Open Access Article
Open Access ArticleSelective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives†
Annika
Herbst
and
Christoph
Janiak
 *
*
Institut für Anorganische Chemie und Strukturchemie, Heinrich-Heine Universität Düsseldorf, Universitätsstraße 1, D-40225 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de
First published on 20th July 2016
Abstract
The catalytic conversion of glucose to 5-hydroxymethylfurfural (5-HMF) is highly desirable, but the 5-HMF yield obtained using heterogeneous catalysts is still low compared to homogeneous catalysts, and the mechanism is not elucidated completely. In addition, the isolation and purification of 5-HMF still present a challenge as degradation reactions take place and side products form. The formation of 5-HMF from glucose has been reported using several solid acid catalysts; still metal–organic framework (MOF) catalysts could, so far, catalyze the cascade reaction of glucose to 5-HMF only in low yields of less than 16%. Glucose conversion using MOFs is little investigated and here sulfonated MIL-101Cr (MIL-SO3H) was found to achieve 29% conversion of glucose to 5-HMF after 24 h in a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 39
v 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. The conversion of maltose resulted in 50% 5-HMF yield (saccharide solutions were 5 wt%). When the reaction was carried out in pure THF using MIL catalysts no product was formed, revealing the indispensability of water for the glucose-to-5-HMF conversion. Importantly, MIL-SO3H preferentially leads to 5-HMF over levulinic acid (molar ratio 1
1) mixture. The conversion of maltose resulted in 50% 5-HMF yield (saccharide solutions were 5 wt%). When the reaction was carried out in pure THF using MIL catalysts no product was formed, revealing the indispensability of water for the glucose-to-5-HMF conversion. Importantly, MIL-SO3H preferentially leads to 5-HMF over levulinic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3), while the catalysts Amberlyst-15 and sulfuric acid form mostly levulinic acid in 5-HMF to levulinic acids ratios of 1
0.3), while the catalysts Amberlyst-15 and sulfuric acid form mostly levulinic acid in 5-HMF to levulinic acids ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. At the same time, MIL-NO2 is the most selective, yielding only 5-HMF and showing no formation of levulinic acid. Using 5-HMF as a substrate did not result in any conversion to levulinic acid in the presence of MIL-SO3H, thereby ruling out the catalytic formation of levulinic acid from 5-HMF. Catalyst recycle experiments showed that MIL-SO3H stays porous and crystalline, but becomes deactivated through fouling by humin formation. With the use of ethanol as an alternative reaction medium, the formation of insoluble humins could be prevented, but the yield of 5-HMF and 5-ethyl-HMF decreased.
10, respectively. At the same time, MIL-NO2 is the most selective, yielding only 5-HMF and showing no formation of levulinic acid. Using 5-HMF as a substrate did not result in any conversion to levulinic acid in the presence of MIL-SO3H, thereby ruling out the catalytic formation of levulinic acid from 5-HMF. Catalyst recycle experiments showed that MIL-SO3H stays porous and crystalline, but becomes deactivated through fouling by humin formation. With the use of ethanol as an alternative reaction medium, the formation of insoluble humins could be prevented, but the yield of 5-HMF and 5-ethyl-HMF decreased.
Introduction
Biomass is highly attractive as a source for valuable chemicals and fuels, to save fossil fuels and reduce CO2 emissions therefrom.1,2 In particular, “waste” biomass from by-product streams (e.g. in agro industry, food sector, paper manufacturing and recycling) is investigated as feedstock for platform chemicals, e.g., 5-hydroxymethylfurfural (5-HMF) (Fig. 1), which can replace the petro-based chemicals and create new valuable products.3 Biomass of plants or algae consists mainly of lignocelluloses, which in turn contain cellulose, lignin and hemicelluloses. Cellulose represents the largest part with up to 50 weight%.4 Therefore the conversion of cellulose, respectively its glucose subunits, is of broad interest. The chemical conversion of glucose can proceed via acid-catalyzed dehydration, which presents challenging requirements to the catalyst, since a variety of products can be obtained (e.g. fructose, levoglucosan, other C5 or C6 monosaccharides, 5-HMF, furfural, lactic acid, levulinic acid and formic acid) (cf.Fig. 2).2 | ||
| Fig. 2 Reaction scheme for 5-HMF production from glucose.10 | ||
Among these glucose-dehydration products, 5-hydroxymethylfurfural (5-HMF) is of special interest because it can be converted to important polymer precursors such as 2,5-furan dicarboxylic acid (replacement of terephthalic acid), 1,6-hexanediol, caprolactam and 2,5-dimethylfuran, which is interesting as a fossil fuel alternative (Fig. 1).5 Consequently, enormous research interest arose with the aim of 5-HMF synthesis from glucose. During the last two years and also very recently, a number of excellent reviews have been published on this topic and provide a thorough overview.4,6
5-HMF can be synthesized from glucose in aqueous media, DMSO, ionic liquids and in biphasic systems. Numerous catalysts have been tested which can be roughly classified into homogeneous Brønsted and Lewis acids (H2SO4, CrCl3 or AlCl3) and solid acids (zeolites, resins and metal organic frameworks). A brief reaction sequence of the conversion of glucose to HMF is depicted in Fig. 2. Generally, 5-HMF is accessible via Lewis-acidic isomerization of glucose to fructose and Brønsted acidic conversion to 5-HMF.7 Tessonnier et al. also investigated the selective isomerization to fructose via base catalysis, using amines, and found the same performance as the state-of-the-art Lewis acid catalysts with a yield of 32% and a selectivity of 63% for fructose after 20 min at 100 °C.8,9 Huber et al. recently proposed a new mechanistic pathway for the formation of 5-HMF from cellulose and glucose which is based on acid catalysis through the intermediate levoglucosan instead of fructose (Fig. 2).10
Interestingly, the best 5-HMF yield of 44% from cellulose was reported for a mixture of THF/water 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, using H2SO4 as the catalyst at 190 °C. As postulated by Vlachos et al., the first step of glucose dehydration to levoglucosan in the Brønsted acidic mechanism is a protonation step (see the Mechanistic aspects section in the ESI†).11
1, using H2SO4 as the catalyst at 190 °C. As postulated by Vlachos et al., the first step of glucose dehydration to levoglucosan in the Brønsted acidic mechanism is a protonation step (see the Mechanistic aspects section in the ESI†).11
Using separable and recyclable solid Brønsted acid instead of homogenous acid catalysts would lead to a further improvement in terms of green chemistry or green catalysis12 which requires the catalysts to be designed for easy separation from the reaction products and multi-time efficient reuse/recycling.13–15
Metal–organic frameworks (MOFs), built from organic ligands (linkers) and metal nodes, are intensively investigated as heterogeneous catalysts.16,17 Usually, metal–organic frameworks are known for their Lewis acidity, which originates from open metal sites at the secondary building units (SBUs).18 Due to their porous structure, there are different possibilities to introduce Brønsted acid functionalities, discussed in a detailed review of Jiang and Yaghi.19 These include encapsulation of Brønsted acidic molecules in the cavity of MOFs, ligation of Brønsted acid groups to metal open sites or (post)synthetic functionalization of linkers with acidic moieties. There are examples for all three types of Brønsted acid functionalities in MOFs with subsequent catalysis.19 At present, there are only a handful of studies dealing with the conversion of biomass using MOFs. Li and Hensen et al. demonstrated the Brønsted acidic conversion of fructose and glucose to 5-HMF in DMSO by encapsulating phosphotungstic acid (PTA) in MIL-101Cr, albeit with a very low yield of 2% from glucose.20 Hatton reported a MOF/polymer composite material from MIL-101Cr and poly(N-bromomaleimide) which was able to convert fructose to 5-HMF in 87% yield.21 A hybrid material containing PTA and ruthenium immobilized on MIL-100Cr was used for the conversion of cellulose and cellobiose into sorbitol.22 Matsuda and Kitagawa showed that linker functionalization in MIL-101Cr with SO3H enabled the hydrolysis of cellulose into monosaccharides in water.23 The same group studied the glucose to fructose isomerization ability of MIL-101Cr derivatives (with bdc–NO2, –NH2, –SO3H; bdc = benzene-1,4-dicarboxylate) in water. MIL-101Cr can have open metal sites that could act as catalysts for glucose to fructose isomerization. The highest activity was found for the NO2- and the SO3H-functionalized MIL-101Cr (18.4 and 21.6% conversion to fructose, respectively, after 24 h, 100 °C, 0.2 g catalyst and 25 mg glucose in 2.0 g H2O).24 Chen et al. investigated the activity of different SO3H-functionalized MOFs (MIL-101Cr-SO3H, UiO-66Zr-SO3H and MIL-53Al-SO3H) for the conversion of fructose into 5-HMF in DMSO with a maximum conversion of 90%.25 To the best of our knowledge, until now no conversion of glucose to 5-HMF using MOF catalysts with yields higher than 16% has been reported. The glucose conversion of 16% was achieved using MIL-101Cr-PMAi-Br with a MIL-101Cr catalyst loading of 6 mol% (or 21 wt%) after 6 h in DMSO solvent, which made the product separation difficult (PMAi-Br = poly(N-bromomaleimide-co-divinylbenzene)).21
The isomerization of glucose to fructose can be carried out enzymatically but consumes over 10 million tons of glucose isomerase annually, representing the largest industrial use of immobilized enzymes.26 Glucose isomerase has a narrow operating window concerning pH and temperature.26 Therefore, it would be highly desirable to carry out a one-pot glucose-to-5-HMF transformation without the need of a separate glucose-to-fructose isomerization.
Recently, we showed that the catalyst activity of nitro-modified MIL-101Cr in benzaldehyde acetalization reaction originates from Brønsted acidity.27 It has been concluded that inside the pores of MIL-101Cr there exists high proton acidity through polarized chromium-coordinated water molecules (aqua ligands). It is a logical extension to investigate the activity of functionalized MIL-101Cr in the acid catalyzed glucose-to-5-HMF dehydration. We are aware of the better catalytic performance of other heterogeneous catalysts like sulfated ZrO2 or zeolite-beta,28 but our aim here is to investigate the potential of MOFs for this cascade reaction. Further, the chosen reaction media is THF/water in order to avoid high boiling solvents (DMSO) and to facilitate the separation of 5-HMF. MIL-101Cr and its derivatives are known for their high water stability and uptake.29,30 MIL-101 is also often used as a catalyst support, e.g., for metal31 or metal oxide nanoparticles.32
In this work, we have examined four functionalized MIL-101Cr derivatives for the acid catalyzed conversion of glucose to 5-HMF using a THF/water system. For the first time, using a metal–organic framework catalyst a significant conversion of glucose to 5-HMF of 29% has been achieved. In addition, the selectivity between the formation of 5-HMF and levulinic acid has been investigated and compared with sulfuric acid and Amberlyst-15 as catalysts.
Experimental
Characterization of catalysts
Powder X-ray diffraction (PXRD) patterns were recorded on a Bruker D2 phaser diffractometer in the Bragg–Brentano configuration with Cu-Kα1/α2 radiation (λ = 1.54184 Å), a nickel filter and stationary flat-panel low background sample holder in the range 2θ = 5–50° (step width 0.02° in 2θ). Simulated powder patterns were based on single-crystal data and calculated using the STOE WinXPOW software package33 or Mercury v. 3.3 software.34 Scanning electron microscopy (SEM) images were measured on a JSM6510 with an LaB6 cathode. The N2 sorption measurements were performed on a Quantachrome NOVA 4200e instrument at 77 K. The samples were degassed under high vacuum (10−5 Torr) at 150 °C for 3 h, prior to each measurement. The BET surface area was calculated from adsorption isotherm data points in the pressure range p/p0 = 0.05–0.2.Preparation of catalysts
All MIL-101Cr materials were synthesized hydrothermally, utilizing an autoclave and programmable oven according to literature procedures.30,35 MIL-SO3H is described here as an example: CrO3 (3.0 g, 30 mmol), H2bdc–SO3Na (6.0 g, 30 mmol) and 12 mol L−1 HCl (2.2 g, 60 mmol) were dissolved in H2O and split into four batches of 30 mL, each of which was transferred into four Teflon-lined stainless steel autoclaves. The solutions were heated at 180 °C for 6 days (heating ramp 5 h; cooling ramp 15 h). For more details, see the ESI.†Standard procedure for catalytic reactions
In a typical experiment, the MIL-101 catalyst (50 mg) was suspended in a solvent mixture (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of tetrahydrofuran (without stabilizer) (4.875 mL) and water (125 μL) with a total volume of 5 mL. The standard glucose loading was 5.0 wt% (223 mg, 1.24 mmol). The temperature was kept constant at 130 °C through a stirred oil bath around the reaction vial. The reaction was stopped by cooling the reaction mixture to 0 °C. The MIL catalysts were separated by centrifugation at 4800 rpm for 10 min and the solution was passed through a syringe filter (0.2 μm diameter).
1) of tetrahydrofuran (without stabilizer) (4.875 mL) and water (125 μL) with a total volume of 5 mL. The standard glucose loading was 5.0 wt% (223 mg, 1.24 mmol). The temperature was kept constant at 130 °C through a stirred oil bath around the reaction vial. The reaction was stopped by cooling the reaction mixture to 0 °C. The MIL catalysts were separated by centrifugation at 4800 rpm for 10 min and the solution was passed through a syringe filter (0.2 μm diameter).
Standard conditions: catalyst 5.22 × 10−5 mol, glucose 223 mg (1.24 mmol), solvent 5 mL (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39
v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 130 °C, 24 h.
1), 130 °C, 24 h.
The concentrations of 5-HMF in the organic THF phase were quantified by GC using external standard calibration (R2 = 0.9991, R = 0.9995). For more details, see the ESI.†
Results and discussion
Synthesis and porosity of MOFs
MIL-101Cr36 is a three-dimensional micro- to mesoporous material based on chromium-terephthalate linkages with the empirical formula [Cr3(O)X(bdc)3(H2O)2] (bdc = benzene-1,4-dicarboxylate, X = OH or F depending on synthesis; here X = OH).37 MIL-101Cr has inner cages of 29 Å and 34 Å diameters with pore aperture windows of diameters up to 16 Å (Fig. 3 and Fig. S1 in ESI†).36 Two of the three Cr(III) octahedra in the trinuclear building unit have terminal aqua ligands, which can potentially be Lewis acid sites.38,39 | ||
| Fig. 3 (a) Trinuclear {Cr3(μ3-O)(OH/F)(H2O)2(O2C–)6} building unit which is assembled by bridging benzene-1,4-dicarboxylate ligands into (b) vertex-sharing supertetrahedra whose centers form the corners of (c) mesoporous cages with diameters of 29 or 34 Å shown here in a zeolite-type framework presentation with the topological connectivity (in green) of the centers of the vertex-sharing supertetrahedra. Not-to-scale drawings (additional ones in Fig. S1, ESI†) were generated from the deposited X-ray data file at the Cambridge Structure Database (CSD-Refcode OCUNAK)36 using the program DIAMOND.40 | ||
MIL-101Cr and its linker-modified derivatives30,41 show remarkable stability towards water which make them most suitable for applications in the presence of moisture/water.29,30 In addition, 2-nitro and 2-sulfo-terephthalic acids were selected to compare the influence of different functional groups. The strong electron-withdrawing effect of 2-nitro-modified MIL-101Cr on the catalyst activity was already shown in the diacetalization of benzaldehyde.27 All modified MIL-101Cr materials were synthesized directly from the linker (2-nitro- or 2-sulfo-terephthalic acid) and CrO3 under acidic hydrothermal conditions (details are given in the ESI†).30,35 For mixed-linker MILs, a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of the linkers H2bdc–NO2/H2bdc–SO3Na and H2bdc/H2bdc–SO3Na was used in the synthesis. The effective linker ratio was then determined by digestive dissolution and 1H NMR analysis (Table 1) (spectra in Fig. S2, ESI†).
1 of the linkers H2bdc–NO2/H2bdc–SO3Na and H2bdc/H2bdc–SO3Na was used in the synthesis. The effective linker ratio was then determined by digestive dissolution and 1H NMR analysis (Table 1) (spectra in Fig. S2, ESI†).
| Materials | BET surface areaa (m2 g−1) | Total pore volumeb (cm3 g−1) | Exp. ratio NO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SO3H or bdc SO3H or bdc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SO3Hc SO3Hc |
Number of Brønsted acid sites, in formula unitd | Particle diameter ± standard deviation (σ)e (nm) |
|---|---|---|---|---|---|
| a Calculated in the pressure range 0.05 < p/p0 < 0.2 from N2 sorption isotherm at 77 K. The BET error margin is 20–50 m2 g−1. b Calculated from N2 sorption isotherm at 77 K (p/p0 = 0.95) for pores ≤40 nm. c Determined by digestion of MOF and 1H-NMR (see Fig. S2, ESI). d From molecular formula based on the possible number of protic H atoms per formula unit (see ESI) of MIL = [Cr3(O)(OH)(bdc-X)3(H2O)2]. e Obtained from SEM measurements, minimum of 50 particles were counted. f Particles are extremely agglomerated, particle size could not be determined (Fig. S3c, ESI). | |||||
| MIL-101Cr | 3049 | 1.50 | — | 2 | 174 ± 35 |
| MIL-NO2 | 2058 | 1.18 | — | 2 | 168 ± 30 |
| MIL-NO2/SO3H | 1480 | 0.75 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.5 | 160 ± 41 |
| MIL-SO3H(0.33) | 1633 | 0.93 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.99 | n.d.f |
| MIL-SO3H | 1333 | 0.70 | — | 5 | 152 ± 43 |
The MIL-101 structure of the linker-modified compounds was verified by positive matching of the simulated and measured powder X-ray diffractograms (Fig. S4, ESI†). N2 sorption isotherms at 77 K showed the typical MIL-101 curvature with two steps up to p/p0 = 0.25 in the otherwise Type Ib isotherm, characteristic of a wider microporous material (Fig. S5, ESI†).42 BET surface areas and pore volumes were calculated from these N2 sorption isotherms. Among the four tested catalysts, MIL-NO2 (2058 m2 g−1) has the highest and MIL-SO3H (1333 m2 g−1) the lowest surface area. In our earlier contribution on the diacetal formation using MIL-101Cr derivatives, it could be shown that the surface area is not the decisive factor for high catalytic activity, but the particle size as well as electron withdrawing or donating functionalities has a higher influence on conversion.27
Conversion of glucose to 5-HMF
Due to the high importance of platform chemicals derived from biomass, several solid acid catalysts have been investigated for the conversion of glucose to 5-HMF.19,28 Metal–organic frameworks were investigated for a number of acid-catalyzed reactions,43 including the conversion of fructose and glucose, albeit in a yield of maximum 16% for the latter.21 Therefore, this work focuses on the conversion of glucose to 5-HMF using MOF catalysts.The conversion of glucose to 5-HMF was performed in the presence of each of the four functionalized MIL catalysts (Fig. 4) and the conversion was followed by gas chromatography (GC). In a typical experiment, 1.24 mmol glucose (223 mg) in 5 mL THF/water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 39
v 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),10 giving a 5 wt% glucose solution, was heated in the presence of the catalyst (5.22 × 10−2 mmol) for a chosen time at 130 °C in a Pyrex glass vial. For all experiments, 4.0 mol% MIL catalyst with respect to glucose was used. In the literature, similar glucose or fructose substrate to MOF-catalyst ratios were used.20,21,25 When glucose was kept in THF/water under the same conditions but in the absence of the MIL material, no formation of 5-HMF or any other products could be detected which shows that the MIL material is necessary for the catalytic reaction.
1),10 giving a 5 wt% glucose solution, was heated in the presence of the catalyst (5.22 × 10−2 mmol) for a chosen time at 130 °C in a Pyrex glass vial. For all experiments, 4.0 mol% MIL catalyst with respect to glucose was used. In the literature, similar glucose or fructose substrate to MOF-catalyst ratios were used.20,21,25 When glucose was kept in THF/water under the same conditions but in the absence of the MIL material, no formation of 5-HMF or any other products could be detected which shows that the MIL material is necessary for the catalytic reaction.
 | ||
Fig. 4 (a) Conversion of glucose to 5-HMF with functionalized MIL-X (catalyst 5.22 × 10−5 mol, 223 mg glucose, 130 °C, 5 mL THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39 v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 24 h). (b) Normalized to theoretical number of Brønsted acid (BA) sites (cf.Table 1). 1, 24 h). (b) Normalized to theoretical number of Brønsted acid (BA) sites (cf.Table 1). | ||
Comparing the catalytic activity of the four MIL-101Cr derivatives, the completely SO3H-functionalized MIL has the highest activity. With 29% yield of 5-HMF (determined by calibrated GC after workup), this result is, to the best of our knowledge, the highest 5-HMF yield obtained using MOFs for the formation of 5-HMF from glucose. Interestingly, the other MOFs, MIL-NO2, MIL-SO3H(0.33) and MIL-NO2/SO3H, give lower, but among them very similar, HMF yields of about 12–13%. Pure MIL-101Cr shows a very low 5-HMF yield of only 2% (standard conditions, 24 h) (Fig. 4a). Interestingly, MIL-NO2, which has no obvious additional Brønsted acid sites compared to MIL-101Cr, shows a significantly higher 5-HMF yield, probably due to the stronger polarized aqua ligands through the electron-withdrawing nitro groups (Fig. 4a). Hence, the electron-withdrawing effect of the NO2 and additional acidic SO3H groups seems to have a strong influence on the catalytic activity of the MIL-101 platform. MIL-SO3H gives the highest yield of 5-HMF and, therefore, this catalyst has been investigated in more detail.
If we assume that Brønsted acid (BA) catalysis takes place in the glucose to 5-HMF conversion and we normalize for the number of protons per formula unit (cf.Table 1) the activity becomes highest for MIL-NO2 with just two BA sites (Fig. 4b). We have tried to determine the number of accessible acidic sites by potentiometric titration according to literature procedures.44 Typically, MIL is stirred in a saturated solution of sodium chloride in order to exchange the protons with sodium. Our experiments revealed that MIL materials are not stable under these conditions (see ESI†), since two inflection points were observed although by the back-titration method only one acid strength (HCl/H2O) should be present. We also followed a recently published approach by Klet and coworkers who used sodium nitrate for proton exchange (see ESI†).45 Attempts to quantify the amount of available or accessible acid sites remained inconclusive (see ESI†), which is why the theoretical number of acid sites was used (Table 1). We can only state from titration experiments that the amount of accessible protons is much smaller than what is calculated from the formula unit.
Stability and recycling test
In order to prove that no leaching occurs, MIL-SO3H was tested in a filtration experiment. The reaction was stopped by cooling to 0 °C after 5 hours and the catalyst was separated by centrifugation at 6000 rpm and subsequent filtration of the filtrate using a 0.2 μm syringe filter. The reaction was continued with the filtrate under identical conditions for different times, followed by the standard workup. No further formation of 5-HMF takes place over a period of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 h (Table 2). Small differences of 1% were within experimental error of the reaction procedure, since for every measuring point a new reaction was started.
45 h (Table 2). Small differences of 1% were within experimental error of the reaction procedure, since for every measuring point a new reaction was started.
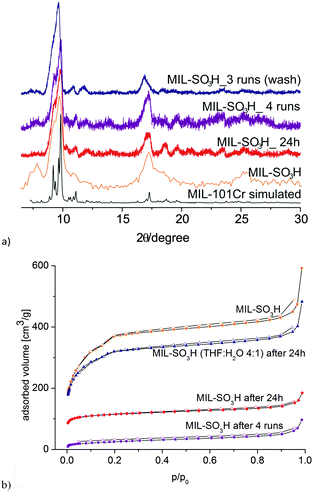
Multiple run experiments showed that MIL-SO3H can be re-used up to 3–4 times with a loss in 5-HMF yield from 29% to 13–16% (Table S3 in the ESI†). In a first series of recycle experiments, MIL-SO3H was re-used without any intermediate washing or reactivation steps. In the fourth run, the 5-HMF yield was reduced to 13%. Therefore, the experiment was repeated such that after each run the catalyst was washed with water and methanol and dried before re-use. After three runs, a 5-HMF yield of 16% was measured. After three runs, the PXRD still shows crystalline MIL-SO3H (Fig. 5a). The surface area was reduced to half after the first run (443 m2 g−1) and essentially lost after four runs (91 m2 g−1) (Fig. 5b). The surface area increased only negligibly from 91 m2 g−1 to 120 m2 g−1 after washing the MIL-SO3H material after the third run with HCl (2 mol L−1) at 85 °C for 20 h, followed by water and drying (140 °C, 15 h).
Effect of water content on 5-HMF yield
It was suggested in a molecular simulation study that MIL-101Cr is able to adsorb glucose from an aqueous solution.46,47 Since the MIL material is known for its high uptake of water,29 this might be a driving force for glucose to diffuse into the pores, due to the much better solubility of glucose in water compared to THF.In order to elucidate the effect of water on the conversion of glucose, the water content was increased gradually from 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results in Table 3 show that with increasing water content the yield of 5-HMF becomes lower.
1. The results in Table 3 show that with increasing water content the yield of 5-HMF becomes lower.
In pure water, only very low formation of 5-HMF was detected (6%) and in pure THF no 5-HMF (and no other products) could be detected by GC. Therefore, a small amount of water is not only beneficial, but even necessary for 5-HMF formation. In this respect, our reaction system differs significantly from the sulfuric acid study of Huber and coworkers, since for conc. (water-free) H2SO4 also in water-free THF, the formation of 5-HMF was detected.10 A possible explanation might be that the development of full acid strength of the MOF catalyst involves the polarization of water at the chromium centers of the MOFs. Glucose is more soluble in water than in THF. When water is adsorbed by the MIL catalyst this might be the driving force resulting in a higher 5-HMF yield, at low amounts of water, having a higher local concentration of glucose in the MIL environment. The glucose concentration in the MOF vicinity would be lowered further with too much or only water as solvent. In addition, THF is known to stabilize 5-HMF and prevent the formation of levulinic acid.48 Abu-Omar et al. showed that by using NaCl in a THF/H2O mixture as a phase separator, the 5-HMF yield from glucose could be increased from 52% to 61% in comparison to the system without NaCl (catalyst AlCl3, HCl, 160 °C). Since the reaction takes place in water a good phase separation and a good continuous transfer of 5-HMF to the organic THF phase is necessary.48 In the case of MIL-SO3H catalysis in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 39
v 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), these requirements are apparently fulfilled best.
1), these requirements are apparently fulfilled best.
Formation of humins and deactivation pathways
The degradation of carbohydrates to humins plays a crucial role in glucose conversion and catalyst degradation. This kind of deactivation can be classified as fouling.49 The formation of humins is not well investigated. Generally, unidentified soluble or insoluble degradation products are called humins.6b,d,50 Recently, Vlachos et al. investigated the effect of reaction conditions, 5-HMF conversion and acid concentration on the molecular structure and formation of humins, using FTIR spectroscopy, SEM and in–situ dynamic light scattering (DLS).51 It was concluded that combined effects of aldol condensation and etherification reactions as well as nucleophilic attack from 5-HMF and aggregation effects lead to insoluble polymers, especially at high 5-HMF yield.51 These by-products can block the pores so that no substrate can migrate in and no product can form or migrate out anymore. If the degradation products are also formed inside the pores, they block the MOF pores, which leads to deactivation.With increasing amount of water in the reaction solvent the yield of 5-HMF became lower (see Table 3). Yet, with increasing water fraction also less insoluble humin products are formed as evidenced from the color retention of the MIL and weight of the solid materials after the reaction (Fig. S6 and Table S2 in the ESI†). To determine the amount of insoluble humins which have formed, the solid material was separated by centrifugation and dried (120 °C, overnight). The weights are listed in Table S2 (ESI†). Also, with increasing water fraction the BET surface area of MIL-SO3H is diminished less after one catalytic run (Fig. 5b). When the catalytic reaction was carried out in a THF/water mixture of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the MIL-SO3H catalyst still had a BET surface area of 1161 m2 g−1 after the first run.
1, the MIL-SO3H catalyst still had a BET surface area of 1161 m2 g−1 after the first run.
Regeneration attempts were performed by washing the catalyst after the reaction with methanol, THF and H2O as well as acid (H2SO4) at room temperature and at 80 °C. No significant improvement of the surface area was measured. Since regeneration attempts failed and calcination is not possible due to instability of the MOF above 350 °C, the most effective way would be to prevent the formation of humins during the catalytic reaction.
A possible strategy to inhibit the formation of insoluble humins may be to deactivate the polymerizable sites of 5-HMF. Therefore, experiments were performed using ethanol in a mixture with THF and water (Table 4). Also in the presence of ethanol, the formation of 5-ethoxymethylfurfural (5-ethyl-HMF) was observed in addition to 5-HMF. The higher the amount of ethanol the higher was the yield of 5-ethyl-HMF.
Solvent mixturea (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) v) |
5-HMF yieldb [%] | 5-Ethyl-HMF yieldb [%] | MIL-SO3H surface area SBET [m2 g−1] after reaction |
|---|---|---|---|
| a Conditions: MIIL-SO3H 50 mg, glucose 223 mg, 5 mL solvent, 24 h, 130 °C. b Determined by calibrated GC. | |||
THF/H2O (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
29 | 0 | 443 |
THF/EtOH/H2O (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
13 | 7 | 1028 |
EtOH/H2O (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5 | 11 | 1239 |
The amount of insoluble humins seemed to be reduced when the reaction was carried out in ethanol/H2O, evidenced by the retention of the MIL porosity, its color and the amount of the catalyst (Fig. S6, S7 and Table S2 in ESI†). Unfortunately, in EtOH/H2O the overall yield and product selectivity are much lower than in the THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 39
v 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (Table 4).
1) mixture (Table 4).
Time profile
The yield of 5-HMF slowly approaches the determined value over the maximum allowed reaction time of 24 h under our standard conditions (Fig. 6). The space--time yield is much lower than with other catalysts such as sulfuric acid,10 AlCl3,52 montmorillonite K-10 clay53 or beta zeolites,54 where saturation is observed within 40 min to 5 h. This difference in the reaction rate can give a hint on the reaction mechanism, since it was suggested with homogeneous AlCl3/solvent/H2O that the catalysis proceeds by glucose to fructose isomerization (Lewis acid catalyzed) and subsequent dehydration to 5-HMF. This reaction route seems to be much faster than only Brønsted acid catalyzed conversion by homogeneous sulfuric acid.11,55 | ||
Fig. 6 Time dependent conversion of glucose to 5-HMF. Standard conditions: catalyst 50 mg, glucose 223 mg, 5 mL THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39 v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C. 1, 130 °C. | ||
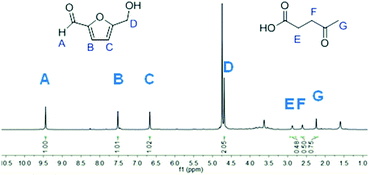
Glucose signals were still detected after 8 and 15 h when the reaction was stopped by cooling after 8, 15 and 24 h and analyzed by NMR in D2O (Fig. S9, ESI†). After 24 h, saccharide signals were not seen any more in the NMR spectrum (Fig. 9).
Assuming that the conversion of glucose takes place inside the pores or at least in the pore mouths of the MOF, diffusion limitations can be the reason for the slow conversion.
When decreasing the temperature to 100 °C under standard conditions the formation of 5-HMF is even slower; after 24 h only 8% can be detected. At 100 °C, only the formation of 5-HMF and the starting material is visible from the NMR spectrum (in D2O); no by-products are formed (Fig. S8d, ESI†).
Conversion of other substrates to 5-HMF
The reaction of maltose (5 wt%, 0.65 mmol) with MIL-SO3H resulted in a conversion of 50% to 5-HMF. Maltose is a glucose- dimer with the two monomer units connected through a glycosyl- bond. The same mass of maltose contains almost the same amount of glucose monomer units as in 223 mg glucose used under our standard conditions. Since the 5-HMF yield from maltose is higher than from glucose, it seems that the (unknown) intermediate state is more easily accessible from the dimer maltose. Also, the possible intermediates levoglucosan and fructose were used as starting materials and the reaction was performed under standard conditions (50 mg MIL-SO3H, 1.24 mmol sugar, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39
v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C). With levoglucosan after 8 h a 5-HMF yield of 12% and after 24 h of 28% was measured by GC. It is evident that 5-HMF can be formed also from levoglucosan, but only to a similar extent as from glucose.
1, 130 °C). With levoglucosan after 8 h a 5-HMF yield of 12% and after 24 h of 28% was measured by GC. It is evident that 5-HMF can be formed also from levoglucosan, but only to a similar extent as from glucose.
It has already been shown by several groups that fructose can be converted to 5-HMF in good yields by Brønsted acidic MOF materials in other solvents.20,25 In our study, a maximum yield of 5-HMF of 47% was obtained after 8 h under standard conditions. As expected, the conversion of fructose proceeds much faster than the conversion of glucose. The formation of humins was also observed with fructose, levoglucosan and maltose by color and weight change.
Cellulose with MIL-SO3H shows almost no formation of 5-HMF (1%). This could indicate that the long polymer chains of cellulose cannot enter the pores of MIL-SO3H for conversion or, alternatively, that the chains are not cleaved to a sufficient extent.
Formation of 5-HMF versus levulinic acid
When 5-HMF is dissolved in THF/water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 39
v 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and reacted at 130 °C for 24 h with the catalyst MIL-SO3H, no reaction is observed; only the starting material 5-HMF can be detected by GC or GC-MS. The 1H NMR spectrum of the reaction solution (without workup) showed only trace amounts of levulinic acid (molar ratio 5-HMF to levulinic acid, 1
1) and reacted at 130 °C for 24 h with the catalyst MIL-SO3H, no reaction is observed; only the starting material 5-HMF can be detected by GC or GC-MS. The 1H NMR spectrum of the reaction solution (without workup) showed only trace amounts of levulinic acid (molar ratio 5-HMF to levulinic acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04). The catalyst also retained its green color instead of changing to brown, which indicates no formation of humins as in the glucose conversion. In contrast, Dumesic and coworkers showed that other catalysts, for instance, H2SO4, Amberlyst-70 or zeolites will give levulinic acid from furfuryl alcohol in THF/H2O solutions with levulinic acid yields of over 70%.57
0.04). The catalyst also retained its green color instead of changing to brown, which indicates no formation of humins as in the glucose conversion. In contrast, Dumesic and coworkers showed that other catalysts, for instance, H2SO4, Amberlyst-70 or zeolites will give levulinic acid from furfuryl alcohol in THF/H2O solutions with levulinic acid yields of over 70%.57
The selectivity of the glucose-to-5-HMF conversion concerning the formation of levulinic acid as a by-product was analyzed by NMR. Since levulinic acid is very soluble in water, the complete reaction solution was dried in vacuum; the residue was re-dissolved in deuterated solvent and then measured. All reactions have been performed under standard conditions for 24 h, unless stated otherwise.
With MIL-SO3H and glucose, the selectivity towards 5-HMF over levulinic acid is 80%, since the molar ratio between 5-HMF and levulinic acid is 1 to 0.25 after 24 h (Table 5 and Fig. 7).
| Time (h) | Integral ratio 5-HMF vs. levulinic acidd | Selectivity of 5-HMF over levulinic acid (%) |
|---|---|---|
a Conditions: 50 mg MIIL-SO3H, 5 wt% glucose, 5 mL THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39 v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C. Determined by 1H-NMR in D2O.
b Fructose as substrate (1.24 mmol).
c Levuglucosan as substrate (1.24 mmol).
d Integral ratio of CH2 groups: D (5-HMF) and the average of the E and F signal (levulinic acid), see Fig. 9. 1, 130 °C. Determined by 1H-NMR in D2O.
b Fructose as substrate (1.24 mmol).
c Levuglucosan as substrate (1.24 mmol).
d Integral ratio of CH2 groups: D (5-HMF) and the average of the E and F signal (levulinic acid), see Fig. 9.
|
||
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 0.13 |
88 |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.16 0.16 |
86 |
| 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
80 |
| 24 hb | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09 0.09 |
92 |
| 24 hc | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.21 0.21 |
83 |
After 8 h and 15 h reaction times, the selectivity towards 5-HMF over levulinic acid is even higher (88% and 86%, respectively) and decreases with time (Table 5 and Fig. S9, ESI†). This indicates that 5-HMF also slowly converts to levulinic acid. As pure 5-HMF with MIL-SO3H shows only a very low conversion over 24 h (see above) other reaction products may assist in this conversion.
For comparison, MIL-SO3H was also reacted with levoglucosan (1.24 mmol) and fructose (1.24 mmol) as substrate and similar ratios between 5-HMF and levulinic acid were obtained (Fig. S8a, b, ESI† and Table 5). Interestingly, the 1H NMR spectrum of the glucose conversion with MIL-NO2 shows signals of 5-HMF, but no levulinic acid is found. Only some signals of unidentified products can be found (Fig. S8c, ESI†). In summary, MIL-SO3H shows the highest yield of 5-HMF which correlates with the highest potential number of acid sites of the MIL-materials (Fig. 4), whereas MIL-NO2 shows a very selective formation of 5-HMF since no levulinic acid was found.
For comparison, chromium(III) nitrate, sulfuric acid and Amberlyst-15 have been tested as catalysts (1.57 × 10−4 mol) for the formation of 5-HMF under the same conditions as the MIL-materials (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v
H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39
v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C, 223 mg glucose) for 24 h. CrCl3 is known to catalyze the isomerization from glucose to fructose. Also, the formation of 5-HMF, levulinic acid and formic acid was observed with CrCl3.7a,56 In contrast to MIL-SO3H, the yield of 5-HMF from glucose catalyzed by Cr(NO3)3 is only 6% after 24 h. Interestingly, no levulinic acid was detected, but a high amount of unidentified products can be observed by NMR and GC-MS. The high formation of by-products in the case of Cr3+ salts is supported by a comprehensive study of Vlachos et al.7a
1, 130 °C, 223 mg glucose) for 24 h. CrCl3 is known to catalyze the isomerization from glucose to fructose. Also, the formation of 5-HMF, levulinic acid and formic acid was observed with CrCl3.7a,56 In contrast to MIL-SO3H, the yield of 5-HMF from glucose catalyzed by Cr(NO3)3 is only 6% after 24 h. Interestingly, no levulinic acid was detected, but a high amount of unidentified products can be observed by NMR and GC-MS. The high formation of by-products in the case of Cr3+ salts is supported by a comprehensive study of Vlachos et al.7a
Comparing the product distribution using MIL-SO3H as a catalyst or H2SO4 as a homogenous catalyst or the strong acidic resin Amberlyst-15, starting from glucose, a significant change can be observed (Fig. 8 and Fig. S10, ESI†). For Amberlyst-15, the selectivity is strongly directed to levulinic acid, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of 5-HMF to levulinic acid (5-HMF selectivity only 25%). For sulfuric acid as a catalyst the major dominant product is also levulinic acid. The ratio between 5-HMF and levulinic acid is 1
3 ratio of 5-HMF to levulinic acid (5-HMF selectivity only 25%). For sulfuric acid as a catalyst the major dominant product is also levulinic acid. The ratio between 5-HMF and levulinic acid is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (5-HMF selectivity 9%).
10 (5-HMF selectivity 9%).
 | ||
Fig. 8 Ratio between 5-HMF and levulinic acid from 1H-NMR. Comparison between MIL-SO3H, H2SO4 and Amberlyst-15H. Conditions: catalyst MIL-SO3H 5.22 × 10−5 mol, glucose 223 mg, 5 mLTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v H2O (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) 39 v) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 130 °C. For H2SO4 and Amberlyst-15 the reaction was conducted with 1.57 × 10−4 mol of the catalyst under the same conditions. The estimated amount of acid of MIL-SO3H, Amberlyst-15 and sulfuric acid is 0.46 mmol H+ per g (see Table S4, ESI†), 4.7 mmol H+ per g (AMBERLYST™ product information) and 10.2 mmol H+ per g, respectively. 1, 130 °C. For H2SO4 and Amberlyst-15 the reaction was conducted with 1.57 × 10−4 mol of the catalyst under the same conditions. The estimated amount of acid of MIL-SO3H, Amberlyst-15 and sulfuric acid is 0.46 mmol H+ per g (see Table S4, ESI†), 4.7 mmol H+ per g (AMBERLYST™ product information) and 10.2 mmol H+ per g, respectively. | ||
Recently, Dumesic et al. reported the conversion of furfuryl alcohol into levulinic acid using different solid acids (including Amberlyst-70 and H-ZSM-5) as well as sulfuric acid in a similar THF/water system.57 Huber et al. reported the formation of levulinic acid as a by-product from glucose conversion to 5-HMF using sulfuric acid (catalyst).10
It is evident that the ratio of 5-HMF to levulinic acid is inversely proportional to the acidity of the catalyst. The significantly lower actual acidity of MIL-SO3H compared to Amberlyst-15 and H2SO4 explains the low-high ratio of 5-HMF to levulinic acid. The even lower acidity of MIL-NO2 then explains the absence of levulinic acid. At present, it is not fully clear if 5-HMF is converted to levulinic acid. Levulinic acid could also form through a 5-HMF independent pathway (see the Mechanistic aspects section in the ESI†).11
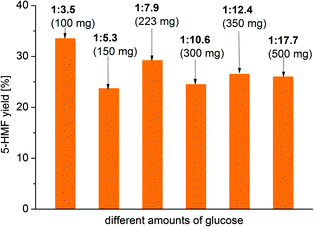
The 5-HMF yield varies only slightly, essentially within experimental error, with the amount of MIL-SO3H catalyst (Fig. 9).
Increasing the ratio of MIL-SO3H to glucose from the standard 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.9 (223 mg glucose) to 1
7.9 (223 mg glucose) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 (100 mg glucose) gives a 5-HMF yield of 34% (up from 29%). By decreasing the ratio of MIL-SO3H/glucose to 1
3.5 (100 mg glucose) gives a 5-HMF yield of 34% (up from 29%). By decreasing the ratio of MIL-SO3H/glucose to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.7 (500 mg), a slight decrease in 5-HMF formation to 26% can be observed (Fig. 9). These results show that the catalyst remains active even at higher concentrations of glucose and the percentage yield does not change significantly with a moderate variation of the substrate ratio.
17.7 (500 mg), a slight decrease in 5-HMF formation to 26% can be observed (Fig. 9). These results show that the catalyst remains active even at higher concentrations of glucose and the percentage yield does not change significantly with a moderate variation of the substrate ratio.
Parallel to this work, Su and coworkers also investigated the activity of MIL-101Cr-SO3H on the glucose to 5-HMF conversion.58 They used a solvent mixture of γ-valerolactone (GVL) and H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 150 °C. The glucose to catalyst ratio can be calculated as 1
1) at 150 °C. The glucose to catalyst ratio can be calculated as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 1
5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (considering 3 Cr ions per formula unit), taking into account that only 60% of the linker had a SO3H moiety. In our study, the glucose to MIL-SO3H ratio was 1
2 (considering 3 Cr ions per formula unit), taking into account that only 60% of the linker had a SO3H moiety. In our study, the glucose to MIL-SO3H ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 or 1
24 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Su et al.58 synthesized MIL-101Cr-SO3H from monosodium sulfoterephthalic acid, HF and Cr(NO3)3 instead of CrO3. The average particle size of MIL-101Cr-SO3H was higher with 400 nm and the BET surface area was 1700 m2 g−1. Under their reported conditions, a highest 5-HMF yield of 45% and selectivity of 46% could be achieved. Acidity was calculated from back-titration experiments with saturated NaCl to 1.61 mmol g−1. Unfortunately, no titration curves were given.58
8. Su et al.58 synthesized MIL-101Cr-SO3H from monosodium sulfoterephthalic acid, HF and Cr(NO3)3 instead of CrO3. The average particle size of MIL-101Cr-SO3H was higher with 400 nm and the BET surface area was 1700 m2 g−1. Under their reported conditions, a highest 5-HMF yield of 45% and selectivity of 46% could be achieved. Acidity was calculated from back-titration experiments with saturated NaCl to 1.61 mmol g−1. Unfortunately, no titration curves were given.58
It was shown that the high boiling solvent GVL is beneficial for the 5-HMF yield over short reaction times (2 h). Similar to our work, lower 5-HMF yields were also found for higher amounts of water. In contrast, in pure GVL also 26% 5-HMF yield was obtained,58 whereas in our study no conversion was observed in pure THF. In terms of the mechanism, the authors referred to the 1,2-hydride shift, proven for Lewis acidic zeolites. Through HPLC experiments small amounts of fructose could be identified, although the possibilities of alternative intermediates are not discussed. They suggest a second order kinetics for the reaction. In fixed bed reactions, a 5-HMF yield between 35 and 45% over 56 h was obtained.58
The scope of using MOFs as catalysts in the synthesis of fine chemicals is summarized elsewhere,16,59 but the advantage of selective catalysis in 5-HMF formation is obvious. Synergistic effects due to reactions catalyzed in confined space are highlighted in the literature60 and could be an important factor explaining the selectivity towards 5-HMF using MILs as catalysts. A defined solvent microenvironment due to the preferred uptake of water by the MIL catalyst can also play a role.
Conclusions
For different nitro- and sulfo-modified MIL-101Cr compounds, MIL-SO3H gives the highest yield of 5-HMF from glucose of 29% after 24 h. This yield is lower than the results obtained using other solid acid catalysts,28 but at present the highest value is reported for the glucose-to-5-HMF conversion catalyzed by metal–organic frameworks. Importantly, it could be shown that the reaction only proceeds with a small amount of water. In pure THF, no product formation was observed. Furthermore, also from pure 5-HMF as a reactant, only very little production of levulinic acid could be detected, in contrast to the results in the literature using sulfuric acid10 or Amberlyst-70.57 Under the same conditions, MIL-SO3H, sulfuric acid and Amberlyst-15 show significantly different 5-HMF/levulinic acid product distributions. MIL-SO3H preferentially forms 5-HMF over levulinic acid in contrast to the other catalysts. Reactivation of the MOF catalyst remains a challenge, since pore blocking effects have been observed in multiple run experiments. Derivatisation to 5-ethyl-HMF is a promising way, avoiding the formation of insoluble humins. At present, the 5-ethyl-HMF yield is low, awaiting the development of more active MOF-based acidic catalysts.Acknowledgements
The work was funded in part by the Deutscher Akademischer Austauschdienst, DAAD, through PPP-project no. 57053987 (China 2j ab 14).References
- (a) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC; (b) S. Dutta and S. Pal, Biomass Bioenergy, 2014, 62, 182–197 CrossRef CAS.
- C. Chatterjee, F. Pong and A. Sen, Green Chem., 2015, 17, 40–71 RSC.
- (a) A. A. Koutinas, A. Vlysidis, D. Pleissner, N. Kopsahelis, I. L. Garcia, I. K. Kookos, S. Papanikolaou, T. H. Kwan and C. S. K. Lin, Chem. Soc. Rev., 2014, 43, 2587–2627 RSC; (b) D. Esposito and M. Antonietti, Chem. Soc. Rev., 2015, 44, 5821–5835 RSC.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- R. Beerthuis, G. Rothenberg and N. R. Shiju, Green Chem., 2015, 17, 1341–1361 RSC.
- (a) B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24–38 RSC; (b) R. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed; (c) S. P. Teong, G. Yi and Y. Zhang, Green Chem., 2014, 16, 2015–2026 RSC; (d) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- (a) V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS PubMed; (b) J. Tang, X. Guo, L. Zhu and C. Hu, ACS Catal., 2015, 5, 5097–5103 CrossRef CAS.
- C. Liu, J. M. Carraher, J. L. Swedberg, C. R. Herndon, C. N. Fleitman and J. Tessonnier, ACS Catal., 2014, 4, 4295–4298 CrossRef CAS.
- J. M. Carraher, C. N. Fleitman and J. Tessonnier, ACS Catal., 2015, 5, 3162–3173 CrossRef CAS.
- R. Weingarten, A. Rodriguez-Beuerman, F. Cao, J. S. Luterbacher, D. Martin Alonso, J. A. Dumesic and G. W. Huber, ChemCatChem, 2014, 6, 2229–2234 CrossRef CAS.
- L. Yang, G. Tsilomelekis, S. Caratzoulas and D. G. Vlachos, ChemSusChem, 2015, 8, 1334–1341 CrossRef CAS PubMed.
- R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- P. Wasserscheid and T. Welton, Ionic Liquid in Synthesis, Wiley-VCH, Weinheim, 2007, vol. 1, pp. 325–350 Search PubMed.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773–3789 ( Angew. Chem. , 2000 , 112 , 3926–3945 ) CrossRef.
- C. van Doorslaer, Y. Schellekens, P. Mertens, K. Binnemanns and D. De Vos, Phys. Chem. Chem. Phys., 2010, 12, 1741–1749 RSC.
- P. Garcia-Garcia, M. Müller and A. Corma, Chem. Sci., 2014, 5, 2979–3007 RSC.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabres i Xamena, ACS Catal., 2014, 4, 361–378 CrossRef CAS.
- (a) F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck and D. E. De Vos, Angew. Chem., Int. Ed., 2012, 51, 4887–4890 CrossRef CAS PubMed; (b) P. Valvekens, F. Vermoortele and D. De Vos, Catal. Sci. Technol., 2013, 3, 1435–1445 RSC.
- J. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966–6997 CrossRef CAS PubMed.
- Y. Zhang, V. Degirmenci, C. Li and E. J. M. Hensen, ChemSusChem, 2011, 4, 59–64 CrossRef CAS PubMed.
- L. Bromberg, X. Su and T. A. Hatton, Chem. Mater., 2014, 26, 6257–6264 CrossRef CAS.
- J. Chen, S. Wang, J. Huang, L. Chen, L. Ma and X. Huang, ChemSusChem, 2013, 6, 1545–1555 CrossRef CAS PubMed.
- G. Akiyama, R. Matsuda, H. Sato, M. Takata and S. Kitagawa, Adv. Mater., 2011, 23, 3294–3297 CrossRef CAS PubMed.
- G. Akiyama, R. Matsuda, H. Sato and S. Kitagawa, Chem. – Asian J., 2014, 9, 2772–2777 CrossRef CAS PubMed.
- J. Chen, K. Li, L. Chen, R. Liu, X. Huang and D. Ye, Green Chem., 2014, 16, 2490–2499 RSC.
- R. DiCosimo, J. McAuliffe, A. J. Poulose and G. Bohlmann, Chem. Soc. Rev., 2013, 42, 6437–6474 RSC.
- A. Herbst, A. Khutia and C. Janiak, Inorg. Chem., 2014, 53, 7319–7333 CrossRef CAS PubMed.
- (a) T. D. Swift, H. Nguyen, Z. Erdman, J. S. Kruger, V. Nikolakis and D. G. Vlachos, J. Catal., 2016, 333, 149–161 CrossRef CAS; (b) I. Jiménez-Morales, A. Teckchandani-Ortiz, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2014, 144, 22–28 CrossRef; (c) J. Wang, J. Ren, X. Liu, J. Xi, Q. Xia, Y. Zu, G. Lu and Y. Wang, Green Chem., 2012, 14, 2506–2512 RSC; (d) H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS.
- (a) A. Khutia, H. Urs Rammelberg, T. Schmidt, S. Henninger and C. Janiak, Chem. Mater., 2013, 25, 790–798 CrossRef CAS; (b) J. Ehrenmann, S. K. Henninger and C. Janiak, Eur. J. Inorg. Chem., 2011, 471–474 CrossRef CAS.
- G. Akiyama, R. Matsuda, H. Sato, A. Hori, M. Takata and S. Kitagawa, Microporous Mesoporous Mater., 2012, 157, 89–93 CrossRef CAS.
- M. Trivedi, Bhaskaran, A. Kumar, G. Singh, A. Kumar and N. P. Rath, New J. Chem., 2016, 40, 3109–3118 RSC; N. Cao, T. Liu, J. Su, X. Wu, W. Luo and G. Cheng, New J. Chem., 2014, 38, 4032–4035 RSC.
- M. Saikia, D. Bhuyan and L. Saikia, New J. Chem., 2015, 39, 64–67 RSC.
- STOE WinXPOW Version 1.10 , STOE and Cie GmbH, Darmstadt, Germany, 2002 Search PubMed.
- Mercury – Program for Crystal Structure Visualisation, Exploration and Analysis, The Cambridge Crystallographic Data Centre (CCDC), Version 3.3.1, copyright CCDC 2001–2016 Search PubMed.
- Y. Lee, Y. Chung and W. Ahn, RSC Adv., 2014, 4, 23064–23067 RSC.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- T. Zhao, F. Jeremias, I. Boldog, B. Nguyen, S. K. Henninger and C. Janiak, Dalton Trans., 2015, 44, 16791–16801 RSC.
- Y. K. Hwang, D.-Y. Hong, J.-S. Chang, S. H. Jhung, Y.-K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144–4148 CrossRef CAS PubMed.
- D.-Y. Hong, Y. K. Hwang, C. Serre, G. Férey and J.-S. Chang, Adv. Funct. Mater., 2009, 19, 1537–1552 CrossRef CAS.
- K. Brandenburg, Diamond (Version 3.2), crystal and molecular structure visualization, Crystal Impact, K. Brandenburg & H. Putz Gbr, Bonn, Germany, 2007–2012 Search PubMed.
- M. Lammert, S. Bernt, F. Vermoortele, D. E. De Vos and N. Stock, Inorg. Chem., 2013, 52, 8521–8528 CrossRef CAS PubMed.
- (a) M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS; (b) K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- A. Dhakshinamoorthy, M. Opanasenko, J. Cejka and H. Garcia, Catal. Sci. Technol., 2013, 3, 2509–2540 CAS.
- (a) J. Chen, K. Li, R. Liu, X. Huang and D. Ye, Green Chem., 2014, 16, 2490–2499 RSC; (b) S. Chen, T. Yokoi, C. Tang, L. Jang, T. Tatsumi, J. C. C. Chan and S. Cheng, Green Chem., 2011, 13, 2920–2930 RSC; (c) Y. Zang, J. Shi, F. Zhang, Y. Zhong and W. Zhu, Catal. Sci. Technol., 2013, 3, 2044–2049 RSC; (d) Z. Hasan, J. W. Jun and S. H. Jhung, Chem. Eng. J., 2015, 278, 265–271 CrossRef CAS.
- R. C. Klet, Y. Liu, T. C. Wang, J. T. Hupp and O. K. Farha, J. Mater. Chem. A, 2016, 4, 1479–1485 CAS.
- K. M. Gupta, K. Zhang and J. Jiang, Sci. Rep., 2015, 5, 12821–12830 CrossRef CAS PubMed.
- P. Bai and J. I. Siepmann, AIChE J., 2013, 59, 3523–3529 CrossRef CAS.
- Y. Yang, C. Hu and M. M. Abu-Omar, Green Chem., 2012, 14, 509–513 RSC.
- I. Sádaba, M. López Granados, A. Riisager and E. Taarning, Green Chem., 2015, 17, 4133–4145 RSC.
- J. Lange, Angew. Chem., 2015, 127, 13382–13394 CrossRef.
- G. Tsilomelekis, M. J. Orella, Z. Lin, Z. Cheng, W. Zheng, V. Nikolakis and D. G. Vlachos, Green Chem., 2016, 18, 1983–1993 RSC.
- Y. J. Pagan-Torres, T. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef CAS.
- Z. Fang, B. Liu, J. Luo, Y. Ren and Z. Zhang, Biomass Bioenergy, 2014, 60, 171–177 CrossRef CAS.
- R. Otomo, T. Yokoi, J. N. Kondo and T. Tatsumi, Appl. Catal., A, 2014, 470, 318–326 CrossRef CAS.
- R. van Putten, J. N. M. Soetedjo, E. A. Pidko, J. C. van der Waal, E. J. M. Hensen, E. de Jong and H. J. Heeres, ChemSusChem, 2013, 6, 1681–1687 CrossRef CAS PubMed.
- P. Wrigstedt, J. Keskiväli, M. Leskelä and T. Repo, ChemCatChem, 2015, 7, 501–507 CrossRef CAS.
- M. A. Mellmer, J. M. R. Gallo, D. M. Alonso and J. A. Dumesic, ACS Catal., 2015, 5, 3354–3359 CrossRef CAS.
- Y. Su, G. Chang, Z. Zhang, H. Xing, B. Su, Q. Yang, Q. Ren, Y. Yang and Z. Bao, AIChE J., 2016 DOI:10.1002/aic.15356.
- A. Dhakshinamoorthy and H. Garcia, ChemSusChem, 2014, 7, 2392–2410 CrossRef CAS PubMed.
- (a) C. Yu and J. He, Chem. Commun., 2012, 48, 4933–4940 RSC; (b) R. De Clercq, M. Dusselier, C. Christiaens, J. Dijkmans, R. I. Iacobescu, Y. Pontikes and B. F. Sels, ACS Catal., 2015, 5, 5803–5811 CrossRef CAS; (c) S. H. A. M. Leenders, R. l. Gramage-Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433–448 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis, methods, catalysis, NMR spectra, SEM images, powder X-ray diffractograms, N2 sorption isotherms, and potentiometric titrations. See DOI: 10.1039/c6nj01399f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |