Small heterocycles as highly luminescent building blocks in the solid state for organic synthesis†
Abstract
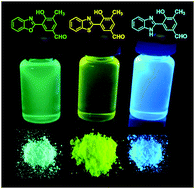
New photoactive mono-formylated benzoxazole derivatives were obtained as stable solids with excellent yields through a Duff functionalization protocol using a simple synthetic methodology. Despite several attempts to obtain the desired formyl compounds through the Duff reaction, which allows different acidic conditions to the studied compounds the key step was to use urotropine and trifluoroacetic acid as reagents. Additionally, the present protocol also allowed us to simplify the purification methodology in spite of the previously reported literature. The compounds present absorption in the UV region (ca. 340 nm) and fluorescence emission with a large Stokes' shift in the blue-yellow regions, related to a phototautomerism in the excited state (ESIPT). In the solid state, main emission bands located in the cyan-green to green regions were observed with higher fluorescence quantum yields. Compound 20 was successfully applied as an optical sensor for amines in solution.

 Please wait while we load your content...
Please wait while we load your content...