The interaction of a β-fused isoindoline–porphyrin conjugate with nucleic acids†
Abstract
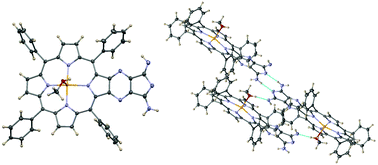
A diiminoisoindolo-pyrazino unit was annulated to the β-pyrrolic position of the Zn complex of tetraphenylporphyrin, giving a macrocycle with an expanded π system in satisfactory yields. The crystal structure of this derivative evidenced the formation of a hydrogen bond network. The interaction of the β-fused isoindoline porphyrin derivative with nucleotides and nucleic acids has been investigated.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...