Integrated copper–nickel oxide mesoporous nanowire arrays for high energy density aqueous asymmetric supercapacitors†
Ruizhi
Li
ab,
Zhijun
Lin
b,
Xin
Ba
b,
Yuanyuan
Li
*c,
Ruimin
Ding
d and
Jinping
Liu
*ab
aSchool of Chemistry, Chemical Engineering and Life Science and State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, Hubei 430070, P. R. China. E-mail: liujp@whut.edu.cn
bInstitute of Nanoscience and Nanotechnology, Department of Physics, Central China Normal University, Wuhan, Hubei 430079, P.R. China
cSchool of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan, Hubei 430074, P. R. China. E-mail: liyynano@hust.edu.cn
dInstitute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi 030001, China
First published on 9th December 2015
Abstract
An integrated (Cu,Ni)O mesoporous nanowire array was fabricated by a simple hydrothermal method with subsequent annealing, which with optimized Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio = 1
Ni ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 delivers a high specific capacitance of 1710 F g−1. The further assembled aqueous asymmetric supercapacitor (Cu,Ni)O(+)//activated carbon(−) demonstrates high energy/power densities and long cycle life.
1 delivers a high specific capacitance of 1710 F g−1. The further assembled aqueous asymmetric supercapacitor (Cu,Ni)O(+)//activated carbon(−) demonstrates high energy/power densities and long cycle life.
Conceptual insightsAsymmetric supercapacitors are promising capacitive devices due to their higher output voltage and energy density as compared to symmetric ones. To construct such asymmetric devices, high-performance Faradaic electrodes are in general required. Herein, a unique integrated (Cu,Ni)O mesoporous nanowire array has been successfully synthesized for this purpose, in which CuO and NiO nanocrystal subunits are hybridized at a nanoscale into a uniform nanowire structure. The electrochemical performance can be readily manipulated by simply tuning the molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni. When optimized, a strong synergistic interaction exists between CuO and NiO, leading to significantly increased electrical conductivity and electroactivity. This synergistic effect combined with the merits of a binder-free mesoporous array architecture (direct electron transport, facile ion diffusion and a robust electrical contact with the current collector) enables superior rate capability, high energy and power densities, and long cycling stability of the assembled asymmetric supercapacitor device of (Cu,Ni)O(+)//AC(−). Our work paves an avenue for boosting the electrochemical performance of supercapacitor electrodes by nanoscale hybridization into an integrated structure. The methodology presented herein would be easily extended to other hybrid electrode systems for diverse electrochemical applications. Ni. When optimized, a strong synergistic interaction exists between CuO and NiO, leading to significantly increased electrical conductivity and electroactivity. This synergistic effect combined with the merits of a binder-free mesoporous array architecture (direct electron transport, facile ion diffusion and a robust electrical contact with the current collector) enables superior rate capability, high energy and power densities, and long cycling stability of the assembled asymmetric supercapacitor device of (Cu,Ni)O(+)//AC(−). Our work paves an avenue for boosting the electrochemical performance of supercapacitor electrodes by nanoscale hybridization into an integrated structure. The methodology presented herein would be easily extended to other hybrid electrode systems for diverse electrochemical applications.
|
The increasing demand for energy in the 21st century has triggered tremendous research efforts for energy storage and conversion from clean and renewable energy sources.1–4 Electrochemical capacitors (ECs) are promising energy storage devices for future portable systems and hybrid electric vehicles due to their high power density, long lifespan, and fast charge–discharge characteristics.5 Therefore, the development of high-performance and inexpensive electrode materials for ECs has attracted increasing interests in recent years.
Transition metal oxides (TMOs) are typical pseudocapacitive materials with high specific capacitance. Among them, copper oxide (CuO) has attractive merits such as abundance of resources, non-toxicity, and easy preparation in diverse shapes of nanospheres, nanoflowers, nanorods, and nanotubes.6–9 More importantly, CuO can deliver a high theoretical pseudocapacitance of ∼1800 F g−1, which is close to that of the state-of-the-art RuO2·nH2O (∼2200 F g−1).10 Unfortunately, the experimentally achieved capacitance of CuO was far from the theoretical values in the literature (typically 500–600 F g−1).11,12 This may be ascribed to the relatively low reversible redox activity as compared to other popular TMOs like MnO2 and Co3O4. In contrast, nickel oxide (NiO) is considered to be a promising candidate for supercapacitors with prominent electrochemical activity, superior reversibility and stability in alkaline electrolytes, in addition to its low cost and high theoretical capacitance.13,14 Therefore, to boost the electrochemical performance of CuO-based electrodes, it is expected that the incorporation of NiO would be effective with the help of further electrode architecture design.
Regarding the design of electrode architectures, great progress has been made in the construction of additive- and binder-free electrodes to avoid the “dead surface” in traditional slurry-derived electrodes and to allow for more efficient charge and mass transport.15–26 To guarantee intimate electrode–electrolyte contact for faster ion diffusion and efficient redox reaction, the directly grown nanostructures of active materials are in general engineered with a large number of mesopores.
On the basis of all the above considerations, we herein report a unique integrated copper–nickel oxide (denoted as (Cu,Ni)O) mesoporous nanowire array electrode for developing high-performance aqueous asymmetric ECs. The merits of such an electrode can be elaborated as follows. Firstly, individual nanowires are always composed of NiO and CuO particulates interconnected with each other uniformly at the nanoscale; the combination of these two active species into one nanostructure gives rise to lower impedance and much higher specific capacitance in comparison with each component. Secondly, the molar ratio of Cu/Ni in this hybrid oxide electrode can be readily adjusted to obtain different electrochemical performances; this will help to understand the roles of each active material and the synergistic interaction between them during electrochemical energy storage. Finally, the direct alignment of hybrid nanowires on the current collector would ensure a robust electrical contact and electron transport, and the interspacing between nanowire individuals can further facilitate the electrolyte penetration and consequently reduce the interfacial resistance.27,28 As a result, the optimized (Cu,Ni)O nanowire electrode achieves a high capacitance of ∼1710 F g−1.
A 1.8 V asymmetric supercapacitor, assembled using our integrated nanowire array as a positive electrode and commercially activated carbon (AC) as a negative electrode, exhibits energy density as high as 50.3 W h kg−1 (superior to many reported metal oxide//AC devices), high power density (5213.4 W kg−1), and excellent rate capability (∼66.9% capacitance retention with the current increasing 72 times) as well as outstanding cycling stability (6000 times with almost 100% capacitance retention). Our work opens up the possibility of tuning the electrochemical performance of the metal oxide electrode by nanoscale hybridization into an integrated structure.
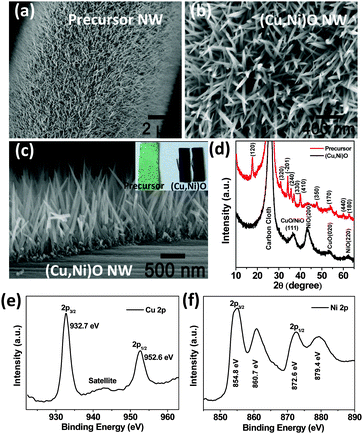
The integrated oxide nanowire array was grown directly on conductive carbon cloth by post-annealing of the precursor nanowire array (see the Experimental section for details, ESI†). Fig. 1a shows a typical scanning electron microscopy (SEM) image of the precursor with a molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The precursor consists of numerous nanowires conformally and uniformly aligned on each carbon fiber. After annealing in Ar gas, a metal oxide nanowire array with a similar shape and orientation can be attained, as displayed in Fig. 1b. The cross-sectional SEM image (Fig. 1c) confirms that the vertically aligned nanowires have average diameter and length of ∼40 nm and 1.5 μm, respectively. The optical images of the precursor and final hybrid oxide nanowire array are shown in the inset of Fig. 1c, which also demonstrate the homogenous growth of the nanowire arrays and provide direct evidence for the intimate contact of the nanowires with the current collector. To rationally determine the composition of the products, X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) were conducted. The XRD spectra of the precursor and oxide nanowire arrays are displayed in Fig. 1d. Besides the signals from carbon cloth, the diffraction peaks of the precursor match well with the standard XRD pattern of (Ni, Cu)2(OH)2CO3 (JCPDS card No. 27-0178), and the peaks of the post-annealing product are indicative of NiO (JCPDS card No. 47-1049) and CuO (JCPDS card No. 48-1548). The chemical composition was further confirmed by XPS analysis, as shown in Fig. 1e and f. In the Cu 2p spectrum, the two peaks at the binding energies of 932.7 and 952.6 eV correspond to Cu 2p3/2 and Cu 2p1/2, respectively. Moreover, the presence of a satellite peak at ∼942.7 eV is the fingerprint of pure CuO crystals.29,30 In Fig. 1f, two obvious shakeup satellite peaks close to two spin–orbit doublets at 854.8 and 872.6 eV are identified as the Ni 2p3/2 and Ni 2p1/2 signals of Ni2+ respectively.31,32
1. The precursor consists of numerous nanowires conformally and uniformly aligned on each carbon fiber. After annealing in Ar gas, a metal oxide nanowire array with a similar shape and orientation can be attained, as displayed in Fig. 1b. The cross-sectional SEM image (Fig. 1c) confirms that the vertically aligned nanowires have average diameter and length of ∼40 nm and 1.5 μm, respectively. The optical images of the precursor and final hybrid oxide nanowire array are shown in the inset of Fig. 1c, which also demonstrate the homogenous growth of the nanowire arrays and provide direct evidence for the intimate contact of the nanowires with the current collector. To rationally determine the composition of the products, X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) were conducted. The XRD spectra of the precursor and oxide nanowire arrays are displayed in Fig. 1d. Besides the signals from carbon cloth, the diffraction peaks of the precursor match well with the standard XRD pattern of (Ni, Cu)2(OH)2CO3 (JCPDS card No. 27-0178), and the peaks of the post-annealing product are indicative of NiO (JCPDS card No. 47-1049) and CuO (JCPDS card No. 48-1548). The chemical composition was further confirmed by XPS analysis, as shown in Fig. 1e and f. In the Cu 2p spectrum, the two peaks at the binding energies of 932.7 and 952.6 eV correspond to Cu 2p3/2 and Cu 2p1/2, respectively. Moreover, the presence of a satellite peak at ∼942.7 eV is the fingerprint of pure CuO crystals.29,30 In Fig. 1f, two obvious shakeup satellite peaks close to two spin–orbit doublets at 854.8 and 872.6 eV are identified as the Ni 2p3/2 and Ni 2p1/2 signals of Ni2+ respectively.31,32
The transmission electron microscopy (TEM) characterization of (Cu,Ni)O nanowires was conducted to gain more structural information. As shown in Fig. 2a, the nanowire is found to have apparent mesopores. The pore size distribution analysis further displays the featured pore size of 2–8 nm (Fig. 2b, see Fig. S1 in the ESI† for more BET details). The mesoporous nanostructure is believed to be formed during the annealing treatment, as CO32− and OH− in the precursor were released into CO2 and water vapour at high temperature, accompanied by the lattice shrinkage and reconstruction. The high-resolution TEM (HRTEM) image in Fig. 2c shows clear lattice fringes with interspacings of 0.23 and 0.24 nm, corresponding to the (111) planes of CuO and NiO, respectively. The CuO and NiO nanocrystals are fused together, forming an integrated mesoporous nanowire structure; this is interesting and can be attributed to the lattice matching between CuO and NiO. Energy dispersive spectroscopy (EDS) elemental mapping clearly illustrates that the homogeneous Ni, Cu and O elements are uniformly distributed throughout the nanowire (Fig. 2d–f), in good agreement with HRTEM results.
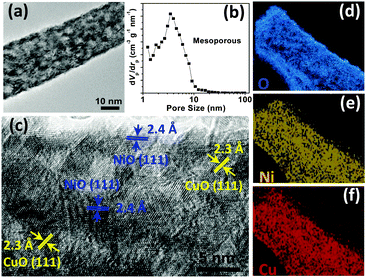 | ||
| Fig. 2 (a) Typical TEM image and (b) pore size distribution of (Cu,Ni)O nanowires. (c) HRTEM image of the nanowire. (d–f) Elemental mapping results of O, Ni and Cu, respectively. | ||
It is well known that the shapes and components of electrode materials have significant influence on the overall electrochemical performance. One of the striking features of our hybrid metal oxide electrode is that the molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni can be controlled via adjusting the reaction solutions, and the resulting electrochemical energy storage property is found to be readily tuned. A series of comparative hydrothermal syntheses were performed with different Cu
Ni can be controlled via adjusting the reaction solutions, and the resulting electrochemical energy storage property is found to be readily tuned. A series of comparative hydrothermal syntheses were performed with different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratios of 0
Ni molar ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (the total mole number of Cu + Ni was kept unchanged; Fig. S2, ESI†). Compared with the product with Cu
0 (the total mole number of Cu + Ni was kept unchanged; Fig. S2, ESI†). Compared with the product with Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1a), the products from Cu
1 (Fig. 1a), the products from Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 0
Ni = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 consist of sparse wire-like films without good vertical alignment. In particular, with Cu
1 consist of sparse wire-like films without good vertical alignment. In particular, with Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (that is, in the absence of nickel salt), only few nanostructures were covered on the carbon cloth. We find that only the product from Cu
0 (that is, in the absence of nickel salt), only few nanostructures were covered on the carbon cloth. We find that only the product from Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 has a similar nanowire array structure to that from Cu
2 has a similar nanowire array structure to that from Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The above results clearly imply that the growth of well-ordered nanowires should be strongly related to the integration of copper and nickel salts in the reaction solutions.
1. The above results clearly imply that the growth of well-ordered nanowires should be strongly related to the integration of copper and nickel salts in the reaction solutions.
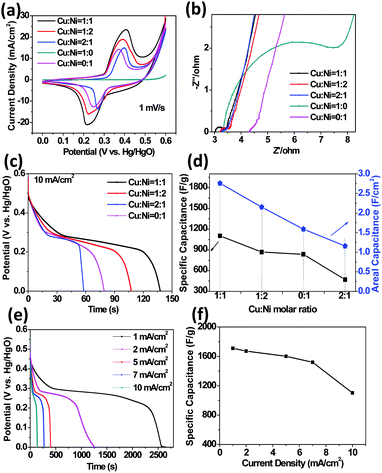
We have further systematically studied the electrochemical performance of the electrodes attained from different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratios in terms of cyclic voltammetry (CV), electrochemical impedance spectrum (EIS), and galvanostatic charge–discharge (CD). All the measurements were conducted in a three-electrode cell in 3 M NaOH aqueous electrolyte (see the Experimental section for details, ESI†). As shown in Fig. 3a, the CV curves of (Cu,Ni)O electrodes typically exhibit a pair of well-defined redox peaks at a scan rate of 1 mV s−1 in the potential range of 0–0.6 V except the Cu
Ni ratios in terms of cyclic voltammetry (CV), electrochemical impedance spectrum (EIS), and galvanostatic charge–discharge (CD). All the measurements were conducted in a three-electrode cell in 3 M NaOH aqueous electrolyte (see the Experimental section for details, ESI†). As shown in Fig. 3a, the CV curves of (Cu,Ni)O electrodes typically exhibit a pair of well-defined redox peaks at a scan rate of 1 mV s−1 in the potential range of 0–0.6 V except the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 electrode that has almost no active materials as discussed above. In addition, the Cu
0 electrode that has almost no active materials as discussed above. In addition, the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode demonstrates strongest redox peaks with the largest CV integrated area. The electrochemical energy storage mechanism in basic electrolytes can be attributed to a combined effect of the following redox processes:33–37
1 electrode demonstrates strongest redox peaks with the largest CV integrated area. The electrochemical energy storage mechanism in basic electrolytes can be attributed to a combined effect of the following redox processes:33–37
| Cu2O + 2OH− ↔ 2CuO + H2O + 2e− |
| NiO + OH− ↔ NiOOH + e− |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni varies, the cathodic peak in CV changes within 0.22–0.27 V and the anodic peak changes within 0.36–0.4 V; this is further indicative of the combined contribution of CuO and NiO to energy storage. In the EIS spectrum, at a high frequency, the intersection of the curve at the real part reflects the resistance of the electrochemical system (Rs) and the semicircle diameter indicates the charge-transfer resistance (Rct), while at a low frequency the slope of the spike reflects the ion diffusion. As illustrated in Fig. 3b, the ion diffusion shows negligible difference for all electrodes. Nevertheless, the Rs values of various (Cu,Ni)O electrodes are smaller than those of the pure metal oxide electrodes (especially NiO, Cu
Ni varies, the cathodic peak in CV changes within 0.22–0.27 V and the anodic peak changes within 0.36–0.4 V; this is further indicative of the combined contribution of CuO and NiO to energy storage. In the EIS spectrum, at a high frequency, the intersection of the curve at the real part reflects the resistance of the electrochemical system (Rs) and the semicircle diameter indicates the charge-transfer resistance (Rct), while at a low frequency the slope of the spike reflects the ion diffusion. As illustrated in Fig. 3b, the ion diffusion shows negligible difference for all electrodes. Nevertheless, the Rs values of various (Cu,Ni)O electrodes are smaller than those of the pure metal oxide electrodes (especially NiO, Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 0
Ni = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), revealing much improved electrical conductivity. In particular, the Cu
1), revealing much improved electrical conductivity. In particular, the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode shows the smallest Rs value of 3.02 Ω (1
1 electrode shows the smallest Rs value of 3.02 Ω (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 3.21 Ω; 2
2, 3.21 Ω; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.27 Ω; 1
1, 3.27 Ω; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 3.34 Ω; 0
0, 3.34 Ω; 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.32 Ω). Furthermore, it displays a much smaller Rct value (0.36 Ω) than that of the 1
1, 4.32 Ω). Furthermore, it displays a much smaller Rct value (0.36 Ω) than that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 electrode (4.34 Ω; pure CuO), indicative of the better electroactivity. Fig. 3c shows the discharging curves of various electrodes at 10 mA cm−2, in which a sloping plateau can always be detected (note that the Cu
0 electrode (4.34 Ω; pure CuO), indicative of the better electroactivity. Fig. 3c shows the discharging curves of various electrodes at 10 mA cm−2, in which a sloping plateau can always be detected (note that the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 electrode was not included since it has almost no capacitance due to the less CuO loading; Fig. S1d, ESI†). The gravimetric and areal capacitances of these electrodes are further calculated and plotted as a function of Cu
0 electrode was not included since it has almost no capacitance due to the less CuO loading; Fig. S1d, ESI†). The gravimetric and areal capacitances of these electrodes are further calculated and plotted as a function of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratio (Fig. 3d). The specific capacitances are 1103.2, 867.7, 835.8, 469.6 F g−1 for the 1
Ni molar ratio (Fig. 3d). The specific capacitances are 1103.2, 867.7, 835.8, 469.6 F g−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1
1. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0
2, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrodes, respectively, while the areal capacitances were 2.76, 2.15, 1.59, 1.16 F cm−2, respectively. The 1
1 electrodes, respectively, while the areal capacitances were 2.76, 2.15, 1.59, 1.16 F cm−2, respectively. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Cu,Ni)O electrode achieves the highest specific capacitance, which is more than twice that of the 2
1 (Cu,Ni)O electrode achieves the highest specific capacitance, which is more than twice that of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode. Simply controlling the Cu
1 electrode. Simply controlling the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio offers an exciting opportunity to optimize the electrochemical performance of our nanowire electrode. The above results ambiguously reveal that the presence of CuO improves the electrical conductivity, while the incorporation of NiO helps to enhance the redox activity (capacitance), manifesting the synergistic interaction between CuO and NiO. To further study the optimized 1
Ni ratio offers an exciting opportunity to optimize the electrochemical performance of our nanowire electrode. The above results ambiguously reveal that the presence of CuO improves the electrical conductivity, while the incorporation of NiO helps to enhance the redox activity (capacitance), manifesting the synergistic interaction between CuO and NiO. To further study the optimized 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode, CDs at various current densities were recorded, as shown in Fig. 3e. The relationship between the specific capacitance and the current density is illustrated in Fig. 3f. When the current density is 1 mA cm−2, its discharge capacitance is measured to be ∼1710 F g−1 (4.28 F cm−2). At higher currents of 2, 5, and 7 mA cm−2, the electrode still has specific capacitances of 1673, 1602, and 1520 F g−1, respectively. The capacitance of 1103 F g−1 can still be maintained even when the current density increases 10 times to 10 mA cm−2 (with 64.5% retention), indicating good rate capability. The capacitance values are much better than those of the previously reported CuO or NiO-based electrodes, such as those of CuO nanobelt (392 F g−1),38 nanowire (88.5, 620 F g−1),11,39 nanosheet (569 F g−1),12 nanoflower (159 F g−1),40 Cu(OH)2 nanowire (750 F g−1);41 NiO nanoflake array (392 F g−1),42 Ni(OH)2 film (357 F g−1),37 and nanoball-like NiOx (951 F g−1).43
1 electrode, CDs at various current densities were recorded, as shown in Fig. 3e. The relationship between the specific capacitance and the current density is illustrated in Fig. 3f. When the current density is 1 mA cm−2, its discharge capacitance is measured to be ∼1710 F g−1 (4.28 F cm−2). At higher currents of 2, 5, and 7 mA cm−2, the electrode still has specific capacitances of 1673, 1602, and 1520 F g−1, respectively. The capacitance of 1103 F g−1 can still be maintained even when the current density increases 10 times to 10 mA cm−2 (with 64.5% retention), indicating good rate capability. The capacitance values are much better than those of the previously reported CuO or NiO-based electrodes, such as those of CuO nanobelt (392 F g−1),38 nanowire (88.5, 620 F g−1),11,39 nanosheet (569 F g−1),12 nanoflower (159 F g−1),40 Cu(OH)2 nanowire (750 F g−1);41 NiO nanoflake array (392 F g−1),42 Ni(OH)2 film (357 F g−1),37 and nanoball-like NiOx (951 F g−1).43
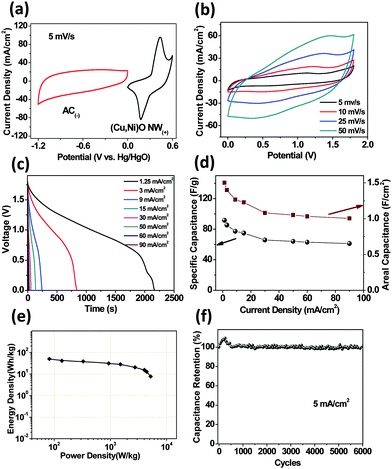
To further evaluate the optimized (Cu,Ni)O nanowire array electrode for device applications, a 2 cm2 asymmetric supercapacitor (Cu,Ni)O(+)//AC(−) was constructed with the 3 M NaOH electrolyte (the mass loading of AC was designed to be 13.9 mg cm−2 to achieve the stored charge matching; (Cu,Ni)O mass: ∼2.5 mg cm−2). Fig. 4a illustrates the CV curves of both (Cu,Ni)O and AC electrodes at 5 mV s−1, in which the electric double-layer capacitive (EDLC) feature of AC can be seen. Fig. 4b shows the CV curves of the asymmetric supercapacitor device at the scan rates from 5 to 50 mV s−1. Unlike the three-electrode electrochemical feature of the integrated mesoporous (Cu,Ni)O electrode, the device always displays a quasi-rectangular CV geometry as EDLC, indicating a capacitive behaviour. The cell voltage is as high as 1.8 V, which is almost twice that of conventional AC-based symmetric capacitors in aqueous electrolytes (0.8–1.0 V). Fig. 4c further displays the discharge curves of devices at various current densities. The discharge profile is relatively linear and reveals a relatively long discharge time of 2166 second at 1.25 mA cm−2. The specific capacitance and areal capacitance at these currents were calculated and plotted in Fig. 4d. The device delivers a high specific capacitance of 91.56 F g−1 (areal capacitance of 1.50 F cm−2) at 1.25 mA cm−2 and 61.23 F g−1 at 90 mA cm−2. By increasing the current 72 times, ∼66.9% of the initial capacitance can be retained, implying its excellent rate capability. Ragone plots are important to evaluate the energy and power performance of supercapacitor devices. On the basis of the above data, the Ragone plot of our device was obtained and is shown in Fig. 4e. Our asymmetric supercapacitor delivers a maximum energy density of 50.3 W h kg−1 at a power density of 82.4 W kg−1. The maximum energy value is considerably higher than those of the symmetric SCs44–46 and is also much superior to those of many recent energy storage devices such as pure NiO//carbon (15–20 W h kg−1),47,48 Cu(OH)2/Cu//AC (18.3 W h kg−1),41 Ni(OH)2//AC (36.2 W h kg−1),49 Ni0.61Co0.39//AC (36.5 W h kg−1),50 NixCo3−xO4//AC (37.4 W h kg−1),51 and NiMoO4·xH2O//AC (34.4 W h kg−1).52 The maximum power density of our device is ∼5213.4 W kg−1. At such a power density, the device is operated within 5.3 s but still maintains a high energy density of 7.68 W h kg−1. The cycle life of the device was further measured by the galvanostatic technique at 5 mA cm−2 (Fig. 4f). The device exhibits excellent cycling stability with the capacitance increase of 0.9% after 6000 cycles. The increase of the capacitance at first cycles is likely due to an “activation process”.4a This result is better than that of the recently reported supercapacitors, such as Co(OH)2//AC (93%, 1000 cycles),53 Ni(OH)2//AC (82%, 1000 cycles),54 Cu(OH)2/Cu//AC (80%, 5000 cycles),41 Ni(OH)2//AC (92%, 1000 cycles),49 Ni0.61Co0.39//AC (62.16%, 1000 cycles),50 Fe3O4//AC (82%, 500 cycles),55 and NiMoO4·xH2O//AC (80.6%, 1000 cycles).52 The achievement of good overall performance of our asymmetric capacitor further manifests the advantages of the integrated (Cu,Ni)O mesoporous nanowire array.
In summary, we have successfully fabricated integrated (Cu,Ni)O mesoporous nanowire arrays directly on carbon cloth, for which the electrochemical performance can be controlled by simply tuning the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratio. The synergetic contribution of the hybrid oxide array can be concluded based on the much improved electroactivity and performance. The optimized (Cu,Ni)O array electrode (Cu
Ni molar ratio. The synergetic contribution of the hybrid oxide array can be concluded based on the much improved electroactivity and performance. The optimized (Cu,Ni)O array electrode (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibits good rate performance and a high specific capacitance of 1710 F g−1. The established (Cu,Ni)O(+)//AC(−) asymmetric supercapacitor device delivers high energy density (50.3 W h kg−1), high power density (5213.4 W kg−1), and excellent cycling stability (6000 cycles, 100.9% retention). Our work will not only shed light on the fabrication of integrated nanowire electrodes via nanoscale hybridization, but also provide a new way of tuning electrode's electrochemical performance for device applications.
1) exhibits good rate performance and a high specific capacitance of 1710 F g−1. The established (Cu,Ni)O(+)//AC(−) asymmetric supercapacitor device delivers high energy density (50.3 W h kg−1), high power density (5213.4 W kg−1), and excellent cycling stability (6000 cycles, 100.9% retention). Our work will not only shed light on the fabrication of integrated nanowire electrodes via nanoscale hybridization, but also provide a new way of tuning electrode's electrochemical performance for device applications.
Acknowledgements
We thank the grants from the National Natural Science Foundation of China (No. 51102105, 11104088), the Science Fund for Distinguished Young Scholars of Hubei Province (No. 2013CFA023), the Youth Chenguang Project of Science and Technology of Wuhan City (No. 2014070404010206), and the Research Start-Up Fund from Wuhan University of Technology.Notes and references
- J. R. Miller and P. Simon, Science, 2008, 321, 651 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- (a) Y. Wang and G. Z. Cao, Adv. Mater., 2008, 20, 2251 CrossRef CAS; (b) J. Liu, G. Z. Cao, Z. G. Yang, D. H. Wang, D. Dubois, X. D. Zhou, G. L. Graff, L. R. Pederson and J. G. Zhang, ChemSusChem, 2008, 1, 676 CrossRef CAS PubMed; (c) H. Xia, C. Y. Hong, B. Li, B. Zhao, Z. X. Lin, M. B. Zheng, S. V. Savilov and S. M. Aldoshin, Adv. Funct. Mater., 2015, 25, 627 CrossRef CAS; (d) Z. Liu, S. W. Tay and X. Li, Chem. Commun., 2011, 47, 12473 RSC.
- (a) C. Zhou, Y. W. Zhang, Y. Y. Li and J. P. Liu, Nano Lett., 2013, 13, 2078 CrossRef CAS PubMed; (b) H. Wang, Y. Liang, M. Gong, Y. Li, W. Chang, T. Mefford, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, Nat. Commun., 2012, 3, 917 CrossRef; (c) G. Huo, X. Lu, Y. Huang, W. Li and G. Liang, J. Eletrochem. Soc., 2014, 161, A1144 CrossRef CAS.
- Z. N. Yu, L. Tetard, L. Zhai and J. Y. Thomas, Energy Environ. Sci., 2015, 8, 702 CAS.
- J. Y. Xiang, J. P. Tu, L. Zhang, Y. Zhou, X. L. Wang and S. J. Shi, J. Power Sources, 2010, 195, 313 CrossRef CAS.
- J. Y. Xiang, J. P. Tu, L. Zhang, Y. Zhou, X. L. Wang and S. J. Shi, Electrochim. Acta, 2010, 55, 1820 CrossRef CAS.
- S. Grugeon, S. Laruelle, R. Herrera-Urbina, L. Dupont, P. Poizot and J. M. Tarascon, J. Electrochem. Soc., 2001, 148, A285 CrossRef CAS.
- X. P. Gao, J. L. Bao, G. L. Pan, H. Y. Zhu, P. X. Huang, F. Wu and D. Y. Song, J. Phys. Chem. B, 2004, 108, 5547 CrossRef CAS.
- B. Vidhyadharan, I. I. Misnon, R. A. Aziz, K. P. Padmasree, M. M. Yusoff and R. Jose, J. Mater. Chem. A, 2014, 2, 6578 CAS.
- J. Huang, H. Wu, D. Cao and G. Wang, Electrochim. Acta, 2012, 75, 208 CrossRef CAS.
- G. L. Wang, J. C. Huang, S. L. Chen, Y. Y. Gao and D. X. Cao, J. Power Sources, 2011, 196, 5756 CrossRef CAS.
- G. Q. Zhang, L. Yu, H. E. Hoster and X. W. Lou, Nanoscale, 2013, 5, 877 RSC.
- G. Zhang, W. Li, K. X. F. Yu and H. Huang, Adv. Funct. Mater., 2013, 23, 3675 CrossRef CAS.
- (a) J. Jiang, Y. Y. Li, J. P. Liu and X. T. Huang, Nanoscale, 2011, 3, 45 RSC; (b) J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- C. C. Hu, K. H. Chang, M. C. Lin and Y. T. Wu, Nano Lett., 2006, 6, 2690 CrossRef CAS.
- (a) B. Liu, J. Zhang, X. F. Wang, G. Chen, D. Chen, C. W. Zhou and G. Z. Shen, Nano Lett., 2012, 12, 3005 CrossRef CAS; (b) W. X. Guo, X. Y. Xue, S. H. Wang, C. J. Lin and Z. L. Wang, Nano Lett., 2012, 12, 2520 CrossRef CAS.
- (a) X. H. Lu, T. Zhai, X. H. Zhang, Y. Q. Shen, L. Y. Yuan, B. Hu, L. Gong, J. Chen, Y. H. Gao, J. Zhou, Y. X. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938 CrossRef CAS; (b) X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, J. Y. Gan, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 1690 CrossRef CAS; (c) Q. Li, Z. L. Wang, G. R. Li, R. Guo, L. X. Ding and Y. X. Tong, Nano Lett., 2012, 12, 3803 CrossRef CAS PubMed.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS.
- J. P. Liu, J. Jiang, M. Bosman and H. J. Fan, J. Mater. Chem., 2012, 22, 2419 RSC.
- R. Z. Li, X. Ren, F. Zhang, C. Du and J. P. Liu, Chem. Commun., 2012, 48, 5010 RSC.
- J. Jiang, J. P. Liu, W. W. Zhou, J. H. Zhu, X. T. Huang, X. Y. Qi, H. Zhang and T. Yu, Energy Environ. Sci., 2011, 4, 5000 CAS.
- J. P. Liu, C. W. Cheng, W. W. Zhou, H. X. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436 RSC.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- J. H. Kim, K. Zhu, Y. F. Yan, C. L. Perkins and A. J. Frank, Nano Lett., 2010, 10, 4099 CrossRef CAS PubMed.
- J. H. Kim, S. H. Kang, K. Zhu, J. Y. Kim, N. R. Neale and A. J. Frank, Chem. Commun., 2011, 47, 5214 RSC.
- X. H. Lu, Y. X. Zeng, M. H. Yu, T. Zhai, C. L. Liang, S. L. Xie, M. Balogun and Y. X. Tong, Adv. Mater., 2014, 26, 3148 CrossRef CAS PubMed.
- (a) X. F. Wang, X. H. Lu, B. Liu, D. Chen, Y. X. Tong and G. Z. Shen, Adv. Mater., 2014, 26, 4763 CrossRef CAS; (b) L. F. Shen, E. Uchaker, X. G. Zhang and G. Z. Cao, Adv. Mater., 2012, 24, 6502 CrossRef CAS; (c) D. Z. Kong, J. S. Luo, Y. L. Wang, W. N. Ren, T. Yu, Y. S. Luo, Y. P. Yang and C. W. Cheng, Adv. Funct. Mater., 2014, 24, 3815 CrossRef CAS.
- S. D. Sun, X. Z. Zhang, Y. X. Sun, S. C. Yang, X. P. Song and Z. M. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 4429 CAS.
- D. Q. Gao, G. J. Yang, J. Y. Li, J. Zhang, J. L. Zhang and D. S. Xue, J. Phys. Chem. C, 2010, 114, 18347 CAS.
- J. W. Lee, T. Ahn, D. Soundararajan, J. M. Ko and J. D. Kim, Chem. Commun., 2011, 47, 6305 RSC.
- H. Chen, L. F. Hu, M. Chen, Y. Yan and L. M. Wu, Adv. Funct. Mater., 2014, 24, 934 CrossRef CAS.
- M. Kang and A. A. Gewirth, J. Phys. Chem. B, 2002, 106, 12211 CrossRef CAS.
- M. Jayalakshmi and K. Balasubramanian, Int. J. Electrochem. Sci., 2008, 3, 1277 CAS.
- S. Komorsky-Lovric, L. Marinic-Pajc, N. Tadej, A. J. M. Horvat and J. Petran, J. Electroanal. Chem., 2008, 623, 75 CrossRef CAS.
- S. Nakayama, A. Kimura, M. Shibata, S. Kuwabata and T. Osakai, J. Electrochem. Soc., 2008, 148, B467 CrossRef.
- G. S. Gund, D. P. Dubal, S. B. Jambure, S. S. Shinde and C. D. Lokhande, J. Mater. Chem. A, 2013, 1, 4793 CAS.
- X. J. Zhang, L. T. Yu, L. L. Wang, R. Ji, G. F. Wang and B. Y. Geng, Phys. Chem. Chem. Phys., 2013, 15, 521 RSC.
- Y. X. Zhang, M. Huang, F. Li and Z. Q. Wen, Int. J. Electrochem. Sci., 2013, 8, 8645 CAS.
- Y. Li, S. Chang, X. Liu, J. Huang, J. Yin, G. Wang and D. Gao, Electrochim. Acta, 2012, 85, 393 CrossRef CAS.
- P. P. Xu, K. Ye, M. M. Du, J. J. Liu, K. Cheng, J. L. Yin, G. L. Wang and D. X. Cao, RSC Adv., 2015, 5, 36656 RSC.
- F. Luan, G. M. Wang, Y. C. Ling, X. H. Lu, H. Y. Wang, Y. X. Tong, X. X. Liu and Y. Li, Nanoscale, 2013, 5, 7984 RSC.
- X. Q. Tian, C. M. Cheng, L. Qian, B. Z. Zheng, H. Y. Yuan, S. P. Xie, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 8029 RSC.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326 CrossRef CAS PubMed.
- X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, S. L. Xie, Y. C. Ling, C. L. Liang, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 5376 CrossRef CAS PubMed.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872 CrossRef CAS PubMed.
- D. W. Wang, F. Li and H. M. Cheng, J. Power Sources, 2008, 185, 1563 CrossRef CAS.
- H. Inoue, Y. Namba and E. Higuchi, J. Power Sources, 2010, 195, 6239 CrossRef CAS.
- J. Huang, P. Xu, D. Cao, X. Zhou, S. Yang, Y. Li and G. Wang, J. Power Sources, 2014, 246, 371 CrossRef CAS.
- Y. Wang, X. Zhang, C. Guo, Y. Zhao, C. Xu and H. Li, J. Mater. Chem. A, 2013, A1, 13290 Search PubMed.
- X. Wang, C. Yan, A. Sumboja and P. S. Lee, Nano Energy, 2014, 3, 119 CrossRef CAS.
- M. C. Liu, L. Kang, L. B. Kong, C. Lu, X. J. Ma, X. M. Li and Y. C. Luo, RSC Adv., 2013, 3, 6472 RSC.
- L. B. Kong, M. Liu, J. W. Lang, Y. C. Luo and L. Kang, J. Electrochem. Soc., 2009, 156, A1000 CrossRef CAS.
- J. W. Lang, L. B. Kong, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2010, 14, 1533 CrossRef CAS.
- X. Du, C. Wang, M. Chen, Y. Jiao and J. Wang, J. Phys. Chem. C, 2009, 113, 2643 CAS.
Footnote |
† Electronic supplementary information (ESI) available: Experimental details and SEM images of (Cu,Ni)O electrodes with different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratios. See DOI: 10.1039/c5nh00100e Ni ratios. See DOI: 10.1039/c5nh00100e |
| This journal is © The Royal Society of Chemistry 2016 |