Hierarchically porous titanium phosphate monoliths and their crystallization behavior in ethylene glycol†
Abstract
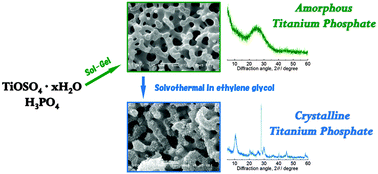
Hierarchically porous titanium phosphate monoliths combining well-defined co-continuous macropores and accessible large mesopores have been synthesized for the first time via a sol–gel process accompanied by phase separation using titanium oxysulfate and phosphoric acid as precursors. The macropore size was tunable over a broad range from 0.6 μm to 10 μm by changing the starting composition, while the mesopore size and the specific surface area of the as-synthesized amorphous monoliths are 21 nm and 320 m2 g−1, respectively. Crystallization of the gel network can be induced in two different ways. Calcination at elevated temperature (800 °C) leads to the formation of TiP2O7 and the collapse of both the macro- and meso-structures, while the solvothermal treatment of the as-synthesized gel in ethylene glycol leads to the formation of platy crystals of titanium phosphate with a layered structure at relatively low temperature. The titanium phosphate monoliths with a hierarchically porous structure are expected to be useful in various applications such as continuous-flow catalysis, water remediation and ion batteries.
- This article is part of the themed collection: The Creative World of Porous Materials

 Please wait while we load your content...
Please wait while we load your content...