Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
4-Perfluoroalkylbutoxybenzene derivatives as liquid crystalline organogelators based on phase-selective gelators†
Banpeng
Cao
ab,
Yuta
Kaneshige
a,
Yuya
Matsue
a,
Yuki
Morita
a and
Hiroaki
Okamoto
*a
aGraduate School of Science and Engineering, Yamaguchi University, 2-16-1, Tokiwadai, Ube Yamaguchi, Japan. E-mail: oka-moto@po.cc.yamaguchi-u.ac.jp
bJiangxi Key Laboratory of Organic Chemistry, Jiangxi Science and Technology Normal University, 605 Fenglin road, Nanchang, Jiangxi province, P. R. China
First published on 7th April 2016
Abstract
4-Perfluoroalkylbutoxybenzene derivatives 1(n) have been prepared as a new class of thermotropic liquid crystalline organogelators incorporating a perfluoroalkyl group without a hydrogen bonding group. Significantly, compounds 1(n) at room temperature act as efficient and rapid phase-selective gelators in a kind of method appropriate for water purification.
Organogels formed by low molecular weight organogelators (LMWOGs) have attracted a great amount of interest and are widely used in industry and pharmacy.1 Research into LMWOGs can be divided into two parts. The first part is the relationship between molecular structure and gelation properties;2 the second is the expansion of LMWOGs' potential applications through novel properties,3 such as liquid crystalline properties in so-called liquid crystalline (LC) organogelators, or phase-selective gelation properties in so-called phase-selective gelators (PSGs).
PSGs were first reported by Bhattacharya and Krishnan-Ghosh.3 Since then PSGs have become a hot topic because they may be applied in water purification, and recovery after crude oil and other chemical oil spills.4 Most PSGs gelate mainly by hydrogen bonding as reported in recent years. The main disadvantage for their application in water purification and oil spill recovery is that CH, OH, and NH hydrogen donors usually coordinate to water molecules in many different combinations leading to unstable organogels.5
In this context, our intention here was to employ a 4-perfluoroalkylbutoxybenzene fragment as a terminal group to synthesize novel multifunctional materials having not only LC properties but also PSG properties without hydrogen bonding. It is to be noted that the modifications of a subtle but significant fluoro substituent are usually accompanied by changes in relation to melting point, transition temperatures, mesophase morphology, and many essential physical properties of soft materials, such as optical properties, dielectric amphiphilicity and visco-elastic properties.6 Herein we report the design and synthesis of 4-perfluoroalkylbutoxybenzene derivatives by combining our past experience in LC and gels.7 Compounds 1(n)–4(5) were synthesized from 4,4′-biphenol or 4-alkoxyphenol with Williamson ether synthesis, free-radical addition and a reduction reaction by LiAlH4 (Scheme 1 and Scheme S1, ESI†).
To analyse the influence of the core systems and the terminal perfluoroalkyl group on LC and gel efficiency, compounds 1(n)–4(5) were synthesized, tested and compared with respect to their LC and gelation properties.
Following the general research into LC organogelators, mesomorphic properties were ascertained by polarized optical microscopy (POM) and differential scanning calorimetry (DSC). Characteristic textures and defect structures, which change when passing a phase transition, reveal the phase type as observed by POM.8 Compound 2(6) is in the liquid state at room temperature and compounds 3(5) and 4(5) do not show any mesophase on cooling. Compounds 1(5) and 1(6) show similar textures for their mesophases (Fig. 1 and Fig. S1, ESI†). The phase transition temperatures and thermodynamic data of compounds 1(5), 1(6) and 3(6) are collected in Fig. S2 and Table S1 (ESI†).
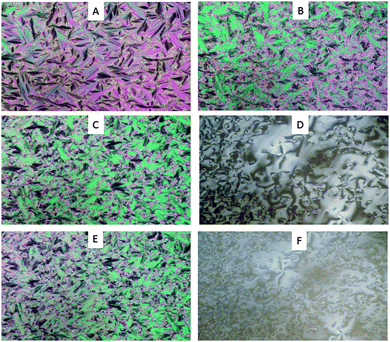 | ||
| Fig. 1 Polarized photomicrographs of compound 1(5) on cooling to: (A) 120 °C, (B) 110 °C, (C and D) 100 °C, (E and F) 90 °C. | ||
On cooling compound 1(5), a typical focal conic fan texture was observed at 163 °C under homogeneously aligned glass surfaces which were maintained at 120 °C (Fig. 1A), and then the texture changed to a broken-fan texture at 112 °C (Fig. 1B). Both mesophases have an orthogonal characteristic. These results suggest that compound 1(5) shows two kinds of smectic A phases (SmA) which were named SmA(2) and SmA(1). The enthalpy change of SmA(1)–SmA(2) also supports the assignment of the SmA.9 At 100 °C, a schlieren texture was observed with homeotropic alignment (Fig. 1D), suggesting that the mesophase should be assigned to a smectic C phase (SmC). The enthalpy change is consistent with the assignment of SmC–SmA.10 However, the lower tilted phase was unidentified. The similar results for compound 1(6) are shown in Table S1 (ESI†) while compound 3(6) only showed SmA with a narrow range of temperature from 106 °C to 108 °C.
To clarify a relationship between the molecular structure and gelation ability, the gelation abilities of compounds 1(n)–4(5) as LMWOGs in various organic solvents (polar and non-polar) and H2O were investigated in a heating–cooling cycle by the “stable to inversion in a test tube” method.11 Compounds 1(5) and 1(6) as LMWOGs can gelate solvents like cyclohexane, octane, 1-octanol, DMF and butyrolactone. Compounds 1(5) and 1(6) tend to precipitate in petroleum ether, acetonitrile, acetone, methanol and ethanol, while they dissolve in ethyl acetate, 3-pentanone, chloroform, 1,2-dichloromethane, toluene and tetrahydrofuran, and are insoluble in H2O. Unfortunately, unlike compounds 1(n), the compounds 2(n), 3(n) and 4(5) cannot form gels in the organic solvents tested (Table S2, ESI†). Although the chemical structures of compounds 1(5) and 1(6) are similar, their gelation abilities are different from each other. In DMSO and propylene carbonate (PC), compound 1(5) can form gels with critical gel concentrations (CGCs) of 2.0 wt% and 1.0 wt% respectively, while compound 1(6) cannot gelate at 5.0 wt% in DMSO and PC.
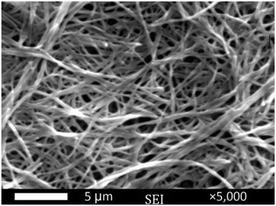
To gain visual insights into the aggregation mode, we selected the gel which was formed by compound 1(5) in PC as a sample to study by scanning electron microscopy (SEM) (Fig. 2). The SEM image showed that three-dimensional (3D) nanofiber networks are formed by the self-assembly of compound 1(5) molecules.
The essential driving force for gelation of compounds 1(n) was also studied by IR and 1H NMR. Infrared spectra of the xerogels of compounds 1(n) in cyclohexane and of pure compounds 1(n) were carried out and are shown in Fig. S3 (ESI†). Pure compounds and xerogels present the same infrared spectral images. The 1H NMR spectra of compound 1(5), in d-DMSO at different temperatures and concentrations in Fig. S4 (ESI†), indicate that hydrogen bonds do not play any significant role in gel self-assembly. As reported in the past, the presence of long alkyl chains and rigid rod-like aromatic segments has been considered to be essential for stable LMOG assemblies.12 Out of compounds 1(n)–4(5), only compounds 1(n) showed gelation ability which means that the gelation by compounds 1(n) with 4-perfluoroalkylbutoxybenzene derivatives results from both the formation of π–π stacking interactions and the weaker intermolecular interactions between the perfluoroalkyl chains.13
The gelation abilities of compounds 1(n) at concentrations ranging from 5 to 1 wt% in amines and oils were also tested (Table S3, Fig. S5 and S6, ESI†). Compounds 1(5) and 1(6) could rapidly gelate primary amines (e.g. aniline, benzylamine, β-phenethylamine). The times taken for secondary amines (e.g. piperidine) to form gels at 1 wt% were longer than those of primary amines in a heating–cooling cycle at room temperature. Hexamethylphosphoric triamide (HMPA) could be gelated only by compound 1(6) at 5 wt% while N-ethyldiisopropyl amine was gelated only by compound 1(5) at the same concentration. In the gelation tests of oil, we chose synthetic lubricant, mineral oil, lamp oil, polyolefin and rape oil to study the gelation abilities of 1(5) and 1(6). Lamp oil required 5 wt%, whereas compounds 1(5) and 1(6) gelated the other oils at 1 wt%.
Amines as important organic bases are widely used in industry and may spill into nature and pollute our rivers and oceans.14 Meanwhile almost every year, accidents involving oil spills happen in oceans and rivers.15 Removal of pollutants from water is arduous but obligatory. Though approaches (e.g. adsorbents, chemical dispersants) which remove pollutants from the environment have been researched, these approaches have some limitations in practice.16 PSGs that can selectively solidify the organic layer from a biphasic mixture of water and an organic layer offer a potential and effective solution to this pollution problem. Generally PSGs with CGCs of 5 wt% show phase-selective properties.17 In the meantime compounds 1(5) and 1(6) are showing efficient gelation abilities even at 1 wt% which encouraged us to analyze their phase-selective properties. We dropped water (1 mL) into each test tube containing gel formed by compounds 1(5) and 1(6) in amines at the CGCs. The mixtures were heated and shaken sharply to ensure homogeneous dispersion of the amines in water. After cooling the mixtures to room temperature, compound 1(5) could selectively gelate the aniline, benzylamine and β-phenethylamine in these aqueous-amine biphasic systems (Fig. S7, ESI†). The gels initially formed by compound 1(6) in benzylamine, β-phenethylamine and HMPA were destroyed, but in aniline, gelation occurred again.
The ability to cause phase-selective gelation in aqueous-oil biphasic systems was also tested. Because oil spills often happen in the ocean and the pH of seawater is about 8.0, we replaced water with 1 mL of a saturated solution of NaHCO3 into which NaCl had been added until it was no longer dissolved. Similar tests to those in the aqueous-amine biphasic systems were carried out. All of the gels formed by compounds 1(n) at CGCs in synthetic lubricant, mineral oil, lamp oil, polyolefin and rape oil selectively solidified in the aqueous-oil biphasic systems after a heating–cooling cycle process (Fig. S8, ESI†). We adjusted the aqueous pH from basic to acidic by the addition of 0.5 mL of 1 M HCl(aq.) and found that compounds 1(n) still could selectively solidify the oil layer (Fig. 3).
 | ||
| Fig. 3 Photograph of gels formed in acidic solutions using compounds 1(5) (left) and 1(6) (right) as gelators. (A) Synthetic lubricant, (B) mineral oil, (C) lamp oil, (D) polyolefin, (E) rape oil. | ||
Compounds 1(n) display excellent selective gelation ability with CGCs of 1% in the aqueous-aniline and aqueous-oil biphasic systems after a heating–cooling process. This inspired us to research their selective gelation abilities at room temperature. We prepared a solution of 10 wt% of compound 1(5) in toluene by heating. The warm toluene solution was dropped into an aqueous-aniline biphasic mixture until the weight percentage of compound 1(5) in the organic layer decreased to 5 wt%. Then the mixture was shaken sharply to make sure that the aniline could mix with the solution. To our surprise, the solution of compound 1(5) could quickly and selectively solidify aniline from the biphasic mixture. Compound 1(6) presented the same result as compound 1(5) (Fig. S9, ESI†).
Biphasic systems (aqueous/synthetic lubricant = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also researched with the same process. The organic layer was quickly and selectively gelated in 1 min. Unfortunately there was a sharp slowdown in the gelation speed when the toluene solution of compound 1(5) was at room temperature. We propose that a warm toluene solution of 10 wt% of compound 1(5) should be dropped into the biphasic systems. It seems that a rapid cooling process quickly forms the 3D nanofiber networks.18 We replaced compound 1(5) with compound 1(6) and found the same result as for compound 1(5). All of the gels formed by dropping toluene solutions retained their gel states for at least three months.
1) were also researched with the same process. The organic layer was quickly and selectively gelated in 1 min. Unfortunately there was a sharp slowdown in the gelation speed when the toluene solution of compound 1(5) was at room temperature. We propose that a warm toluene solution of 10 wt% of compound 1(5) should be dropped into the biphasic systems. It seems that a rapid cooling process quickly forms the 3D nanofiber networks.18 We replaced compound 1(5) with compound 1(6) and found the same result as for compound 1(5). All of the gels formed by dropping toluene solutions retained their gel states for at least three months.
Compound 1(5) at 10 wt% successfully gelated in toluene after overnight storage, but it failed to gelate at 5 wt%. The morphology of the xerogel gelated by compound 1(5) at 10 wt% was investigated by SEM. There were many 3D nanofiber networks (Fig. 4A), although at the same time, clusters (Fig. 4B) and zero-dimensional (0D) nano dots (Fig. 4C) were also found in the same sample at different places. We propose that the stability of the gel formed by compound 1(5) at 10 wt% is not very good.
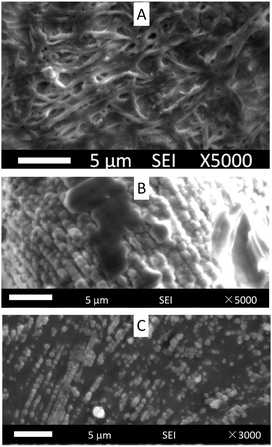 | ||
| Fig. 4 SEM images of compound 1(5) in toluene (10 wt%). (A) 3D nanofiber networks, (B) cluster and (C) 0D nano dots. | ||
In summary, to the best of our knowledge, new liquid crystalline low molecular weight organogelators based on 4-perfluoroalkylbutoxybenzene derivatives without hydrogen bonding have been synthesized for the first time. The gelation abilities of compounds 1(n) are due to π–π stacking interactions and the weaker intermolecular interaction around the perfluoroalkyl chains. Interestingly and importantly, the gelators can selectively gelate organic layers not only in aqueous-amine but also in aqueous-oil systems. Of particular note is the efficient and rapid gelation by toluene solutions of compounds 1(n) in biphasic systems at room temperature. In aqueous-oil systems, compounds 1(n) can show phase-selective properties at extremes of pH. 4-Perfluoroalkylbutoxybenzene derivatives as liquid crystalline physical gels could offer expanded applications in environmental recovery.
Acknowledgements
We are grateful to the Industrial Technology Research Grant Program in 2006 from New Energy and Industrial technology Development Organization (NEDO) of Japan, Kumagai Foundation for Science and Technology, Electric Technology Research Foundation of Chugoku, The Yamagin Regional Enterprise Support Foundation and ISIJ Research Promotion Grant, for financial support. We also extend our thanks to Cosmo Oil Lubricants Co., Ltd for a gift of synthetic lubricant and mineral oil.Notes and references
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS PubMed; K. K. Kartha, R. D. Mukhopadhyay and A. Ajayaghosh, Chimia, 2013, 67, 51–63 CrossRef PubMed; K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellam and M. Marlow, Soft Matter, 2014, 10, 237–256 RSC; K. Skilling, A. Ndungu, B. Kellam, M. Ashford, T. Bradshaw and M. Marlow, J. Mater. Chem. B, 2014, 2, 8412–8417 RSC; S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef PubMed; X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef PubMed.
- Y. Wang, L. Tang and J. Yu, J. Colloid Interface Sci., 2008, 319, 357–364 CrossRef CAS PubMed; J. Liu, J. Ma and C. Chen, Tetrahedron, 2011, 67, 85–91 CrossRef.
- J. Mamiya, K. Kanie, T. Hiyama, T. Ikeda and T. Kato, Chem. Commun., 2002, 1870–1871 RSC; M. Hashimoto, S. Ujiie and A. Mori, Adv. Mater., 2003, 15, 797–800 CrossRef CAS; K. Kubo, A. Mori, S. Ujiie and C. Tschierske, J. Oleo Sci., 2004, 53, 575–579 CrossRef; M. Yoshio, R. Konishi, T. Sakamoto and T. Kato, New J. Chem., 2013, 37, 143–147 RSC; A. Zep, M. Salamonczyk, N. Vaupotič, D. Pociecha and E. Gorecka, Chem. Commun., 2013, 49, 3119–3121 RSC; S. Bhattacharya and Y. K. Ghosh, Chem. Commun., 2001, 185–186 RSC; H. Yu, B. Liu, Y. Wang, J. Wang and Q. Hao, Soft Matter, 2011, 7, 5113–5115 RSC; V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef PubMed.
- S. R. Jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., 2010, 122, 7861–7864 CrossRef CAS; S. Basak, J. Nanda and A. Banerjee, J. Mater. Chem., 2012, 22, 11658–11664 RSC; A. Prathap and K. M. Sureshan, Chem. Commun., 2012, 48, 5250–5252 RSC; R. Rajaganesh, A. Gopal, T. Mohan Das and A. Ajayaghosh, Org. Lett., 2012, 14, 748–751 CrossRef PubMed; S. Mukherjee, C. Shang, X. Chen, X. Chang, K. Liu, C. Yu and Y. Fang, Chem. Commun., 2014, 50, 13940–13943 RSC; V. A. Mallia, D. L. Blair and R. G. Weiss, Ind. Eng. Chem. Res., 2016, 55, 954–960 CrossRef.
- T. Steiner and W. Saenger, J. Am. Chem. Soc., 1993, 115, 4540–4547 CrossRef CAS.
- M. Hird, Chem. Soc. Rev., 2007, 36, 2070–2095 RSC; R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Chem. Soc. Rev., 2011, 40, 3496–3508 RSC; E. Krieg, H. Weissman, E. Shimoni, A. Bar On and B. Rybtchinski, J. Am. Chem. Soc., 2014, 136, 9443–9452 CrossRef CAS PubMed; S. Prasanthkumar, W. Zhang, W. Jin, T. Fukushima and T. Aida, Angew. Chem., 2015, 127, 11320–11324 CrossRef.
- H. Okamoto, H. Murai and S. Takenaka, Bull. Chem. Soc. Jpn., 1997, 70, 3163–3166 CrossRef CAS; H. Okamoto, N. Yamada and S. Takenaka, J. Fluorine Chem., 1998, 91, 125–132 CrossRef; M. Yano, T. Taketsugu, K. Hori, H. Okamoto and S. Takenaka, Chem. – Eur. J., 2004, 10, 3991–3999 CrossRef PubMed; T. Yoshida, T. Hirakawa, T. Nakamura, Y. Yamada, H. Tatsuno, Y. Morita and H. Okamoto, ECS Trans., 2013, 50, 95–102 CrossRef; T. Yoshida, T. Nakamura, Y. Morita and H. Okamoto, Chem. Lett., 2015, 44, 512–514 CrossRef; T. Yoshida, T. Hirakawa, T. Nakamura, Y. Yamada, H. Tatsuno, M. Hirai, Y. Morita and H. Okamoto, Bull. Chem. Soc. Jpn., 2015, 88, 1447–1452 CrossRef.
- I. Dierking, Textures of liquid crystals. Editor, John Wiley & Sons, New York, 2006, pp. 33–34 Search PubMed.
- Y. Park, T. Lubensky, P. Barois and J. Prost, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 37, 2197 CrossRef.
- J. C. Roberts, N. Kapernaum, Q. Song, D. Nonnenmacher, K. Ayub, F. Giesselmann and R. P. Lemieux, J. Am. Chem. Soc., 2010, 132, 364–370 CrossRef CAS PubMed.
- J. G. Hardy, A. R. Hirst, D. K. Smith, C. Brennan and I. Ashworth, Chem. Commun., 2005, 385–387 RSC.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3160 CrossRef CAS PubMed; S. Bhattacharya and S. G. Acharya, Chem. Mater., 1999, 11, 3121–3132 CrossRef; H. J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2010, 44, 72–82 CrossRef PubMed.
- S. S. Babu, V. K. Praveen, S. Prasanthkumar and A. Ajayaghosh, Chem. – Eur. J., 2008, 14, 9577–9584 CrossRef CAS PubMed; D. M. Ryan, T. M. Doran and B. L. Nilsson, Langmuir, 2011, 27, 11145–11156 CrossRef PubMed; S. Prasanthkumar, W. Zhang, W. Jin, T. Fukushima and T. Aida, Angew. Chem., Int. Ed., 2015, 54, 11168–11172 CrossRef PubMed.
- D. Han, H. Yan and K. H. Row, J. Sep. Sci., 2011, 34, 1184–1189 CrossRef CAS PubMed.
- A. Hamad, E. Al-Zubaidy and M. E. Fayed, J. Environ. Manage., 2005, 74, 153–159 CrossRef CAS PubMed; A. Willing, Chemosphere, 2001, 43, 89–98 CrossRef PubMed; I. B. Ivshina, M. S. Kuyukina, A. V. Krivoruchko, A. A. Elkin, S. O. Makarov, C. J. Cunningham, T. A. Peshkur, R. M. Atlas and J. C. Philp, Environ. Sci.: Processes Impacts, 2015, 17, 1201–1219 Search PubMed.
- X. Zhang, J. Song, W. Ji, N. Xu, N. Gao, X. Zhang and H. Yu, J. Mater. Chem. A, 2015, 3, 18953–18962 CAS.
- Y. Ma, M. Deng, C. Liang and S. Jiang, Soft Matter, 2015, 11, 5059–5100 Search PubMed.
- N. Kusukawa, M. V. Ostrovsky and M. M. Garner, Electrophoresis, 1999, 20, 1455–1461 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra; polarized photomicrographs; differential scanning calorimetry; IR spectra. See DOI: 10.1039/c6nj00741d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |