New generation of camptothecin derivatives spontaneously alkylating DNA†
Abstract
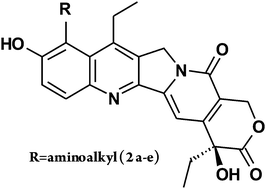
9-Aminomethyl derivatives of 7-ethyl-10-hydroxycamptothecin were synthesized. Interaction with a natural DNA octamer was checked using 1H NMR, PFGSE NMR and MALDI-TOF experiments, which proved covalent binding. Compounds were tested against human cancer cell lines in vitro. All compounds show high activity comparable to SN38. The obtained products are well soluble in water.

 Please wait while we load your content...
Please wait while we load your content...