Dually actuated atomic force microscope with miniaturized magnetic bead-actuators for single-molecule force measurements
Semih
Sevim†
a,
Sevil
Ozer†
a,
Luying
Feng
a,
Joel
Wurzel
b,
Arielle
Fakhraee
c,
Naveen
Shamsudhin
d,
Bumjin
Jang
d,
Carlos
Alcantara
d,
Olgaç
Ergeneman
d,
Eva
Pellicer
e,
Jordi
Sort
f,
Tessa
Lühmann
b,
Salvador
Pané
*d,
Bradley J.
Nelson
d and
Hamdi
Torun
 *a
*a
aDepartment of Electrical and Electronics Engineering, Bogazici University, Bebek, 34342 Istanbul, Turkey. E-mail: hamdi.torun@boun.edu.tr
bInstitute of Pharmacy and Food Chemistry, University of Würzburg, Würzburg, Germany
cAeon Scientific AG, Schlieren, 8952, Zurich, Switzerland
dMulti Scale Robotics Lab, Institute of Robotics and Intelligent Systems, ETH Zurich, 8092, Zurich, Switzerland. E-mail: vidalp@ethz.ch
eDepartament de Física, Facultat de Ciències, Universitat Autònoma de Barcelona, E-08193 Bellaterra, Spain
fInstitució Catalana de Recerca i Estudis Avançats (ICREA) and Departament de Física, Universitat Autònoma de Barcelona, E-08193 Bellaterra, Spain
First published on 9th September 2016
Abstract
We report a novel atomic force microscopy (AFM) technique with dual actuation capabilities using both piezo and magnetic bead actuation for advanced single-molecule force spectroscopy experiments. The experiments are performed by manipulating magnetic microbeads using an electromagnet against a stationary cantilever. Magnetic actuation has been demonstrated before to actuate cantilevers, but here we keep the cantilever stationary and accomplish actuation via free-manipulated microstructures. The developed method benefits from significant reduction of drift, since the experiments are performed without a substrate contact and the measured force is inherently differential. In addition, shrinking the size of the actuator can minimize hydrodynamic forces affecting the cantilever. The new method reported herein allows for the application of constant force to perform force-clamp experiments without any active feedback, profiled for a deeper understanding of biomolecular interactions.
Conceptual insightsWe present a novel atomic force microscopy (AFM) technique and instrument for advanced single-molecule experiments. Instead of engineering the actuator of an AFM system, we replace it with miniaturized magnetic particles potentially down to the size of a single molecule. Thus the footprint of the actuator is unconventionally small offering numerous advantages, considering the dynamics, resolution and stability. We integrate the AFM system with a magnetic manipulator to actuate the functionalized beads against a stationary cantilever. Unlike a conventional AFM setup, the cantilever in our system operates away from a stationary wall. This improves the dynamics of the cantilever and removes the detrimental effects of such a wall on force resolution and loading rate. In addition, there is no need for a larger-scale piezo actuator that introduces drift and system complexity. The new method reported herein allows for the application of constant force on biomolecules performing force-clamp experiments without any active feedback, profiled for a deeper understanding of biomolecular interactions. The introduced method combines the technological advancements of AFM and magnetic tweezers. New capabilities can be introduced for the manipulation and detection of protein molecules by reducing the size of the actuator down to the size of a protein molecule. |
1. Introduction
Atomic force microscopy (AFM) has been used as a versatile force measurement tool in molecular biophysics for the last three decades. AFM offers a force resolution of a few pico-Newtons in liquid environments at the single-molecule level.1 The potential of the technology has been demonstrated successfully for various biomolecular measurements, such as antibody–antigen binding, receptor–ligand interaction, and protein folding/unfolding measurements at single-molecule levels using the biomolecular force spectroscopy technique.2–5 Fundamentally, the force spectroscopy technique involves a cantilever that is functionalized with a specific type of biomolecules. The cantilever, typically actuated using a piezoelectric transducer, is brought in repeated contact with a sample surface that is also functionalized with biomolecules. The force on the cantilever is detected by optical means. The technique allows for the investigation of the specific interactions between single pairs of biomolecules located at the functionalized tip and the sample surface in aqueous environments. It is feasible to quantitatively study the structure, function and energy landscape of biomolecular interactions. The cantilever is actuated over a wide range of speeds, loading the molecular bond before it breaks, and the corresponding forces of biomolecular interactions are recorded. Thus, it is advantageous to have low drift, high-bandwidth actuators to enable both low-speed and high-speed measurements.The external piezoactuator is a limiting factor for high-bandwidth biomolecular experiments as it introduces additional mechanical modes into the cantilever and limits high-speed measurements.6 In addition, cantilever size and geometry have a direct influence on the limitation of the actuation speed due to the hydrodynamic drag effects.7,8 To mitigate these effects, several methods for direct cantilever actuation have been proposed, such as magnetic actuation,9 photothermal actuation,10 acoustic pressure actuation11 and capacitive actuation.12 Magnetic actuation can be achieved using ferromagnetic thin-film coated cantilevers13 or conventional cantilevers attached with magnetic particles,14 coupled with an electromagnet or a permanent magnet in the AFM mechanical assembly.
Drift in the system negatively affects the measurements, especially at low actuation speeds, by inducing spurious deflections on the cantilever. Sources of drift include mechanical instabilities and thermally induced deflection of the cantilever. Undesirable deflection of the cantilever towards the substrate increases the contact forces on the biomolecules and eventually can damage the biological specimens.15,16 In addition, drift-induced deflection of the cantilever results in false force readings. Some of the methods proposed to overcome the detrimental effects of thermal drift problem include use of modified cantilever geometries,17–19 reference cantilevers20,21 and thermomechanically compensating microstages.15
In this study, we present a novel AFM technique with dual actuation capability. The AFM employs a conventional piezotube actuator together with a new approach where we use magnetic beads as a secondary actuator. In this architecture, a molecule attached to an AFM cantilever is probed with a counter-interacting molecule attached to a magnetic bead. The cantilever is kept stationary and is used as a force sensor. Under an externally controlled magnetic field and field gradient, it is possible to manipulate the magnetic bead without a substrate surface. Magnetic actuation has previously been demonstrated for AFM to actuate cantilevers;13,14 however, we report for the first time a method where the cantilever is kept stationary and free-manipulated microstructures are actuated against the stationary cantilever. Manipulation tasks with magnetic objects varying in dimensions from nanometres to centimetres, with five degrees-of-freedom and with high resolution that is limited by imaging technology have already been demonstrated with electromagnetic manipulation setups.22 The footprint of the magnetic actuator with a diameter of a few microns is orders of magnitude smaller than the commonly used piezotubes. Use of miniaturized mechanical actuator improves system dynamics, because the cantilever is kept stationary and a dynamic magnetic bead encounters drastically smaller hydrodynamic drag forces in viscous fluids as compared to larger structures. The drag (hence damping) on a mechanical system is an indication of thermal noise, according to the fluctuation dissipation theorem. Reduced hydrodynamic drag improves force resolution and the stability of the system significantly.
Our design includes a custom built AFM head integrated with an electromagnetic manipulator.23 Previously, we used the magnetic actuation capability of the setup to actuate AFM cantilevers. We glued ferromagnetic beads with a diameter of 30 μm to actuate commercially available silicon nitride cantilevers under controlled magnetic fields and gradients using the electromagnetic manipulator. That setup is suitable to realize the new method we introduce in this study. In our new approach, we use the cantilevers as stationary force sensors against unattached magnetic beads with a diameter of few microns that serve as miniaturized main actuators. In addition, there is no need of a contact with a rigid substrate during biomolecular experiments. So, minimizing the actuator size and keeping the cantilever and its holder stationary, the drift and negative influence of hydrodynamic effect in the system are minimized. Furthermore, the new method is advantageous in force-clamp experiments. It is possible to perform force-clamp experiments without any active feedback since the force on a magnetic bead is constant under constant field and gradient.
2. Results and discussion
2.1. Characterization of the system
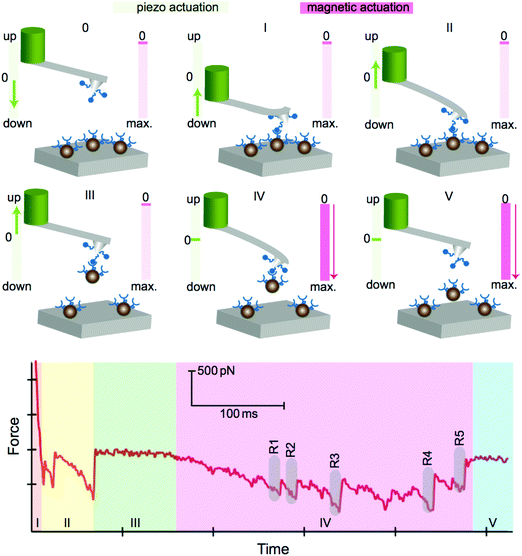
The AFM system, including an integrated electromagnet and a magnetic bead-based actuator, is shown in Fig. 1. The AFM head consists of a piezoactuator and the space below the cantilever plane is designed to be empty, so that the AFM head can be integrated with a magnetic manipulator. A soft-magnetic core based electromagnet is used to actuate a magnetic particle in one-dimensional translational motion for biomolecular pulling measurements.In a typical force spectroscopy experiment, the magnetic bead is actuated against the cantilever to probe the interactions between the molecules attached to the bead and the cantilever. The experiment using the piezoactuation of the cantilever and magnetic actuation of the magnetic bead is illustrated schematically in Fig. 2. The cantilever is functionalized with molecule A and tested against molecule B attached to the magnetic beads in an aqueous environment at room temperature. First, the functionalized cantilever is brought down using the piezoactuator to the substrate, which is covered with magnetic beads (0). The cantilever is initially pressed into the substrate to allow molecules to form bonds. Then, the cantilever is pulled away from the substrate using the piezoactuator (I). As the cantilever moves out of contact with the substrate, non-specific adhesions can be observed (II). Finally, the cantilever moves completely out of contact and then the piezoactuator stops (III). After the piezoactuator is turned off, the electromagnet is driven to pull the magnetic beads back to the substrate (IV). Electromagnetic actuation leads to the unbinding of the molecules located at the cantilever and the magnetic beads, which manifest as multiple rupture events (labeled as R1–R5) and are observed in region IV, while the current is running through the coil. The unbinding forces of the rupture events range from 100 to 500 pN on this specific force curve. The last phase (V) on the force curve indicates the detachment of all beads from the cantilever.
The number of events in one approach cycle can be controlled with the numbers of beads located on the substrate. The time and force resolution of the system allows for the identification of single events and for measuring both the loading rate of the molecular bond and the unbinding force. There are five distinct events evident on the sample force curve shown in region IV of Fig. 2. The temporal resolution of the system allows that the events can be resolved individually and the unbinding forces between single pairs of molecules can be detected. A typical force time trace, showing an unbinding event with a force of 50 pN at a loading rate of 70 pN s−1, is depicted in Fig. 3. The demonstrated loading rate is significantly low; however, the system allows the accurate measurement of force curves at low loading rates and low force levels.
Our method of magnetic actuation eliminates the need for the interaction between the cantilever and a sample surface, while measuring the unbinding events. The capability of performing experiments away from the sample surface is advantageous, because the large mechanical loop between the cantilever and the sample surface is broken. In a typical AFM setup, the cantilever and the sample surface are located on different planes and the stability of the relative position of the planes are determined by the rigidity of all the mechanical components in the mechanical loop connecting these planes. The mechanical loop is a significant source of drift in conventional AFM setups.20,24 In addition, keeping the cantilever away from the sample surface improves its dynamics by eliminating the hydrodynamic wall effect.13,25 We performed long-timescale drift experiments by utilizing our new AFM system in comparison with a commercial AFM system (Dimension Edge, Bruker). We actuated an AFM cantilever (MLCT-C type, V shaped Silicon Nitride cantilever, Bruker) in and out-off contact with a coverslip that was clamped to the base of the AFM stage using the piezoactuator of the commercial system to characterize drift in the setup. For this purpose, we applied a square wave signal with constant amplitude to the piezo and monitored the deflection of the cantilever. Fig. 4 (black line) shows the force on the cantilever. The detected signal follows a square wave with increasing amplitude. The increase in the force amplitude is an indication of the drift in the system as the position of the cantilever changes relative to the coverslip in time. The amplitude increases by a factor of 2.5 in 500 s for the specific experiment. Apparently, the distance between the base of the cantilever and the sample surface decreased due to the drift in the system. This resulted in an increase in the contact force. An increase in contact force is detrimental, especially when the tip of the cantilever is functionalized with biomolecules, as they can be damaged. Simultaneously, we conducted a similar experiment with our new AFM setup. We glued a ferromagnetic bead with a diameter of 30 μm on an identical cantilever and actuated the bead-attached cantilever by applying a square wave signal with constant amplitude to the electromagnet, while observing the deflection. We performed both experiments simultaneously to ensure identical effects due to environmental disturbance. The red line in Fig. 4 represents the deflection of the cantilever due to the magnetic actuation. The amplitude of deflection signal keeps constant during the entire experiment. The mean value of the deflection signal decreases with respect to time, due to the temperature response of the cantilever. On the other hand, the differential amplitude of the force on the cantilever remains the same. Measurements of biomolecular forces rely on the differential force; therefore, the method of actuation minimizes the detrimental effects of drift in terms of detected force levels. Also, the rigid mechanical loop between the cantilever and the substrate is eliminated. So, the biomolecules do not experience excessive force due to the thermally induced forces on cantilever that displace the cantilever into the biomolecules.
Another inherent advantage of the magnetic bead actuation method is the capability of performing force clamp experiments without the need for active feedback. The current applied to the electromagnet determines the strength of the magnetic field and gradient, which is proportional to the force applied to the beads. So, the force applied on magnetic beads is constant when the electromagnet is driven with a constant current signal. Immediately after the functionalized cantilever grabs a magnetic bead (see Fig. 2-III), force-clamp experiments can be performed by applying a constant force on the bead until an unbinding event is observed. The duration of time between the instance when the force is applied and the instance of unbinding, i.e. lifetime, is recorded. A typical force time trace for a force-clamp experiment is shown in Fig. 5 without any feedback. We set a clamping force of 60 pN between the molecules and measured a life-time of 600 ms.
 | ||
| Fig. 5 A sample force time trace in a force-clamp setting by applying constant current to the magnetic manipulator without any feedback loop requirements. | ||
In a conventional AFM setup, the measurement of lifetimes is possible using an active feedback control to maintain the force between the molecules constant. The drift in the setup requires accurate control of the piezoactuator. Fig. 6 depicts a typical force time trace together with the piezo displacement in a force-clamp setting. We used a biotinylated cantilever and a cover slip incubated with streptavidin for the demonstration experiment. We set the clamping force to ∼200 pN between the molecules and adjusted the controller settings for the piezoactuator of the cantilever. The displacement data clearly shows the piezo was actuated >30 nm to keep the force constant during the clamping period. In addition, the closed-loop feedback control typically reduces the detection bandwidth. On the contrary, the method of magnetic bead actuation does not need an active feedback control for force-clamp experiments and offers improved force-clamp measurements.
2.2. Biomolecular experiment
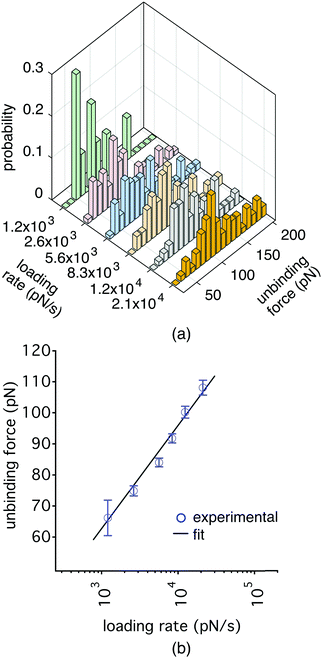
We performed a biomolecular experiment using the dual actuation capability of our AFM setup as proof-of-principle. We used a biotin-coated cantilever and tested it against streptavidin functionalized magnetic beads with a diameter of 2.8 μm. We followed the protocol described in Fig. 2 at room temperature during the experiment. First, we actuated the cantilever using the piezo towards a hard surface, where the beads were located. Then, we retracted the cantilever away from the surface and turned off the piezo. Finally, we turned on the magnetic actuator to observe unbinding events, in the cases where the cantilever had picked up beads in the approach cycle. We collected several hundreds of force curves at different loading rates. The force histograms are shown in Fig. 7(a). The probability of specific events was recorded to be less than 30%. Fig. 7(b) shows the unbinding forces as a function of loading rates. The results of the experiments can be used to calculate the kinetic off-rate (koff) and the energy barrier width (xβ) based on the Bell–Evans model.26,27 We measured koff and xβ as 1.08 s−1 and 0.28 nm, respectively for a loading rate range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pN s−1. The biotin–(strept)avidin interactions have been vastly studied in the literature and the extracted Bell–Evans parameters were summarized previously.28 The measured values of the unbinding forces between biotin and streptavidin and the extracted values of koff and xβ are comparable to the results published in literature. We also performed control experiments by saturating biotin on the cantilever with excess streptavidin. The addition of excess streptavidin on the cantilever reduces the probability of the specific events down to 1%, validating the experimental protocol.
000 pN s−1. The biotin–(strept)avidin interactions have been vastly studied in the literature and the extracted Bell–Evans parameters were summarized previously.28 The measured values of the unbinding forces between biotin and streptavidin and the extracted values of koff and xβ are comparable to the results published in literature. We also performed control experiments by saturating biotin on the cantilever with excess streptavidin. The addition of excess streptavidin on the cantilever reduces the probability of the specific events down to 1%, validating the experimental protocol.
We suggest that mostly single events are being detected because time and force resolution of our system allows us to detect the single unbinding events one by one as shown in Fig. 2. It is possible to have multiple molecular pairs attached between the cantilever tip and the bead(s) due to the possibility of picking up more than one bead at one trial or due to the possibility of multiple bond formation between the cantilever and an individual bead. Nevertheless, single events are resolved one by one in time domain with their loading rates and unbinding force levels. In a typical experiment, we do not observe more than a few events for each cycle.
3. Conclusion
We have developed a novel high resolution AFM technique for single-molecule force spectroscopy experiments to overcome current limitations. We use stationary cantilevers as force sensors and use unattached, surface functionalized magnetic microbeads as the main actuator. We employ an AFM system that has dual actuation capabilities to implement the new method using a conventional piezoactuator and a magnetic actuator. The piezoactuator of the AFM system is mainly used to position the AFM cantilever and is turned off during the data acquisition phase. Instead of actuating the cantilever, a magnetic bead is manipulated against the cantilever by an electromagnet, comprising of a coil and a core, in the demonstrated novel AFM technique. The bead size deployed herein was 2.8 μm in diameter and can be reduced in future experiments. The advantages of such a small mechanical actuator are numerous with regards to dynamics, resolution and stability. Using a free-manipulated micron-sized bead minimizes the effects of drift and hydrodynamic forces on the force-sensing cantilever. In addition, the force spectroscopy can be performed far away from rigid surfaces, which could contribute to denaturation of the biomolecule, thus generating artefacts during force spectroscopy analysis. The introduced method combines the technological advancements of AFM and magnetic tweezers. The force measurement exploits the versatility and force sensitivity of the AFM as it is performed using a stationary cantilever. On the other hand, the actuation relies on the manipulation of a micron-sized bead that benefits from the stability and force resolution of magnetic beads. The size of the actuator beads can potentially be reduced down to the sizes comparable with single molecules. So, it is possible to minimize further the effects of drag and drift. However, some practical consequences of the use of nanoscale particles should be addressed. First, the force on a magnetic particle scales with its volume. Thus the external magnetic field and gradient or the magnetization of the particles should be increased to compensate the decreased volume. Second, we observed magnetic agglomeration with particles that are 100 nm in radius. Lastly, it is difficult to find and pick up individual nanoscale particles inside the meniscus. Nevertheless, here we have demonstrated for the first time the concept of the new method and the new instrument. The size of the actuator is minimized and the large mechanical loop between the cantilever and any rigid substrate is broken. Hence, using the magnetic actuation method, low loading rates down to 101 pN s−1 with single-molecules were demonstrated. We obtained the dynamic strength spectrum between biotin and streptavidin using the novel AFM technique and the results were in good agreement with the conventional piezo actuation methods. In addition, the ability to apply a constant force on the magnetic bead leads to the capability to perform force-clamp experiments without the need for an active feedback control. In summary, we have combined the distinct advantages of AFM and magnetic tweezers into our experimental platform. The new instrument opens exciting new opportunities to study biomolecular interactions in the range of low loading rates at physiological relevant forces.4. Method
4.1. Setup
Our design includes a custom built AFM head integrated with an electromagnetic manipulator, as shown in Fig. 1. The cantilever is attached to a piezoactuator (PI-841.1 with a vertical range of 15 μm, Physik Instrumente GmbH) and its deflection is monitored using a quadrature photodetector (Pacific Silicon Sensor, CA, USA). The cantilever is illuminated with a fiber pigtailed laser (Oz Optics, Ottowa, Canada).The electromagnet consists of an optimized ferromagnetic core geometry and copper winding. The core has a sharp tip to localize the magnetic field and maximize the generated magnetic gradient at a working distance of 100–150 μm from the tip. We measured the tip radius of the core as 100 μm using a white light interferometer. The exchangeable core-piece is made of FeCo alloy (Vacoflux50™) because of its high magnetization saturation (Ms = 2.35 T), which is capable of generating a magnetic field of 0.55 T at a working distance of 100 μm from the tip for a current density of 4 A mm−2.
Since biomolecular experiments have strict requirements on temperature control and stability, we placed the electromagnet in a temperature stabilizer unit comprising a thermoelectric cooler (UEPT-440-127-079E120, Uwe Electronic GmbH) for fine-regulation of temperature, which is mounted on a water-based cooling system. The thermoelectric cooler holds the coverslips during the experiments.
We implemented a software-based controller (NI PXI-8102, National Instruments, TX, USA) via an expansion card with 8 analog inputs (Fs = 1.25 MS s−1), 2 analog outputs (Fs = 3.33 MS s−1) and 24 digital inputs/outputs. We programmed the controller and the user interface in the NI-LabVIEW environment.
4.2. AFM measurements
We used commercially available biotin-coated cantilevers (CT.BIO, Novascan, Ames IA USA) for all experiments. The supplier prepares the cantilevers using silane-based self-assembled monolayers covalently attached to the surface of commercially available AFM probes. Silane monolayers, including APTES, are tethered on the surface of the cantilevers for biotin functionalization. We calibrated the spring constant and the sensitivity of the cantilevers before and after the experiments.We tested these cantilevers against streptavidin functionalized magnetic beads with a diameter of 2.8 μm (Dynabeads, M-280 Streptavidin, ThermoFisher Scientific). The beads are superparamagnetic and have a monolayer of recombinant streptavidin covalently coupled to the surface and further blocked with BSA. The binding capacity of the beads to biotin is reported as 650–900 pmoles mg−1. The supplier provided the streptavidin-functionalized beads at a concentration of 10 mg mL−1. We diluted the original suspension by mixing 1 μL suspension with 1 mL standard phosphate-buffer saline (PBS). Finally, we performed the experiment in 100 μL PBS by adding 5 μL diluted bead suspension.
We align the cantilever with the tip of the core before each experiment. We observe the effect of optical interference at the detector when the core and the cantilever are aligned perfectly, since the readout laser beam off of the cantilever and the core interferes. The tip radius of the core is 100 μm and this allows us to locate the cantilever precisely just over the coil tip. The current running through the coil concentrate the beads over the tip radius under the cantilever. This guarantees the alignment of the beads and the cantilever.
Author contributions
SS and SO designed the AFM head. AF, NS, BJ and CA designed the magnetic manipulator. JW and TL performed the bio-functionalization. SS, SO and LF conducted the experiments. SS, SO and HT wrote the main manuscript text. EP and JS characterized magnetic particles. OE, EP, JS, TL, SP, BJN and HT planned and organized the project. All authors reviewed the manuscript.Acknowledgements
Authors would like to acknowledge funding from the EC (ICT FET-Open) under the MANAQA Project, Grant no. 296679.References
- K. C. Neumanand and A. Nagy, Single-molecule forcespectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy, Nat. Methods, 2008, 5, 491–505 CrossRef PubMed.
- E. M. Puchner and H. E. Gaub, Force and function: probing proteins with AFM-based force spectroscopy, Curr. Opin. Struct. Biol., 2009, 19, 605–614 CrossRef CAS PubMed.
- D. J. Muller and Y. F. Dufrene, Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology, Nat. Nanotechnol., 2008, 3, 261–269 CrossRef PubMed.
- I. Popa, P. Kosuri, J. Alegre-Cebollada, S. Garcia-Manyes and J. M. Fernandez, Force dependency of biochemical reactions measured by single-molecule force-clamp spectroscopy, Nat. Protoc., 2013, 8, 1261–1276 CrossRef CAS PubMed.
- Z. N. Scholl, Q. Li and P. E. Marszalek, Single molecule mechanical manipulation for studying biological properties of proteins, DNA, and sugars, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2014, 6, 211–229 CrossRef CAS PubMed.
- C. Carrasco, P. Ares, P. J. de Pablo and J. Gómez-Herrero, Cutting down the forest of peaks in acoustic dynamic atomic force microscopy in liquid, Rev. Sci. Instrum., 2008, 79, 126106 CrossRef CAS PubMed.
- H. Janovjak, J. Struckmeier and D. Müller, Hydrodynamic effects in fast AFM single-molecule force measurements, Eur. Biophys. J., 2005, 34, 91–96 CrossRef CAS PubMed.
- K. Sarangapani, H. Torun, O. Finkler, C. Zhu and L. Degertekin, Membrane-based actuation for high-speed single molecule force spectroscopy studies using AFM, Eur. Biophys. J., 2010, 39, 1219–1227 CrossRef CAS PubMed.
- C. Vančura, J. Lichtenberg, A. Hierlemann and F. Josse, Characterization of magnetically actuated resonant cantilevers in viscous fluids, Appl. Phys. Lett., 2005, 87, 162510 CrossRef.
- S. W. Stahl, E. M. Puchner and H. E. Gaub, Photothermal cantilever actuation for fast single-molecule force spectroscopy, Rev. Sci. Instrum., 2009, 80, 073702 CrossRef PubMed.
- F. L. Degertekin, B. Hadimioglu, T. Sulchek and C. F. Quate, Actuation and characterization of atomic force microscope cantilevers in fluids by acoustic radiation pressure, Appl. Phys. Lett., 2001, 78, 1628–1630 CrossRef CAS.
- J. Zhang, D. M. Czajkowsky, Y. Shen, J. Sun, C. Fan, J. Hu and Z. Shao, Bias controlled capacitive driven cantilever oscillation for high resolution dynamic force microscopy, Appl. Phys. Lett., 2013, 102, 073110 CrossRef.
- C. Rankl, V. Pastushenko, F. Kienberger, C. M. Stroh and P. Hinterdorfer, Hydrodynamic damping of a magnetically oscillated cantilever close to a surface, Ultramicroscopy, 2004, 100, 301–308 CrossRef CAS PubMed.
- G. R. Jayanth, Y. Jeong and C.-H. Menq, Direct tip-position control using magnetic actuation for achieving fast scanning in tapping mode atomic force microscopy, Rev. Sci. Instrum., 2006, 77, 053704 CrossRef.
- S. Sevim, S. Tolunay and H. Torun, Micromachined sample stages to reduce thermal drift in atomic force microscopy, Microsyst. Technol., 2014, 21, 1559–1566 CrossRef.
- H. Torun, O. Finkler and F. L. Degertekin, Athermalization in atomic force microscope based force spectroscopy using matched microstructure coupling, Rev. Sci. Instrum., 2009, 80, 076103 CrossRef CAS PubMed.
- A. Beyder, C. Spagnoli and F. Sachs, Reducing probe dependent drift in atomic force microscope with symmetrically supported torsion levers, Rev. Sci. Instrum., 2006, 77, 056103 CrossRef.
- P. P. Weafer, J. P. McGarry, M. H. van Es, J. Kilpatrick, W. Ronan, D. R. Nolan and S. P. Jarvis, Stability enhancement of an atomic force microscope for long-term force measurement including cantilever modification for whole cell deformation, Rev. Sci. Instrum., 2006, 83, 093709 CrossRef PubMed.
- L. Wenzler, G. Moyes and T. Beebe, Improvements to atomic force microscopy cantilevers for increased stability, Rev. Sci. Instrum., 1996, 67, 4191–4197 CrossRef CAS.
- S. M. Altmann, P. F. Lenne and J. K. Heinrich Horber, Multiple sensor stabilization system for local probe microscopes, Rev. Sci. Instrum., 1996, 72, 142–149 CrossRef.
- J. L. Choy, S. H. Parekh, O. Chaudhuri, A. P. Liu, C. Bustamente, M. J. Footer, J. A. Theriot and D. A. Fletcher, Differential force microscope for long time-scale biophysical measurements, Rev. Sci. Instrum., 2007, 78, 043711 CrossRef PubMed.
- J. Pokki, O. Ergeneman, S. Sevim, V. Enzmann, H. Torun and B. J. Nelson, Measuring localized viscoelasticity of the vitreous body using intraocular microprobes, Biomed. Microdevices, 2015, 17, 1–9 CrossRef PubMed.
- S. Sevim, N. Shamsudhin, S. Ozer, L. Feng, A. Fakhraee, O. Ergeneman, S. Pané, B. J. Nelson and H. Torun, An Atomic Force Microscope with Dual Actuation Capability for Biomolecular Experiments, Sci. Rep., 2016, 6, 27567 CrossRef CAS PubMed.
- J. H. Kindt, J. B. Thompson, M. B. Viani and P. K. Hansma, Atomic force microscope detector drift compensation by correlation of similar traces acquired at different setpoints, Rev. Sci. Instrum., 2002, 73, 2305 CrossRef CAS.
- E. Bonaccurso, M. Kappl and H.-J. Butt, Hydrodynamic Force Measurements: Boundary Slip of Water on Hydrophilic Surfaces and Electrokinetic Effects, Phys. Rev. Lett., 2002, 88, 076103 CrossRef PubMed.
- G. I. Bell., Models for the specific adhesion of cells to cells, Science, 1978, 200, 618 CAS.
- E. Evans and K. Ritchie, Dynamic strength of molecular adhesion bonds, Biophys. J., 1997, 72, 1541 CrossRef CAS PubMed.
- J. M. Teulon, Y. Delcuze, M. Odorico, S. W. Chen, P. Parot and J. L. Pellequer, Single and multiple bonds in (strept)avidin-biotin interactions, J. Mol. Recognit., 2011, 24, 490 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |