Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fourfold action of surfactants with superacid head groups: polyoxometalate–silicone nanocomposites as promising candidates for proton-conducting materials†
Alexander
Klaiber
a,
Steve
Landsmann
ab,
Tobias
Löffler
a and
Sebastian
Polarz
*a
aDepartment of Chemistry, University of Konstanz, Universitätsstrasse 10, 78457 Konstanz, Germany. E-mail: sebastian.polarz@uni-konstanz.de
bEMPA-Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland
First published on 13th August 2015
Abstract
Conventional, organic surfactants normally fulfill only one task associated with their amphiphilic character. The situation changes and multifunctionality is granted for surfactants containing inorganic entities, so-called I-SURFs. Here, we demonstrate that an I-SURF with a heteropolyacid head fulfills four tasks at once. It acts as an effective emulsification agent, it catalyzes the polymerization of silicone monomers, it provides proton-conductivity in a PDMS/polyoxometalate hybrid, and it acts as a precursor for a nanostructured WO3 material.
One of the major challenges societies face today and in the near future is securing sufficient resources and energy in the context of exponentially growing population and industries, and at the same time avoiding an irreversible impact on our ecological environment. Because the residue in hydrogen technology is water, substantial scientific activity was invested for more than one decade.1,2 The key element of hydrogen technology is the fuel-cell converting chemical energy into electrical energy. Significant research was done in materials science regarding the optimization of every component of fuels cells, anode, cathode and electrolyte. Still, the so-called Nafion, a polymer-based, proton-conducting material is a standard as an electrolyte.3–5 Nafion, developed in the 1960s by Dupont, is a Teflon derivative with side chains containing sulfonic acid groups. Thus, it has amphiphilic properties resulting in a structure with water-filled nano-channels, in which proton-conduction takes place.6 Because the preparation of Nafion is demanding and expensive, there is still a high demand of promising materials as substitutes.7–10
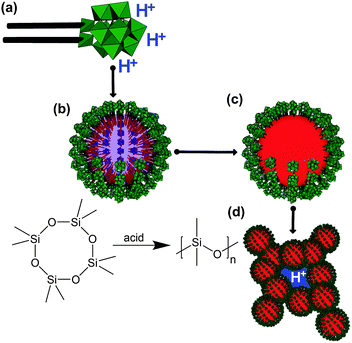
Here, we present a unique approach towards novel proton-conducting materials containing special polyoxometalate compounds (POMs).11,12 A special sub-group of polyoxometalates, the so-called heteropolyacids, contain protons as counter-ions to the negatively charged metal-oxo cluster and are known for their high acidity due to pKa values in the range of superacids, which makes them interesting in catalysis.13,14 Very recently, there have been a few papers reporting about beneficial, proton-conducting materials containing heteropolyacids. For instance Borros et al. have combined H3Mo12PO40 with a benzimidazole polymer and phosphoric acid,15 or the polyoxometalate could be incorporated into the pores of metal–organic framework materials (MOFs) as demonstrated by Banerjee et al.10 However, due to the weak interaction between the POM and the support, the large drawback of the latter approaches is that the heteropolyacid could quickly leach out (on the basis of days), which will negatively affect the durability of the materials. The route presented in the current paper is schematically depicted in Scheme 1.
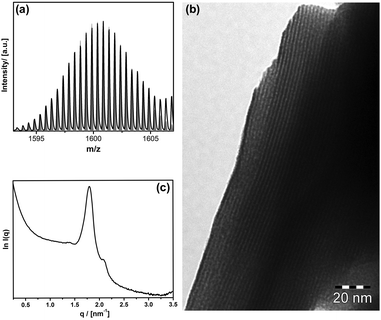
First a special type of surfactant with a heteropolyacid head group (Scheme 1a) was prepared according to a method developed in our group.16–20 The lacunary Keggin [PW11O39]7− is reacted with two equivalents of the alkoxy-organosilane (MeO)3Si(CH2)15CH3, followed by cation exchange with protons. The identity of the desired compounds [(C16H33Si)2PW11O39](H)3 (1) was proven by a combination of analytical techniques (see also ESI,† S1). In electrospray ionization mass spectrometry (ESI-MS) the main signal at 1600 m/z can be assigned to the doubly-deprotonated form of (1) (see Fig. 1a). In the NMR-spectra given in ESI,† S1 (1H, 29Si, 31P, 183W) all signals can be assigned to (1), and in particular the occurrence of a broad signal at δ(1H) = 5.46 ppm proves the existence of protons, respectively H3O+ ions.
The surfactant character of (1) can be seen from its ability to form lyotropic liquid crystals (LCC) at high concentration in contact with water (Fig. 1b and c). Microscopy under crossed polarizers (see ESI,† S1e) shows a birefringent phase. The texture is in agreement with a lamellar phase. The lamellar character is confirmed by transmission electron microscopy (TEM; Fig. 1b). The interlamellar spacing (d = 3.5 nm) can be determined more precisely from the main signal in small angle X-ray scattering (SAXS) shown in Fig. 1c. Because d is less than the double length of the surfactant, there is presumably a partial interdigitation of the alkyl chains in the LCC phase as shown in ESI,† S1f.
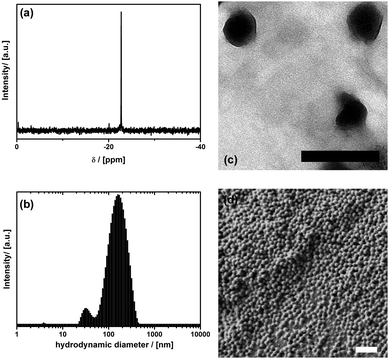
At lower concentration (1) forms micellar solutions with an average aggregate diameter of 3.6 nm determined from dynamic light scattering (DLS) (see ESI,† S1g). However, it is much more important for the current work that the POM-surfactant can also be used for the generation of stable emulsions of octamethylcyclotetrasiloxane (TSIL) in water. The dispersions are stable over weeks and months (see ESI,† S2a). TSIL was used as a cyclic monomer for the acid-catalyzed generation of polydimethylsiloxane (PDMS), with additional PhSi(OEt)3 used as a crosslinker. However, no additional acid needed to be added, because the acidity of the surfactant is high enough to self-catalyse the reaction. The successful consumption of TSIL and formation of PDMS was monitored using 29Si-NMR spectroscopy. With time the signal at δ = −20.1 ppm for TSIL disappears almost completely and the new signal for PDMS (δ = −22.7 ppm) emerges (Fig. 2a). The resulting dispersion contains almost spherical ≈155 nm large particles with fairly narrow size distribution as found by DLS and TEM (Fig. 2b and c). The DLS also shows a weak second size distribution around 30 nm. The origin of this peak is not clear at the moment. Eventually there are also some smaller particles formed in due course of the emulsion polymerization, or one sees vesicular structure, which are known to occur for polyoxometalate surfactants.17 Also these dispersions are stable for weeks, which demonstrates the effective steric stabilization due to the negatively charged POM-surfactant at the surfaces of the particles. Furthermore, we could not detect any leaching of tungstate species and degradation of the surfactant into the aqueous phase, as long as it is secured that the pH-value remains acidic. The reason for the stability of the material is that the alkyl-chain of the surfactant is tightly locked inside the PDMS matrix (see Scheme 1c).
Aggregation of these particles can either be induced by addition of salt, or by drying. We have used freeze-drying until all water has been removed. Solid, highly elastic, rubber-like materials were obtained (see also ESI,† S3). The particles in these solids have formed a fairly porous network shown via scanning electron microscopy (SEM) in Fig. 2d. The porosity and inner surface area (ABET = 33 m2 g−1) was determined using N2 physisorption analysis (isotherm shown in ESI,† S3). It is important to note that the porous network is stable and particles cannot be re-dispersed, no matter which solvent is used (see also Fig. 3). This is caused by linkage between the particles due to partial fusion, which can be seen from magnified SEM images (ESI,† S3). The homogeneity of the resulting material was also investigated in a further analytical technique. As expected there are no crystalline domains found by powder X-ray diffraction (PXRD) and no periodicities on the meso-scale seen by small angle X-ray scattering (SAXS); both data not shown. However, using FT-IR and FT-Raman spectroscopy provided in ESI,† S3, it can be proven that the material is composed exclusively of the POM-surfactant and the siloxane polymer. No other constituents are present. Further proof that the mentioned material still contains the POM constituent was provided by a combined TGA (thermogravimetric analysis) PXRD experiment. The mass loss observed in TGA in air (see ESI,† S3) is larger than expected for the decomposition and removal of volatile components originating from the organosilsesquioxane species alone, and as a product a mixture of amorphous SiO2 and crystalline WO3 (data given in ESI,† S4) was obtained. Considering modern applications of WO3,21,22 an interesting aspect, which however is beyond the scope of the current manuscript, is that the obtained SiO2/WO3 hybrid material is also nanostructured and resembles the morphology of the PDMS/POM-surfactant phase (see ESI,† S4).
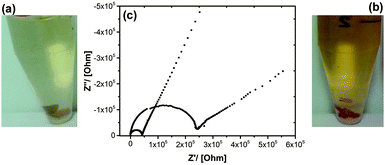
Coming back to the PDMS/POM nanohybrid there are some obvious similarities to Nafion. We have a hydrophobic backbone with highly acidic, water compatible groups covering exclusively the surfaces of a porous structure (Scheme 1). It now needs to be probed, if the material is conducting protons. A first, phenomenological proof is coming from adding the material to an aqueous solution containing a pH-indicator (see Fig. 3a and b). In comparison to a PDMS material prepared with a conventional, surfactant (SDS) instead of (1) there is clearly an acidic reaction not only in solution, but also directly inside the solid material.
Furthermore, the materials (after equilibration of samples with water)23 were characterized using impedance spectroscopy, which is a good method for the investigation of proton conduction in solid materials.24,25Fig. 3c shows the Nyquist diagram of the data, which were evaluated using literature-known methods.25,26 The proton conductivity is calculated from the low frequency real-axis intercept of the semicircular arc by scaling the resistance appropriately with pellet thickness and pellet diameter. As expected the proton conductivities depend on the relative amount of POM-surfactant to PDMS polymer, respectively, the ratio of protons provided by (1) and Si from the polymer; (σ(H+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si = 1
Si = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) = 5.9 × 10−5 S cm−1); (σ(H+
90) = 5.9 × 10−5 S cm−1); (σ(H+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si = 1
Si = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) = 7.5 × 10−3 S cm−1).
20) = 7.5 × 10−3 S cm−1).
In the current paper we discuss a surfactant containing a special inorganic entity as a head group, a so-called heteropolyacid. This polyoxometalate surfactant [(C16H33Si)2PW11O39](H)3 exhibits fourfold action at once. First of all it is an effective emulsification agent for monomers leading to polysiloxane polymers (silicones). Secondly, its protic character catalyses the polymerization of the monomers, which leads to spherical PDMS/POM-surfactant particles. Solvent removal allows us to assemble a nanoporous network from these particles. Because the POM-surfactants are located at the surfaces of this network, their third function is to provide proton-conductivity inside the channels of the materials. Finally, the POM head could be used as a precursor for WO3, and interesting, nanostructured silica–WO3 hybrid materials were obtained that are briefly discussed in the paper.
Unfortunately, the material cannot compete yet with Nafion as the most prominent proton-conducting material used in fuel cells. Depending on the literature source and conditions the conductivity of Nafion is reported in the region 10−1–10−2 S cm−1,27 whereas our material is roughly 1 and 2 orders of magnitude worse. This requires some optimization processes in the future. In particular the degree of PDMS cross-linking needs to be adjusted for controlling and increasing the porosity of the PDMS/POM-surfactant nanohybrid. However we could show that PDMS/POM materials are promising candidates for proton-conducting membranes, as they exhibit good proton conductivity with respect to their low inner surface area, which indicates a high proton mobility within the material. We are aware that the porosity of the material is not the only factor for high performance proton-conduction materials as Nafion beads exhibit BET surfaces of only 0.02 m2 g−1.28 In addition to applications as proton-conducting membranes, one can also imagine that the materials presented here are interesting in heterogeneous catalysis.29
Experimental section
An emulsion is prepared by sonication (using a Bandelin TT13; 5 min) of a mixture of the POM-surfactant (1) (e.g. 0.031 mmol), water (16 mL), TSIL (e.g. 1.7 mmol) and PhSi(OEt)3 as a cross-linker. The resulting dispersion is stirred for 20 h at 80 °C. Impedance spectroscopy was performed using a Zahner IM6 workstation over a frequency range of 1–10−7 Hz with an oscillating voltage 50–500 mV.Acknowledgements
The current work was funded by the European Research Council within the ERC consolidator grant ‘ISURF’.Notes and references
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345–352 CrossRef CAS PubMed.
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K. I. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS PubMed.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- D. J. Kim, M. J. Jo and S. Y. Nam, J. Ind. Eng. Chem., 2015, 21, 36–52 CrossRef CAS.
- D. K. Paul, A. Fraser and K. Karan, Electrochem. Commun., 2011, 13, 774–777 CrossRef CAS.
- J. A. Asensio, E. M. Sanchez and P. Gomez-Romero, Chem. Soc. Rev., 2010, 39, 3210–3239 RSC.
- T. J. Peckham and S. Holdcroft, Adv. Mater., 2010, 22, 4667–4690 CrossRef CAS PubMed.
- S. Liu, Z. Yue and Y. Liu, Dalton Trans., 2015, 44, 12976–12980 RSC.
- C. Dey, T. Kundu and R. Banerjee, Chem. Commun., 2012, 48, 266–268 RSC.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009–6048 CrossRef CAS PubMed.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS PubMed.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199–217 CrossRef CAS PubMed.
- P. Gomez-Romero, J. A. Asensio and S. Borros, Electrochim. Acta, 2005, 50, 4715–4720 CrossRef CAS.
- S. Polarz, S. Landsmann and A. Klaiber, Angew. Chem., Int. Ed., 2014, 53, 946–954 CrossRef CAS PubMed.
- S. Landsmann, M. Luka and S. Polarz, Nat. Commun., 2012, 3, 6 Search PubMed.
- J. J. Giner-Casares, G. Brezesinski, H. Mohwald, S. Landsmann and S. Polarz, J. Phys. Chem. Lett., 2012, 3, 322–326 CrossRef CAS PubMed.
- S. Landsmann, M. Wessig, M. Schmid, H. Colfen and S. Polarz, Angew. Chem., Int. Ed., 2012, 51, 5995–5999 CrossRef CAS PubMed.
- S. Landsmann, C. Lizandara-Pueyo and S. Polarz, J. Am. Chem. Soc., 2010, 132, 5315–5321 CrossRef CAS PubMed.
- C. Santato, M. Odziemkowski, M. Ulmann and J. Augustynski, J. Am. Chem. Soc., 2001, 123, 10639–10649 CrossRef CAS PubMed.
- S. K. Deb, Sol. Energy Mater. Sol. Cells, 2008, 92, 245–258 CrossRef CAS.
- B. A. Holmberg and Y. S. Yan, J. Electrochem. Soc., 2006, 153, A146–A149 CrossRef CAS.
- G. Inzelt, M. Pineri, J. W. Schultze and M. A. Vorotyntsev, Electrochim. Acta, 2000, 45, 2403–2421 CrossRef CAS.
- S. M. J. Zaidi, S. D. Mikhailenko, G. P. Robertson, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2000, 173, 17–34 CrossRef CAS.
- P. X. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko and S. Kaliaguine, Macromolecules, 2004, 37, 7960–7967 CrossRef CAS.
- K. D. Kreuer, J. Membr. Sci., 2001, 185, 29–39 CrossRef CAS.
- M. A. Harmer, W. E. Farneth and Q. Sun, J. Am. Chem. Soc., 1996, 118, 7708–7715 CrossRef CAS.
- A. Corma and H. Garcia, Adv. Synth. Catal., 2006, 348, 1391–1412 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: S1. Additional information for the surfactant with a heteropolyacid head [(C16H33Si)2PW11O39](H)3 (1). Additional information for the emulsion formed with TSIL, water and (1) as an emulsifier. S3. Additional data for the solid PDMS–POM–surfactant nanohybrid. S4. Additional data for the material obtained after calcination of the PDMS–POM–surfactant nanohybrid. See DOI: 10.1039/c5nj01544h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |