 Open Access Article
Open Access ArticlePlatinum(II) complexes with hybrid amine-imidazolin-2-imine ligands and their reactivity toward bio-molecules†
Jovana
Bogojeski
*a,
Jeroen
Volbeda
b,
Živadin D.
Bugarčić
a,
Matthias
Freytag
b and
Matthias
Tamm
b
aFaculty of Science, University of Kragujevac, R. Domanovića 12, P. O. Box 60, 34000 Kragujevac, Serbia. E-mail: jrosic@kg.ac.rs; Fax: +381 (0)34335040; Tel: +381 (0)34336223
bInstitut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Hagenring 30, 38106 Braunschweig, Germany
First published on 15th March 2016
Abstract
Two new Pt(II) complexes with imidazolin-2-imines as ancillary ligands, [Pt(DMEAImiPr)Cl2] and [Pt(DPENImiPr)Cl2], were synthesized and characterized. Substitution reactions of these complexes with nucleophiles – thiourea (TU), L-methionine (L-Met), L-histidine (L-His) and guanosine-5′-monophosphate (5′-GMP) – were carried out in 25 mM Hepes buffer in the presence of 30 mM NaCl. The reactions were monitored using variable-temperature UV-Vis spectrophotometry and were followed under pseudo-first-order conditions with a large excess of nucleophiles. A slightly higher reactivity was found for [Pt(DMEAImiPr)Cl2], while the reactivity of the nucleophiles decreased in the order TU > L-Met > L-His > 5′-GMP. The negative values reported for the entropy of activation confirmed an associative substitution mode. Spectrophotometric acid–base titrations were performed to determine the pKa values of the coordinated water molecules in the diaqua complexes [Pt(DMEAImiPr)(H2O)2]2+ and [Pt(DPENImiPr)(H2O)2]2+. Solubility measurements revealed good solubility of the studied imidazolin-2-imine complexes in water. The crystal structure of [Pt(DMEAImiPr)Cl2] was determined by X-ray diffraction analysis. The coordination geometries around the platinum atoms are distorted square-planar; the [Pt(DMEAImiPr)Cl2] complex displays Pt–N distances of 2.0162(19) and 2.0663(19) Å. Attempts to coordinate Au(III) ions to different imidazolin-2-imine ligands did not result in the formation of coordination complexes, but rather in the reduction of the Au(III) precursor. This was evidenced by the X-ray crystal structure of [(DACH(ImiPrH)2)(AuCl2)2], which formed during the reaction of KAuCl4 with the ligand DACH(ImiPr)2.
1. Introduction
The platinum group metals, Pt(II), Pd(II) and Au(III), are valence isoelectronic and often form characteristic isostructural square-planar complexes.1 Accordingly, the chemical behavior in solution of structurally analogous Pt(II) and Pd(II) complexes is very similar.2,3 Since the late 1970s, platinum(II) complexes have been well-known for their anti-tumor activity.4,5 In the past 50 years a vast variety of structurally different platinum complexes were synthesized and tested, but platinum drug resistance and toxic side effects represent a limiting factor and continuing challenge.4–8 The research field of platinum complexes as anti-tumor drugs is still growing and going in the direction of designing novel platinum drugs with distinctly different structural and mechanistic profiles in comparison with cisplatin. In addition, research has been focused on the synthesis and investigation of complexes of different metal ions with the goal to establish compounds with good anti-tumor properties but with no side-effects and lower resistance compared to cisplatin.6–8 In recent years, the anti-tumor activity of some Pd(II) complexes has been confirmed,9–11 and also square-planar Au(III) compounds have shown to be excellent candidates for anticancer evaluation.12,13Factors that influence the properties of the complexes such as geometry, steric hindrance, flexibility, electronic effects, and lipophilicity are important to be considered during drug design. In an earlier publication,14 we were able to show that the use of hybrid amine-imidazolin-2-imines as strong N-donor ligands15–17 afforded Pd(II) complexes with improved properties such as water solubility and lower reactivity toward small bio-molecules, which should lead to a more selective distribution of these complexes in the human body.14 Therefore, it was of interest to introduce imidazolin-2-imines in the coordination sphere of Pt(II) and Au(III) complexes and follow the way how this will affect the characteristics of such complexes under physiological conditions as well as their reactivity towards small bio-molecules.
In this paper, we present the synthesis of imidazolin-2-imine Pt(II) complexes and their interactions with small bio-molecules, in an attempt to define preliminary structure–function relationships within this new class of imidazolin-2-imine complexes. In addition, we report attempts to prepare imidazolin-2-imine Au(III) complexes.
2. Results and discussion
2.1. Pt(II) imidazolin-2-imine complexes
Orange single-crystals of [Pt(DMEAImiPr)Cl2] suitable for X-ray diffraction analysis were obtained from chloroform/diethyl ether solution, and the molecular structure is shown in Fig. 2. The molecule crystallizes in the monoclinic space group P21/n, with two co-crystallized molecules of CHCl3 per unit. The diimine ligand is coordinated to the platinum(II) ion in a chelating, bidentate fashion with an N1–Pt–N2 bite angle of 83.04(8)°. The Pt–N bond lengths are 2.0162(19) Å and 2.0663(19) Å for Pt–N1 and Pt–N2, respectively, indicating stronger coordination of the more basic imine nitrogen to the Pt atom. The electron-donating capacity of imidazolin-2-imine is also reflected in the different Pt–Cl bond lengths, with the Pt–Cl1 (2.3368(6) Å) trans to the imine nitrogen being notably longer than the Pt–Cl2 (2.3076(5) Å) trans to the tertiary amine. These structural parameters are comparable to those observed for [Pd(DMEAImiPr)Cl2], [Pd(DPENImiPr)Cl2] and [Pd(BLiPr)Cl2].14,19
 | ||
| Fig. 1 Structures of the investigated Pt(II) complexes and nucleophiles, along with their abbreviations. | ||
The pKa values of the complexes in aqueous solution were determined. This was performed via UV-Vis spectrophotometric pH titration with NaOH as a base in the pH range between 2 and 12. Each pKa titration was performed twice and the average of both values was taken. Fig. 3 and Fig. S2 (ESI†) show plots of absorbance versus pH at specific wavelengths, which were used to determine the pKa values of the coordinated water molecules. The data were fitted using a nonlinear least-squares procedure, as shown in the insets in Fig. 3 and Fig. S2 (ESI†). The overall process can be represented by eqn (2) and (3). The titration data for the complexes were fitted to the following eqn (1) for the determination of both pKa values,14,20–22 and the obtained data are presented in Table 2.
| y = a + (b − a))/(1 + 2.718 × ((x − pKa1/m) + (c − b)/(1 + 2.718 × ((x − pKa2)/n)) | (1) |
 | ||
| Fig. 3 UV-vis spectra recorded for 0.1 mM [Pt(DMEAImiPr)(H2O)2]2+ in the pH range of 2 to 12 at 25 °C. Inset: The plot of absorbance vs. pH at 230 nm. | ||
The parameter a represents the value of the absorbance at the beginning of the titration, b represents the absorbance during the titration and c is the absorbance at the end of the titration. The parameters m and n are used to optimize the titration curve. In this equation y represents the absorbance value and x refers to the pH. The data obtained for the pKa values are summarized in Table 2.
 | (2) |
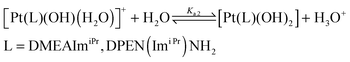 | (3) |
The substitution reactions of square-planar metal complexes can proceed according to two parallel pathways.26 One involves the formation of a solvent-coordinated complex, e.g. a diaqua complex, followed by rapid substitution of the coordinated solvent by the entering nucleophile (solvolytic pathway), whilst the other involves a direct nucleophilic attack by the entering nucleophile. To suppress the solvolytic pathway, a 30 mM NaCl solution was added (see Fig. S3, ESI†). The rate constants for substitution could be determined, under pseudo-first-order conditions from a plot of the linear dependence of kobsdversus the total nucleophile concentration, according to eqn (4) and (5). The slope of the line represents k1 or k2, whilst the intercept represents k−1[Cl−] or k−2[Cl−]. The results are summarized in Table 3 and Table S10 (ESI†).
| kobsd1 = k1[Nu] + k−1[Cl−] | (4) |
| kobsd2 = k2[Nu] + k−2[Cl−] | (5) |
| [Pt(DMEAImiPr)Cl2] | [Pt(DPENImiPr)Cl2] | |||
|---|---|---|---|---|
| First step k1 [M−1 s−1] | Second step k2 [M−1 s−1] | First step k1 [M−1 s−1] | Second step k2 [M−1 s−1] | |
| TU | (41.10 ± 0.10) × 10−2 | (6.00 ± 0.10) × 10−2 | (20.90 ± 0.20) × 10−2 | (4.88 ± 0.20) × 10−2 |
| L-Met | (14.30 ± 0.20) × 10−2 | — | (11.90 ± 0.10) × 10−2 | — |
| L-His | (3.50 ± 0.10) × 10−3 | (3.00 ± 0.10) × 10−4 | (2.80 ± 0.10) × 10−3 | (2.16 ± 0.20) × 10−4 |
| 5′-GMP | (3.30 ± 0.10) × 10−3 | (2.91 ± 0.20) × 10−4 | (2.59 ± 0.20) × 10−3 | (1.59 ± 0.10) × 10−4 |
Nu = TU, L-Met, L-His and 5′-GMP
Fig. 4 shows the dependence of kobsd on the nucleophile concentration for the [Pt(DMEAImiPr)Cl2] complex (see also Fig. S4 and S5, ESI†).
[Pt(DMEAImiPr)Cl2] reacts faster than [Pt(DPENImiPr)Cl2] which is in accordance with the order of reactivity observed for analogous Pd(II) complexes.14 The second substitution step is slower than the first substitution for both complexes. It can be assumed that the first substitution would take place next to the less sterically hindered side of the ligand viz. next to the amine donor, while, the second substitution would have to take place next to the bulky imidazolin-2-imine donor. In addition, the first substitution would result in a less electrophilic Pt(II) center, reducing the reaction rate for the second substitution.
The order of the reactivity of the investigated nucleophiles for the first reaction step is: TU > L-Met > L-His > 5′-GMP (Table 3). Thus, the sulfur-donor nucleophiles react faster with Pt(II) complexes than the nitrogen-donor nucleophiles.
The order of the reactivity for the second substitution step is: TU > L-His > 5′-GMP. Kinetic traces for reactions with L-Met gave fits with a double exponential function. However, when the constants, kobsd1 and kobsd2 were plotted against the concentration of the entering L-Met, it was observed that kobsd1 shows a linear dependence on the nucleophile concentration, while kobsd2 was found to be independent of the L-Met concentration, suggesting a chelate formation process as presented in Scheme 2 and Fig. 5.
 | ||
| Fig. 5 Plots of kobsdversusL-Met concentration for the [Pt(DMEAImiPr)Cl2] complex (pH = 7.2, 310 K, 25 mM Hepes buffer, 30 mM NaCl). | ||
The substitution reactions of the investigated Pt(II) complexes with L-Met proceed as shown in Scheme 2 with the formation of a six-membered ring via the sulphur and nitrogen atoms of L-Met. Ring-closure and formation of a six-membered ring also occur in the substitution reactions of analogous Pd(II) complexes with L-Met.14 To confirm that the second step is chelation, the kinetics were studied using the Pt(II) complexes in excess rather than L-Met. This would mean that a two-step reaction can only occur if ring closure is involved. The obtained kinetic traces for such reactions gave fits to a double exponential function. Similar values for the rate constants were obtained, as were observed in the experiments in which L-Met was added in excess (see Fig. S6, ESI†).
The activation parameters ΔH≠ and ΔS≠ (Table S1, ESI†) were calculated using the Eyring equation for the reactions with TU for the first and second reaction step. The activation parameters support an associative mechanism for each of these reactions which is supported by the significantly negative activation entropies.
The Pd(II) imidazolin-2-imine complexes shows a huge slowdown in reactivity compared with other Pd(II) complexes. Thus, [Pd(DMEAImiPr)Cl2] and [Pd(DPENImiPr)Cl2] react 100 times slower than [Pd(en)Cl2] (en = ethylenediamine) and their reactivity approaches the reactivity of Pt(II) aqua complexes.14 However, the analogous Pt(II) complexes do not show such a pronounced decrease in reactivity as they react only 1.5–2.0 times slower compared to [Pt(en)Cl2], Table 4.
| L-His × 103k1 [M−1 s−1] | 5′-GMP × 103k1 [M−1 s−1] | |
|---|---|---|
| [Pt(en)Cl2]27 | 7.90 ± 0.70 | 4.40 ± 0.30 |
| [Pt(DMEAImiPr)Cl2] | 3.50 ± 0.10 | 3.30 ± 0.10 |
| [Pt(DPENImiPr)Cl2] | 2.80 ± 0.10 | 2.59 ± 0.20 |
The observed intercepts in Fig. 4 and Fig. S4, S5 (ESI†) are ascribed to the back reaction with the excess of chloride present in solution. The obtained values of these rate constants are summarized in Table S10 (ESI†) and they are much smaller in comparison to the values of rate constants for the direct reactions.
2.2. Au(III) and imidazolin-2-imines
Gold(III) complexes can be significantly stabilized, even at neutral pH, by the appropriate choice of the ligand, preserving its interesting biological properties. It was shown that the presence of at least two nitrogen donors directly coordinated with the Au(III) center leads to a significant decrease in the redox potential of such complexes.12,13,28 Keeping this in mind, we attempted to use imidazolin-2-imines as spectator ligands in the synthesis of Au(III) complexes and to find out whether these ligands can stabilize Au(III) ions. However, the isolation of stable complexes was not achieved.The only stable product which we were able to isolate is the product of the reaction between the bis(imidazolin-2-imine) ligand DACH(ImiPr)218 and KAuCl4 in THF. The product of this reaction was obtained as a yellow solid, 1H and 13C NMR spectroscopy shows characteristic signals of the ligand18 (see ESI†).
Yellow crystals suitable for X-ray diffraction analysis were isolated from a chloroform/diethyl ether solution, and the molecular structure of [(DACH(ImiPrH)2)](AuCl2)2 is shown in Fig. 6. The molecule crystallizes in the monoclinic space group P21 as a chloroform solvate. The protonated ligand shows H⋯Cl contacts with the AuCl2 counterions of 2.54(7) Å (H01–Cl1) and 2.46(8) Å (H02–Cl4). In addition, the protonation of the imine nitrogen atoms causes clear delocalization of the imine double bond, which is indicated by the C–N bond distances of the imines of 1.381(6) Å (C7–N1) and 1.373(6) Å (C18–N2).
Apparently, the reaction between DACH(ImiPr)2 and KAuCl4 leads to reduction of Au(III) to Au(I), indicating that electron-rich imidazolin-2-imine ligands are not suited for the complexation of Au(III). This reactivity can be ascribed to the reducing nature of the imidazolin-2-imine ligands and the oxidizing nature of Au(III), this is also in agreement with the redox properties reported for guanidine-type ligands.29–32
3. Conclusion
In this study, two novel platinum(II) complexes with mono(imidazolin-2-imines) were synthesized and characterized; for [Pt(DMEAImiPr)Cl2], a crystal structure could be determined. Furthermore, the influence of the imidazolin-2-imine ligands on the solubility, acid–base characteristics and reactivity towards bio-molecules of these complexes were studied. We performed spectrophotometric acid–base titrations to determine the pKa values for the coordinated water ligands in the respective diaqua complexes. In general, we found two pKa values for both studied Pt(II) complexes. Solubility measurements have shown that Pt(II) complexes with imidazolin-2-imines revealed good solubility in water, which is higher than that of cisplatin and oxaliplatin. The performed kinetic measurements have shown that the complex [Pt(DMEAImiPr)Cl2] reacts faster than [Pt(DPENImiPr)Cl2]. The reactivity of the studied bio-molecules decreases in the order TU > L-Met > L-His > 5′-GMP. The investigated nitrogen-donor bio-molecules react with the Pt(II) complexes in two successive reaction steps that are both dependent on the nucleophile concentration. However, L-methionine reacts by forming a six-membered ring via its sulphur and nitrogen atoms. The negative values reported for the entropy of activation confirmed an associative substitution mode. The Pd(II) imidazolin-2-imine complexes show a large decrease of reactivity compared with other Pd(II) complexes, and their reactivity approaches that of Pt(II) aqua complexes. However, the analogous Pt(II) complexes do not show such a pronounced decrease of reactivity as they react 1.5–2.0 times slower compared to [Pt(en)Cl2]. Our attempts to coordinate Au(III) ions to imidazolin-2-imines were unsuccessful; instead, reduction to Au(I) was observed.4. Experimental
4.1. Chemicals and solutions
Thiourea, L-methionine, L-histidine, guanosine-5′-monophosphate sodium salt, N,N-dimethylethylenediamine, (1S,2S)-(−)-1,2-diphenylethylenediamine, (1R,2R)-(−)-1,2-diaminocyclohexane, NaBF4, KF, NaNH2, KOtBu, K2PtCl4 and KAuCl4 were obtained from Acros Organics or Sigma Aldrich, and were used without further purification. Hepes buffer (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) was obtained from Sigma Aldrich. All the other chemicals were of the highest purity commercially available and were used without further purification. Ultra-pure water was used in all experiments. Nucleophile stock solutions were prepared shortly before use by dissolving the chemicals.4.2. Preparation of the complexes
All reactions were performed in a glove box, under a dry argon atmosphere (MBraun 200B) or on a high-vacuum line using standard Schlenk techniques, unless noted otherwise. Commercial grade solvents were purified by use of a solvent purification system from MBraun GmbH and stored over molecular sieves (4 Å) under a dry argon atmosphere. The ligands, DMEAImiPr, DPENImiPr and DACH(ImiPr)2 were prepared according to the literature procedure.14,18
1H NMR (300 MHz; CDCl3): δ = 5.41 (sept, 2H, JHH 7.2 Hz, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 2.93 (s, 6H, N(C
Me2), 2.93 (s, 6H, N(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 2.75 (t, 2H, JHH 4.5 Hz, C
3)2), 2.75 (t, 2H, JHH 4.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC
NC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH2), 2.45 (t, 2H, JHH 4.5 Hz, CH2C
2CH2), 2.45 (t, 2H, JHH 4.5 Hz, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2NMe2), 2.10 (s, 6H, CC
2NMe2), 2.10 (s, 6H, CC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.55 (d, 6H, JHH 7.0 Hz, CH(C
3), 1.55 (d, 6H, JHH 7.0 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.43 (d, 6H, JHH 7.0 Hz, CH(C
3)2), 1.43 (d, 6H, JHH 7.0 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2) ppm.
3)2) ppm.
13C NMR (100 MHz; CDCl3): δ = 151.8 (N2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 119.2 (
N), 119.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Me), 67.5 (CH2
Me), 67.5 (CH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2NMe2), 51.5 (C
H2NMe2), 51.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH2), 50.8 (N(
H2CH2), 50.8 (N(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 48.1 (
H3)2), 48.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HMe2), 22.4 (CH(
HMe2), 22.4 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 21.9 (CH(
H3)2), 21.9 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 10.3 (C
H3)2), 10.3 (C![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3) ppm.
H3) ppm.
Anal. calcd for (C15H30Cl2N4Pt) C: 33.84; H: 5.68; N: 10.52. Found: C: 33.53; H: 5.49; N: 10.11.
1H NMR (300 MHz; CDCl3): δ 7.37–6.85 (m, 10H, HAr), 5.29 (sept, 2H, JHH 7.0 Hz, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 4.96 (d, 1H, JHH 9.0 Hz, NH2HC
Me2), 4.96 (d, 1H, JHH 9.0 Hz, NH2HC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (Ph)CH), 4.32 (d, 1H, JHH 9.0 Hz, CNHC
(Ph)CH), 4.32 (d, 1H, JHH 9.0 Hz, CNHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (Ph)CH), 2.14 (s, 6H, CC
(Ph)CH), 2.14 (s, 6H, CC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 2.10 (s, 2H, N
3), 2.10 (s, 2H, N![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2), 1.64 (d, 3H, JHH 7.1 Hz, CH(C
2), 1.64 (d, 3H, JHH 7.1 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2), 1.43(d, 3H, JHH 7.1 Hz, CH(C
3)2), 1.43(d, 3H, JHH 7.1 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2), 1.03 (d, 3H, JHH 7.1 Hz, CH(C
3)2), 1.03 (d, 3H, JHH 7.1 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2), 0.84 (d, 3H, JHH 7.1 Hz, CH(C
3)2), 0.84 (d, 3H, JHH 7.1 Hz, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2), ppm.
3)2), ppm.
13C NMR (100 MHz; CDCl3): δ 156.8 (N2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 140.1 (ipso-
N), 140.1 (ipso-![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar(CHNH2)), 133.2 (ipso-
Ar(CHNH2)), 133.2 (ipso-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) Ar(CHN), 125.5 (
Ar(CHN), 125.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar), 123.1 (
Ar), 123.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar), 122.2 (
Ar), 122.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar), 124.7 (m-
Ar), 124.7 (m-![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar(CHNH2)), 124.2 (m-
Ar(CHNH2)), 124.2 (m-![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar(CHNH), 123.9 (
Ar(CHNH), 123.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Ar), 119.8 (
Ar), 119.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) Me) 73.5 (CNH
Me) 73.5 (CNH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(Ph)CH), 60.4 (NH2H
H(Ph)CH), 60.4 (NH2H![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(Ph)CH), 49.1 (
H(Ph)CH), 49.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HMe2), 48.0 (
HMe2), 48.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HMe2), 22.8 (CH(
HMe2), 22.8 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 21.9 (CH(
H3)2), 21.9 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 21.3 (CH(
H3)2), 21.3 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.1 (CH(
H3)2), 20.1 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 11.2 (C
H3)2), 11.2 (C![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 10.8 (C
H3), 10.8 (C![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3) ppm.
H3) ppm.
Anal. calcd for (C25H34Cl2N4Pt) C: 45.73; H: 5.22; N: 8.53. Found: C: 45.96; H: 5.37; N: 8.71.
4.3. Instrumentation and measurements
NMR spectra were recorded on Bruker DPX 200 and AV 300 devices. Chemicals shifts (δ) are reported in ppm and referenced to tetramethylsilane. Coupling constants (J) are reported in Hertz (Hz) and splitting patterns are indicated as s (singlet), d (doublet), t (triplet), sept (septet), bs (broad signal) and m (multiplet). Elemental analyses (C, H, N) were performed by combustion and gas chromatographic analysis using an Elementar Vario MICRO elemental analyzer. High resolution electron spray ionization (ESI) mass spectroscopy was performed on a Finnigan MAT 95 XL Trap device. pH measurements were carried out using a Mettler Delta 350 digital pH meter with a resolution of ±0.01 mV, equipped with a combination glass electrode. This electrode was calibrated using standard buffer solutions of pH 4, 7 and 9 obtained from Sigma. Kinetic measurements of the Pt(II) complex were carried out on a PerkinElmer Lamda 25 and 35 double-beam spectrophotometer in thermostated 1.00 cm quartz Suprasil cells. The temperature was controlled to ±0.1 °C. All kinetic measurements were performed under pseudo-first-order conditions, i.e., at least a 10-fold excess of the nucleophile was used.4.4. Determination of the pKa value of the Pt(II) complexes
Spectrophotometric pH titrations of the solutions of the complexes were performed with NaOH as a base at 298 K. To avoid absorbance corrections due to dilution, a large volume (300 mL) of the complex solution was used in the titration. The change in pH from 2 to approximately 3 was achieved by addition of known amounts of crushed pellets of NaOH. The consecutive pH changes were obtained by adding drops of saturated solutions of NaOH, 1 or 0.1 M, using a micropipette. To avoid contamination caused by the pH electrode, it was necessary to take 2 mL aliquots from the solution into narrow vials for pH measurements. The aliquots were discarded after the measurements. The total reversibility of the titration could be achieved by subsequent addition of HClO4.4.5. Solubility measurements
The concentrations of saturated solutions of the studied Pt(II) complexes were determined by UV-Vis spectrophotometry. The specific absorptivity of the compounds in water was determined first. This was measured using five dilution series (5, 10, 30, 40, and 50 mM) of the studied complexes, and then the calibration curve was calculated using the Lambert–Beer law. The slope of the curve gave specific absorptivity.The required quantity of water solution was added to the 5 ml volumetric flask. The solution was heated up to 298 K. A previously weighed quantity of Pt(II) complexes was added to the volumetric flask until the saturation point occurs. Stirring was continued up to 7 hours at 298 K. The sample was filtered through a 0.20 μm membrane filter. A measured quantity of the filtered sample was transferred into another volumetric flask and further dilutions were made. The absorbance was measured using UV-Vis spectrophotometry. The same process was repeated two times.
4.6. Kinetic measurements
The kinetics of the substitution of the coordinated chlorides were followed spectrophotometrically by following the change in absorbance at suitable wavelengths as a function of time. The working wavelengths were determined by recording the spectra of the reaction mixture over the wavelength range 220 to 450 nm. All kinetic experiments were performed under pseudo-first-order conditions, for which the concentration of the nucleophile was always in at least a 20-fold excess. The reactions were initiated by mixing 0.5 ml of the Pt(II) complex solution with 2.5 ml of thermally equilibrated nucleophile solution in the UV-Vis cuvette, and reactions were followed for at least 8 half-lives. The observed pseudo-first-order rate constants, kobsd, represent an average value of two to four independent kinetic runs for each experimental condition. Some of the reactions were studied at three temperatures (288, 298 and 308 K).The experimental data are summarized in the ESI,† Tables S2–S9. The values of the constants and other thermodynamic parameters were determined using the computer programs Microsoft Excel 2007 and OriginPro 8.
4.7. X-ray diffraction studies
Data were recorded at 100(2) K using an Oxford Diffraction Eos diffractometer with monochromated Mo Kα radiation. The structures were refined anisotropically using the SHELXL-97 program.34 Hydrogen atoms were either (i) located and refined isotropically (NH), (ii) included as idealized methyl groups allowed to rotate but not tip or (iii) placed geometrically and allowed to ride on their attached carbon atoms. CCDC 1431738 and 1431739.| [Pt(DMEAImiPr)Cl2]·2(CHCl3) | [(DACH(ImiPrH)2)](AuCl2)2·(CHCl3) | |
|---|---|---|
| Empirical formula | C17H32Cl8N4Pt | C29H53Au2Cl7N6 |
| Formula weight | 771.16 | 1127.86 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21 |
| a/Å | 11.5923(3) | 11.9748(3) |
| b/Å | 16.9429(3) | 10.2420(3) |
| c/Å | 14.1236(3) | 16.6859(4) |
| β/° | 90.040(2) | 97.307(3) |
| Volume [Å3] | 2773.98(10) | 2029.84(9) |
| Z | 4 | 2 |
| Reflections collected | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 084 084 |
143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 811 811 |
| Independent reflections | 8427 [Rint = 0.0558] | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219 [Rint = 0.0786] 219 [Rint = 0.0786] |
| ρ C/g cm−3 | 1.846 | 1.845 |
| μ/mm−1 | 5.843 | 7.708 |
| R(Fo), [I > 2σ(I)] | 0.0231 | 0.0365 |
| R w (Fo2) | 0.0419 | 0.0789 |
| Goodness of fit on (F2) | 1.065 | 1.055 |
| Flack parameter | — | 0.009(5) |
| Δρ/e Å−3 | 1.530/−1.080 | 1.630/−1.486 |
Abbreviations
| en | Ethylendiamine |
| DMEAImiPr | N 2-[1,3-Diisopropyl-4,5-dimethylimidazolin-2-ylidene]-N1,N1-dimethyl-1,2-ethanediamine |
| DPENImiPr | N 1-[1,3-Diisopropyl-4,5-dimethylimidazolin-2-ylidene]-1,2-diphenyl-(1S,2S)-1,2-ethanediamine |
| DACH(ImiPr)2 | N 1,N2-Bis[1,3-diisopropyl-4,5-dimethylimidazolin-2-ylidene]-(1R,2R)-1,2-cyclohexanediamine |
| TU | Thiourea |
| L-Met | L-Methionine |
| L-His | L-Histidine |
| 5′-GMP | Guanosine-5′-monophosphate |
Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Education, Science and Technological Development Serbia, project No. 172011 and the Deutsche Forschungsgemeinschaft (DFG).References
- Inorganic Chemistry, ed. Z. E. Housecroft and A. G. Sharp, Essex, England, 2005 Search PubMed.
- Ž. D. Bugarčić, J. Bogojeski and R. van Eldik, Coord. Chem. Rev, 2015, 292, 91 CrossRef.
- Ž. D. Bugarčić, J. Bogojeski, B. Petrović, S. Hochreuther and R. van Eldik, Dalton Trans., 2012, 41, 12329–12345 RSC.
- (a) B. Rosenberg and L. V. Camp, Nature, 1965, 205, 698 CrossRef CAS PubMed; (b) B. Rosenberg, L. V. Camp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385 CrossRef CAS PubMed; (c) B. Rosenberg and L. V. Camp, Cancer Res., 1970, 30, 1799 CAS; (d) B. Rosenberg, L. V. Camp, E. B. Grimley and A. J. Thomson, J. Biol. Chem., 1967, 242, 1347 CAS.
- Cisplatin, Chemistry and Biochemistry of Leading Antitumor Drugs, ed. B. Lippert, Wiley-VCH, Zürich, 1999 Search PubMed.
- Bioinorganic Medicinal Chemistry, ed. E. Alessio, Wiley-VCH, Weinheim, 2011, ch. 1–4 Search PubMed.
- N. P. E. Barry and P. J. Sadler, Chem. Commun., 2013, 49, 5106 RSC.
- L. Ronconi and P. J. Sadler, Coord. Chem. Rev., 2007, 251, 1633 CrossRef CAS.
- A. Bechara, C. M. V. Barbosa, E. J. Paredes-Gamero, D. M. Garcia, L. S. Silva, A. L. Matsuo, F. D. Nascimento, E. G. Rodrigues, A. C. F. Caires, S. S. Smaili and C. Bincoletto, Eur. J. Med. Chem., 2014, 79, 24 CrossRef CAS PubMed.
- E. Gao, C. Liu, M. Zhu, H. Lin, Q. Wu and L. Liu, Anticancer Agents Med. Chem., 2009, 9, 356 CrossRef CAS PubMed.
- E. Gao, M. Zhu, H. Yin, L. Liu, Q. Wu and Y. Sun, J. Inorg. Biochem., 2008, 102, 1958 CrossRef CAS PubMed.
- A. Bindoli, M. Pia Rigobello, G. Scutari, C. Gabbiani, A. Casini and L. Messori, Coord. Chem. Rev., 2009, 253, 1692–1707 CrossRef CAS.
- (a) A. N. Wein, A. T. Stockhausen, K. I. Hardcastle, M. Reza Saadein, S. Peng, D. Wang, D. M. Shin, Z. Chen and J. F. Eichler, J. Inorg. Biochem., 2011, 105, 663 CrossRef CAS PubMed; (b) S. J. Berners-Price and A. Filipovska, Metallomics, 2011, 3, 863 RSC.
- J. Bogojeski, J. Volbeda, M. Freytag, M. Tamm and Ž. D. Bugarčić, Dalton Trans., 2015, 44, 17346 RSC.
- X. Wu and M. Tamm, Coord. Chem. Rev., 2014, 260, 116 CrossRef CAS.
- T. Glöge, D. Petrović, C. G. Hrib, C. Daniliuc, E. Herdtweck, P. G. Jones and M. Tamm, Z. Anorg. Allg. Chem., 2010, 636, 2303 CrossRef.
- T. K. Panda, S. Randoll, C. G. Hrib, P. G. Jones, T. Bannenberg and M. Tamm, Chem. Commun., 2007, 5007 RSC; T. K. Panda, D. Petrovic, T. Bannenberg, C. G. Hrib, P. G. Jones and M. Tamm, Inorg. Chim. Acta, 2008, 361, 2236 CrossRef CAS; T. K. Panda, A. G. Trambitas, T. Bannenberg, C. G. Hrib, S. Randoll, P. G. Jones and M. Tamm, Inorg. Chem., 2009, 48, 5462 CrossRef PubMed; A. G. Trambitas, T. K. Panda, J. Jenter, P. Roesky, C. G. Daniliuc, C. G. Hrib, P. G. Jones and M. Tamm, Inorg. Chem., 2010, 49, 2435 CrossRef PubMed; T. K. Panda, C. G. Hrib, P. G. Jones and M. Tamm, J. Organomet. Chem., 2010, 695, 2768 CrossRef; M. Tamm, A. G. Trambitas, C. G. Hrib and P. G. Jones, Terrae Rarae, 2010, 7, 1 Search PubMed; A. G. Trambitas, J. Yang, D. Melcher, C. G. Daniliuc, P. G. Jones, Z. Xie and M. Tamm, Organometallics, 2011, 30, 1122 CrossRef; A. G. Trambitas, D. Melcher, L. Hartenstein, P. W. Roesky, C. Daniliuc, P. G. Jones and M. Tamm, Inorg. Chem., 2012, 51, 6753 CrossRef PubMed.
- J. Volbeda, P. G. Jones and M. Tamm, Inorg. Chim. Acta, 2014, 422, 158 CrossRef CAS.
- J. Bogojeski, R. Jelić, D. Petrović, E. Herdtweck, P. G. Jones, M. Tamm and Ž. D. Bugarčić, Dalton Trans., 2011, 40, 6515 RSC.
- T. Soldatović, S. Jovanović, Ž. D. Bugarčić and R. van Eldik, Dalton Trans., 2012, 41, 876 RSC.
- A. Mambanda, D. Jaganyi, S. Hochreutther and R. van Eldik, Dalton Trans., 2010, 39, 3595 RSC.
- (a) A. Hofmann and R. van Eldik, Dalton Trans., 2003, 2979 RSC; (b) H. Erturk, A. Hofmann, R. Puchta and R. van Eldik, Dalton Trans., 2007, 2295 RSC; (c) H. Erturk, J. Maigut, R. Puchta and R. van Eldik, Dalton Trans., 2008, 2759 RSC; (d) H. Erturk, R. Puchta and R. van Eldik, Eur. J. Inorg. Chem., 2009, 1334 Search PubMed.
- S. J. Barton, K. J. Barnham, A. Habtemariam, R. E. Sue and R. J. Sadler, Inorg. Chim. Acta, 1998, 273, 8 CrossRef CAS.
- J. Reedijk, Chem. Commun., 1996, 801 RSC.
- J. H. Burchenal, K. Kalaher, K. Dew, L. Lokys and G. Gale, Biochimie, 1978, 60, 961 CrossRef CAS.
- Inorganic Reaction Mechanism, ed. M. L. Tobe and J. Burgess, Longman, England, 1999, pp. 70, 364 Search PubMed.
- J. Bogojeski, Ž. D. Bugarčić, R. Puchta and R. van Eldik, Eur. J. Inorg. Chem., 2010, 5439 CrossRef CAS.
- I. Ott, Coord. Chem. Rev., 2009, 253, 167–1681 CrossRef.
- F. Oton, A. Tarraga and P. Molina, Org. Lett., 2006, 8, 2107 CrossRef CAS PubMed.
- K. G. Caulton, Eur. J. Inorg. Chem., 2012, 435 CrossRef CAS.
- S. Stang, A. Lebkücher, P. Walter, E. Kaifer and H.-J. Himmel, Eur. J. Inorg. Chem., 2012, 4833 CrossRef CAS.
- H.-J. Himmel, Topics in Heterocyclic Chemistry, Springer, Berlin, Heidelberg, 2015, pp. 1–39 Search PubMed.
- T. G. Appleton, J. R. Hall, S. F. Ralph and C. S. M. Thompson, Inorg. Chem., 1984, 23, 3521 CrossRef CAS.
- G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structure from Diffraction Data, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1431738 and 1431739. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5nj03360h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |





