarity was observed (Fig. 1b), that is, the ΦF value significantly decreases with increasing the empirical solvent polarity parameter (ENT) from toluene (ENT = 0.099, ΦF = 62%) to acetonitrile (ENT = 0.460, ΦF = 2%). Thus, on the basis of the fact that there is no change in λfl by changing the solvent polarity, which reveals the decrease in ΦF value with increasing the solvent polarity is not attributed to the intramolecular charge transfer (ICT) properties and the change of photoexcited state,15 this result indicates that in non-polar solvents such as toluene and 1,4-dioxane the formation of strong boron–nitrogen (B–N) interaction16 between BPin and a tertiary amino group in MH-1(BN) can lead to the suppression of the PET process from the electron-donating tertiary amino group to the photoexcited BODIPY core due to the lowering of the HOMO levels of the tertiary amino group, resulting in a high ΦF value (Scheme 1b). Moreover, it is revealed by the fact that 2,6-diethyl-1,3,5,7-tetramethyl-8-[(N,N-dimethylamino)methyl] BODIPY (ΦF = 5% in THF)17 suffers from a drastic fluorescence attenuation due to the PET process from the electron-donating tertiary amino group to the photoexcited BODIPY core when compared to the corresponding 1,3,5,7,8-pentamethyl-2,6-diethyl BODIPY (PM567) without the amino group (ΦF = 75% in THF).18 On the other hand, in polar solvents such as acetone and acetonitrile the weakened B–N interaction accompanied with the promotion of intramolecular rotation of PhenylBPin in MH-1 due to the polar environment can lead to the activation of the PET process, resulting in a low ΦF value.
| Solvent |
E
NT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
λ abs/nm | ε/M−1 cm−1 | λ fl/nm | Φ F |
|---|---|---|---|---|---|
| a Empirical solvent polarity parameter (ref. 19). b Fluorescence quantum yields (ΦF) were determined by using a calibrated integrating sphere system (λex = 490 nm). | |||||
| Toluene | 0.099 | 540 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
556 | 62 |
| 1,4-Dioxane | 0.164 | 537 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
552 | 51 |
| THF | 0.207 | 537 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
552 | 27 |
| Acetone | 0.355 | 534 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
549 | 4 |
| Acetonitrile | 0.460 | 534 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
548 | 2 |
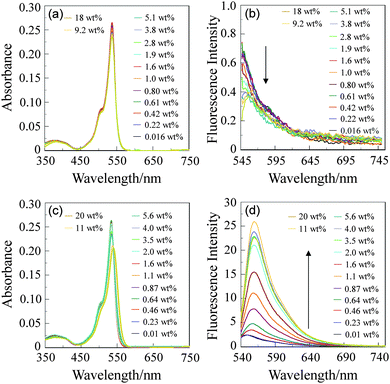
In order to investigate the sensing ability of MH-1 for water in solvents, the absorption and fluorescence spectra of MH-1 were measured in 1,4-dioxane, THF, acetone and acetonitrile that contained various concentrations of water (Fig. 2 for 1,4-dioxane and acetonitrile, Fig. S2, ESI†). In the water content region below 20 wt% for all the four solvents, the absorption spectra of MH-1 did not undergo appreciable changes upon addition of water to the solution. On the other hand, for 1,4-dioxane and THF the florescence spectra of MH-1 underwent a decrease in fluorescence intensity with a slight red-shift (ca. 5 nm) of the fluorescence peak wavelength at 552 nm upon addition of water to the solution. The decrease in fluorescence peak intensity levels off in the water content region greater than 4.0–5.0 wt%. In contrast, for acetone and acetonitrile the fluorescence spectra exhibited an enhancement of fluorescence intensity with a slight red-shift (ca. 8 nm) of the fluorescence peak wavelength at around 548 nm upon addition of water to acetone or acetonitrile solution (Fig. 2d). The enhancement of the fluorescence peak intensity levels off in the water content region greater than 2.0–5.0 wt%. These results indicate that the addition of water to a polar solvent (acetone or acetonitrile) containing MH-1 causes the efficient formation of the fluorescent ionic structure. Thus, as shown in Scheme 1b, MH-1(H2O) with a stable fluorescent ionic structure between the protonated tertiary amino group and the hydroxylated PhenylBPin by the addition of water molecules is stably formed in a polar solvent, because, as described in an earlier MH-1, the weakened B–N interaction can facilitate the formation of MH-1(H2O) in the polar envelopment. Consequently, for polar solvents such as acetone and acetonitrile the fluorescence enhancement of MH-1 with increasing water content in the solution can be attributed to the suppression of the PET process by the efficient formation of MH-1(H2O), which is due to the lowering of the HOMO level of tertiary amino group by the addition of water molecules, as with the case of anthracene-based sensor OF-2. On the other hand, for non-polar solvents such as 1,4-dioxane or THF the decrease in fluorescence intensity upon addition of water to the solution is attributed to the disruption of B–N interaction in MH-1(BN) associated with the formation of ionic species MH-1(H2O) in the solvent/water mixture.20 Therefore, this work reveals that for MH-1 the formation of B–N interaction can effectively inactivate the PET process, rather than the formation of an ionic structure between the protonated tertiary amino group and the hydroxylated PhenylBPin, that is, the HOMO level of the tertiary amino group in MH-1(BN) due to the B–N interaction is lower than that in MH-1(H2O). Consequently, this work demonstrates that MH-1 can act as a fluorescent sensor for trace amounts of water based on the PET method with both fluorescence enhancement and attenuation systems.
To estimate the sensitivity and accuracy characteristics of MH-1 for the detection of water in the low water content region, the changes in fluorescence intensity (at ca. 550 nm) and ΦF value are plotted against the water fraction in these four organic solvents (Fig. 3 for 1,4-dioxane and acetonitrile, and Fig. S3 (ESI†) for THF and acetone). These plots demonstrated that for 1,4-dioxane and THF the fluorescence intensity and ΦF value decreased almost linearly as a function of the water content, but for acetone and acetonitrile the fluorescence intensity and ΦF value increased linearly as a function of the water content. Moreover, it is worth mentioning here that the ΦF value levels off at 20–30% in the water content region greater than 2.0–5.0 wt% for all the four solvents, that is, this result strongly indicates the formation of MH-1(H2O) upon addition of water to all the four solvents. The DL determined from the plot of the fluorescence intensity as a function of the water content is 0.3 wt% for acetonitrile and over 1.0 wt% for 1,4-dioxane, respectively (DL = 3.3σ/ms, where σ is the standard deviation of the blank sample and ms is the slope of the calibration curve in the low water content region below 5.0 wt% for 1,4-dioxane and 2.0 wt% for acetonitrile, respectively). Therefore, this work indicates that the fluorescence behavior of MH-1 for the detection of water based on the PET method with both fluorescence enhancement and attenuation systems exhibited a good linear response in the low water content region, indicating that BODIPY MH-1 with a PET structure can act as a dual function-based fluorescent sensor for trace amounts of water possessing fluorescence intensity and ΦF value as a function of detection of water, although the DL for water based on the PET characteristics of MH-1 is not superior to that based on the PET method in our previous work.9
In conclusion, we revealed that BODIPY MH-1 with a PET structure can act as a dual function-based fluorescent sensor possessing fluorescence intensity and ΦF value as a function of detection of trace amounts of water. It was found that the fluorescence enhancement of MH-1 with increasing water content in a polar solvent can be attributed to the suppression of the PET process associated with the formation of fluorescent ionic species MH-1(H2O) by the addition of water molecules. In contrast, the fluorescence attenuation of MH-1 with increasing water content in a non-polar solvent can be attributed to the disruption of boron–nitrogen (B–N) interaction between BPin and a tertiary amino group in MH-1(BN) associated with the formation of MH-1(H2O) by the addition of water molecules. We propose that this organic fluorescent dye with a PET structure which is capable of efficiently forming the fluorescence ionic structure by the addition of water molecules could be a promising candidate as a fluorescent sensor for a trace amount of water based on the PET method. Consequently, this work provides new insight into the molecular design for the development of high-sensitivity and -accuracy optical sensors for water based on the PET method with both fluorescence enhancement and attenuation systems.
The absorption spectra were recorded using a Shimadzu UV-2910 spectrophotometer. The fluorescence spectra were recorded using a Hitachi F-4500 spectrophotometer. The addition of water to organic solvents containing MH-1 was made in terms of weight percent (wt%). The determination of water in 1,4-dioxane, THF, acetone and acetonitrile was done using a MKC-610 and MKA-610 Karl Fischer moisture titrator (Kyoto Electronics manufacturing Co., Ltd) based on Karl Fischer coulometric titration for below 1.0 wt% and volumetric titration for above 1.0 wt%, respectively.
This work was supported by an Iketani Science and Technology Foundation's research grant.
References
- H. S. Jung, P. Verwilst, W. Y. Kim and J. S. Kim, Chem. Soc. Rev., 2016, 45, 1242 RSC.
- J. Lee, M. Pyo, S. Lee, J. Kim, M. Ra, W.-Y. Kim, B. J. Park, C. W. Lee and J.-M. Kim, Nat. Commun., 2014, 5, 3736 CAS.
- A. Douvali, A. C. Tsipis, S. V. Eliseeva, S. Petoud, G. S. Papaefstathiou, C. D. Malliakas, I. Papadas, G. S. Armatas, I. Margiolaki, M. G. Kanatzidis, T. lazarides and M. J. Manos, Angew. Chem., Int. Ed., 2015, 54, 1651 CrossRef CAS PubMed.
- W.-E. Lee, Y.-J. Jin, L.-S. Park and G. Kwak, Adv. Mater., 2012, 24, 5604 CrossRef CAS PubMed.
- L. Ding, Z. Zhang, X. Li and J. Su, Chem. Commun., 2013, 49, 7319 RSC.
- Q. Deng, Y. Li, J. Wu, Y. Liu, G. Fang, S. Wang and Y. Zhang, Chem. Commun., 2012, 48, 3009 RSC.
- D. Citterio, K. Minamihashi, Y. Kuniyoshi, H. Hisamoto, S. Sasaki and K. Suzuki, Anal. Chem., 2001, 73, 5339 CrossRef CAS PubMed.
- H. Mishra, V. Misra, M. S. Mehata, T. C. Pant and H. B. Tripathi, J. Phys. Chem. A, 2004, 108, 2346 CrossRef CAS.
- (a) A. P. de Silva and S. A. de Silva, J. Chem. Soc., Chem. Commun., 1986, 1709 RSC; (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; (c) A. P. de Silva, T. P. Vance, M. E. S. West and G. D. Wright, Org. Biomol. Chem., 2008, 6, 2468 RSC; (d) A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385 RSC; (e) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Nature, 1995, 374, 345 CrossRef CAS; (f) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 477 RSC; (g) T. D. James, K. R. A. S. Sandanayake, R. Iguchi and S. Shinkai, J. Am. Chem. Soc., 1995, 117, 8982 CrossRef CAS; (h) S. Franzen, W. Ni and B. Wang, J. Phys. Chem. B, 2003, 107, 12942 CrossRef CAS; (i) W. Ni, G. Kaur. G. Springsteen, B. Wang and S. Franzen, Bioorg. Chem., 2004, 32, 571 CrossRef CAS PubMed; (j) G. Kaur, H. Fang, X. Gao, H. Li and B. Wang, Tetrahedron, 2006, 62, 2583 CrossRef CAS.
- (a) Y. Ooyama, K. Furue, K. Uenaka and J. Ohshita, RSC Adv., 2014, 4, 25330 RSC; (b) Y. Ooyama, A. Matsugasako, K. Oka, T. Nagano, M. Sumomogi, K. Komaguchi, I. Imae and Y. Harima, Chem. Commun., 2011, 47, 4448 RSC; (c) Y. Ooyama, A. Matsugasako, Y. Hagiwara, J. Ohshita and Y. Harima, RSC Adv., 2012, 2, 7666 RSC; (d) Y. Ooyama, K. Uenaka, A. Matsugasako, Y. Harima and J. Ohshita, RSC Adv., 2013, 3, 23255 RSC.
- Y. Hong, J. W. Y. Lama and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- Y. Zhang, D. Li, Y. Li and J. Yu, Chem. Sci., 2014, 5, 2710 RSC.
- W. Chen, Z. Zhang, X. Li, H. Agren and J. Su, RSC Adv., 2015, 5, 12191 RSC.
- B. Valeur, Molecular Fluorescence, Wiley-VCH, Weinheim, 2002 Search PubMed.
- (a) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Nature, 1995, 374, 345 CrossRef CAS; (b) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 477 RSC; (c) T. D. James, K. R. A. S. Sandanayake, R. Iguchi and S. Shinkai, J. Am. Chem. Soc., 1995, 117, 8982 CrossRef CAS.
- E. Palao, S. de la Moya, A. R. Agarrabeitia, I. Esnal, J. Bañuelos, I. L. Arbelo and M. J. Ortiz, Org. Lett., 2014, 16, 4364 CrossRef CAS PubMed.
- F. L. Arbeloa, T. L. Arbeloa, I. L. Arbeloa, I. G. Moreno, A. Costela, R. Sastre and F. A. Guerri, Chem. Phys., 1998, 236, 331 CrossRef.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH, Weinheim, 2002 Search PubMed.
- L. Zhu, S. H. Shabbir, M. Gray, V. M. Lynch, S. Sorey and E. V. Anslyn, J. Am. Chem. Soc., 2006, 128, 1222 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj01467d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |