Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enlarging the chemical space of anti-leishmanials: a structure–activity relationship study of peptoids against Leishmania mexicana, a causative agent of cutaneous leishmaniasis†‡
H. L.
Bolt§
a,
G. A.
Eggimann§
a,
Paul W.
Denny
*ab and
Steven L.
Cobb
*a
aDepartment of Chemistry, Biophysical Sciences Institute, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: s.l.cobb@durham.ac.uk
bSchool of Medicine, Pharmacy and Health, Durham University, Queen's Campus, Stockton-on-Tees, TS17 6BH, UK. E-mail: p.w.denny@durham.ac.uk
First published on 15th March 2016
Abstract
Peptoids, a class of peptide mimetics, have emerged as promising anti-infective agents against a range of bacterial and fungal infections. Recently we have shown peptoids to be novel anti-parasitic and, specifically, anti-leishmanial, compounds. In this study, we have expanded the chemical space of our peptoid library and have identified peptoids with low micromolar activity against Leishmania mexicana axenic amastigotes and significantly, the first peptoids with promising activity against intracellular amastigotes, which are the clinical cause of cutaneous leishmaniasis.
Leishmaniasis is a neglected tropical disease caused by insect vector borne protozoan parasites and is endemic in over 80 countries worldwide. It is estimated that more than 12 million people are currently infected and over 350 million people live at risk of infection. The vast majority of suffers live in areas of poverty where access to health care is often severely limited. Distinct Leishmania species can cause different forms of the disease; cutaneous leishmaniasis (CL), which leads to significant scaring and mucosal damage (mucocutaneous leishmaniasis), or visceral leishmaniasis (VL) causing life-threatening organ damage.1,2
At present, there is no vaccine available for the prevention of CL and VL, and current treatments rely on a limited selection of drugs, such as sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime). These antimonials, requiring reduction to the trivalent Sb(III) form but with an unclear mode of action, have been in clinical use for over 70 years despite showing severe side-effects and requiring parenteral administration.3–5 Furthermore, the long usage of pentavalent antimonials in the treatment of leishmaniasis is leading to the emergence of drug resistance. Although resistance is not yet widespread, Leishmania spp. resistance to these drugs can be easily induced in a laboratory environment.4,5 Therefore second-line drugs such as amphotericin B (Fungizone, a polyene antibiotic which binds parasite specific sterols) and the aromatic diamidine pentamidine (mechanism of action unclear) have been used increasingly.4,5 Both have been in clinical use for over 30 years, share the severe side-effects of antimonials and also require parenteral administration. The newest and only oral drug for VL, miltefosine (a phospholipid, originally developed as an anticancer drug, with an unverified mode of action against Leishmania spp.), is limited by its teratogenicity.4,5 Parasitic resistance towards these alternative drugs has not yet been conclusively confirmed in the field, but there are indications that it may only be a matter of time.4,6 Given the issues surrounding the use of the current first- and second-line drugs to treat leishmaniasis, there is clearly an urgent need to develop new and effective therapies.
Recently, there has been considerable interest in the discovery and development of safer, more effective treatments for VL. In the 2012 London Declaration on neglected tropical diseases, public and private partnerships pledged to help control VL by 2020.7 In contrast CL, a disease that causes 1.2 million new cases each year, remains relatively ignored. Although CL is usually non-fatal, it results in ulcerated lesions that can cause severe disfigurement and lead to other significant medical problems, disability and social exclusion.
Antimicrobial peptides (AMPs) have been proposed as one potential solution to the development of new topical agents to treat CL.8–11 However, their inherent chemical and biological instability presents a major hurdle and only a few AMPs are currently in clinical trials as anti-bacterials.12
Peptoids, a class of peptide-mimetics, offer a better opportunity for the development of new topical anti-leishmanial therapeutics; they are cheaper to manufacture than peptide based drugs and are much more stable under physiological conditions with an improved biological lifetime. The in vitro activity of certain peptoids against both Gram-positive and Gram-negative bacteria and other clinically relevant targets are very similar to those of the leading AMPs. In addition, peptoids display the hallmark broad-spectrum activity of AMPs, suggesting a strong mechanistic similarity.13–17 We have recently shown that peptoids (or poly-N-substituted glycines) have potential as new anti-parasitic compounds against Leishmania mexicana, the causative agent of cutaneous leishmaniasis.18 In this study, we identified several peptoids with low micromolar activities, with the best ‘hit’ having an ED50 of 17 μM against axenic L. mexicana the clinically relevant, mammalian stage of the parasite.
Herein, we have expanded our peptoid library to undertake a more detailed structure–activity relationship (SAR) study. Over 30 novel peptoids were synthesised and tested against L. mexicana promastigotes (insect stage parasites) and axenic amastigotes (mammalian stage parasites). Some peptoids from this library showed improved activity compared to our first library. The most promising peptoid compounds were also successfully screened, for the first time, against intracellular amastigotes (an in vitro model of disease) and their cytotoxicity investigated. Our results provide further evidence that peptoids may be a promising new class of anti-infectives, particularly in the search for improved therapeutics for the treatment of cutaneous leishmanaisis. These peptoids are both more stable to enzymatic degradation than previously analysed antimicrobial peptides, and show more promising activity against the clinically relevant amastigotes.8
Results and discussion
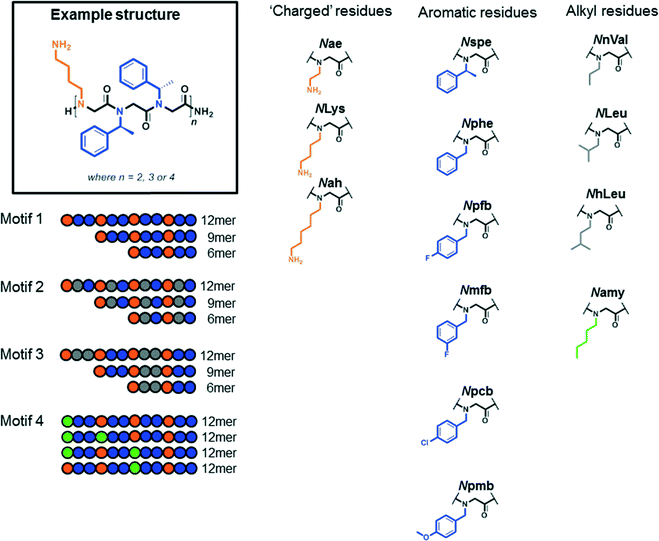
In this extended SAR study, over 30 novel compounds were synthesised to further investigate the biological activity profile of peptoids against L. mexicana. The mode of action of peptoids is suspected to be membrane disruption so to add diversity to this library the sequences were designed around four motifs (see Fig. 1) with varying side chain substituents. We chose to add a range of substituted aromatic monomers to provide variation compared to our previous library18 and selected those derived from commercially available amines.In motif 1, peptoids include the subunit NxNyNy, which was repeated two, three or four times to give 6, 9 or 12 residue peptoids respectively. For Ny different aromatic building blocks were chosen (see Fig. 1 for the structure of the side chains). For the positively charged building block Nx, lysine-type amines with different side chain lengths were chosen to study their influence on anti-leishmanial activity.
In a further iteration, as shown in Fig. 1 with motif 2 (NxNyNz), two different Ny/Nz building blocks were used within the sequence. These residues were uncharged and either aromatic or alkyl amines. To investigate if the sequence specific position of Ny/Nz with their different side chains influenced the activity, they were also placed in a co-block manner (motif 3). In a final design (motif 4), the overall positive charge of a 12mer peptoid was reduced by replacing either one or two charged NLys residues with the uncharged Namy. Namy has the same molecular weight to NLys so differs only in the chemical functionality.
The peptoid library was synthesized manually on resin with the submonomer method using a shaker (50 °C) and 15 min for each coupling or displacement step. All peptoids were purified by RP-HPLC to obtain a library of 49 compounds (for full details regarding synthesis and characterization, please see the ESI‡). All peptoids were screened for activity against L. mexicana promastigotes and amastigotes. These results are shown as ED50 values in Table 1.
| Peptoid | Sequence | ED50 (μM) | |
|---|---|---|---|
| Promastigotes | Amastigotes | ||
| a This data was previously reported by our group18 and is included here for comparison. | |||
| 1 | (NahNpheNphe)4 | 21 | >100 |
| 2 | (NahNpheNphe)3 | >100 | >100 |
| 3 | (NahNpheNphe)2 | >100 | >100 |
| 4 | (NLysNpheNphe)4 | 15 | >100 |
| 5 | (NLysNpheNphe)3 | >100 | >100 |
| 6 | (NLysNpheNphe)2 | >100 | >100 |
| 7 | (NaeNpheNphe)4 | 21 | >100 |
| 8 | (NaeNpheNphe)3 | >100 | >100 |
| 9 | (NaeNpheNphe)2 | >100 | >100 |
| 10 | (NahNspeNspe)4 | 11 | >100 |
| 11 | (NahNspeNspe)3 | 25 | >100 |
| 12 | (NahNspeNspe)2 | >100 | >100 |
| 13 | (NLysNspeNspe)4 | 8 | >100 |
| 14 | (NLysNspeNspe)3 | 15 | >100 |
| 15 | (NLysNspeNspe)2 | >100 | >100 |
| 16 | (NaeNspeNspe)4 | 7 | 17 |
| 17 | (NaeNspeNspe)3 | 10 | >100 |
| 18 | (NaeNspeNspe)2 | >100 | >100 |
| 19 | (NLysNpmbNpmb)4 | 42 | >100 |
| 20 | (NLysNpmbNpmb)3 | >100 | >100 |
| 21 | (NLysNpmbNpmb)2 | >100 | >100 |
| 22 | (NLysNpcbNpcb)4 | 28 | 44 |
| 23 | (NLysNpcbNpcb)3 | 22 | 85 |
| 24 | (NLysNpcbNpcb)2 | 29 | >100 |
| 25 | (NLysNpfbNpfb)4 | 15 | 75 |
| 26 | (NLysNpfbNpfb)3 | 19 | >100 |
| 27 | (NLysNpfbNpfb)2 | >100 | >100 |
| 28 | (NLysNmfbNmfb)4 | 14 | 69 |
| 29 | (NLysNmfbNmfb)3 | 17 | >100 |
| 30 | (NLysNmfbNmfb)2 | >100 | >100 |
| 31 | (NLysNpfbNspe)4 | 8 | 27 |
| 32 | (NLysNpfbNspe)3 | 13 | >100 |
| 33 | (NLysNpfbNspe)2 | >100 | >100 |
| 34 | [(NLysNpfbNpfb)(NLysNspeNspe)]2 | 6 | 21 |
| 35 | (NLysNspeNspe)(NLysNpfbNpfb)(NLysNspeNspe) | 13 | >100 |
| 36 | (NLysNpfbNpfb)(NLysNspeNspe) | >100 | >100 |
| 37 | (NLysNnValNspe)4 | >100 | >100 |
| 38 | (NLysNnValNspe)3 | >100 | >100 |
| 39 | (NLysNnValNspe)2 | >100 | >100 |
| 40 | (NLysNLeuNspe)4 | >100 | >100 |
| 41 | (NLysNLeuNspe)3 | >100 | >100 |
| 42 | (NLysNLeuNspe)2 | >100 | >100 |
| 43 | (NLysNhLeuNspe)4 | 12 | >100 |
| 44 | (NLysNhLeuNspe)3 | 52 | >100 |
| 45 | (NLysNhLeuNspe)2 | >100 | >100 |
| 46 | (NamyNspeNspe)[(NLysNspeNspe)]3 | 8 | 21 |
| 47 | (NamyNspeNspe)2(NLysNspeNspe)2 | 11 | 16 |
| 48 | [(NamyNspeNspe)(NLysNspeNspe)]2 | 10 | 17 |
| 49 | (NLysNspeNspe)2(NamyNspeNspe)(NLysNspeNspe) | 10 | 15 |
After having found active peptoids against the L. mexicana parasite in an earlier library,18 other aromatic building blocks were included within our motif of NLysNyNy (Table 1). Using the building block Npmb in the 12mer peptoid 19, the activity against promastigotes reduced significantly by 3 fold (ED50 = 42 μM) compared to analogues containing Nphe (peptoid 4, ED50 = 15 μM) or Nspe (peptoid 13, ED50 = 8 μM). The shorter 6- and 9mer versions (peptoid 21 and 20 respectively) did not show any activity against the promastigotes and no activity could be found against amastigotes for any length of the Npmb peptoids.
In contrast, peptoids with chlorinated and fluorinated aromatic building blocks (Npcb, Npfb and Nmfb; peptoids 22–30) showed enhanced activity against the axenic amastigotes, whilst maintaining activity against promastigotes, the insect form of the parasite. Peptoids 22–24 have a chloro substituent in the para position of the phenyl ring (Npcb). These were all active against the promastigotes, but only the longer 9- and 12mer (peptoids 22 and 23) also showed any activity against the axenic amastigotes with ED50 values of 85 or 44 μM, respectively. In case of the peptoids with fluorine atoms in the para or meta positions, only the longest 12 residue peptoids showed modest toxicity against the amastigotes (peptoid 25: ED50 = 75 μM and peptoid 28: ED50 = 69 μM). However, fluorinated peptoids had a good efficiency against promastigotes, with similarly low ED50 values as the non-halogenated analogues. Even the shorter 9 residue peptoids, 26 and 29, showed anti-parasitic activity against promastigotes in the low μM range. The short 6mers (peptoid 27 and 30) did not show any activity against either stage of the parasite.
Our previous study18 showed an increase in activity when the chiral Nspe residue was included within the sequence compared to Nphe. Therefore, the chiral aromatic building block Nspe was then combined with the fluorinated building block Npfb in either motif 2 or 3 (peptoids 31–36). Interestingly, the analogues of both motifs had similar activity against both promastigotes and axenic amastigotes. The longest 12 residue peptoids, 31 and 34, showed very good activity against promastigotes (ED50 values of 8 and 6 μM). This activity was similar to the Nspe-only template peptoid 13 (ED50 = 8 μM) and about 2 fold better compared to the Npfb-only peptoid 25 (ED50 = 15 μM). However, the difference in activity between 31/34 and 13 was very different against the axenic amastigote form; the Nspe-Npfb-mixed peptoids 31 and 34 showed good activity (ED50 values of 27 and 21 μM respectively) whereas the Nspe-only peptoid 13 was completely inactive and the Npfb-only peptoid 25 showed only moderate activity with an ED50 of 75 μM. Therefore, the combination of two building blocks with different activities against promastigotes and axenic amastigotes can help to improve the overall activity against the parasite. Peptoid 34 was the most active compound from this part of the SAR study with ED50 values of 6 μM (promastigotes) and 21 μM (axenic amastigotes).
The library was then extended to include sequences that combine one aromatic residue and one residue with an alkyl side chain within the same peptoid. These peptoids were based on motif 2 and the monomers NnVal, NLeu and NhLeu were used to synthesize peptoids 37–45. As shown in Table 1, none of these peptoids showed much activity against either lifecycle stage of L. mexicana. Only the 12 and 9 residue peptoids containing the NhLeu building block were active against the promastigote form, (peptoid 43 ED50 = 12 μM, peptoid 44 ED50 = 52 μM). These results highlight that the size and chemical functionality of residues in the motif NxNyNz is important for anti-leishmanial efficacy.
Our previous study showed that the hydrophobicity of side chains in the peptoid sequence influenced the anti-parasitic activity of the compound; the shorter Nae residue significantly increased the biological activity against the amastigote form of the parasite compared to the NLys building block.18 To extend the SAR study, NLys residues were replaced by the alkyl building block Namy in several sequences. In these peptoids, the terminal –NH2 group of one or two NLys side chains is replaced by a –CH3 group. Thus, the net positive charge of the peptoid is reduced, but has the same molecular weight as the NLys analogues, as shown in Table 1 (peptoids 46–49).
For most compounds in this library (peptoids 1–45), the promastigotes are more susceptible to the peptoids than amastigotes. We believe this is due to the large differences in the cell surface coat of each parasite.19 However, surprisingly, all four Namy peptoids (46–49) showed very similar activity not only against the promastigotes, but also against the amastigotes. For the promastigotes, ED50 values were between 8–10 μM and therefore comparable to our previous ‘hit’, peptoid 16 (ED50 = 7 μM). The activity against the amastigotes was also in the low μM range with ED50 values between 15–21 μM and similar to peptoid 16 (ED50 = 17 μM). These results suggest that the increased hydrophobicity and reduced charge of the Namy residues can increase biological activity compared to shorter alkyl substituents and it does not matter if the overall positive charge is reduced by 1 or 2 or which residue is replaced.19,20
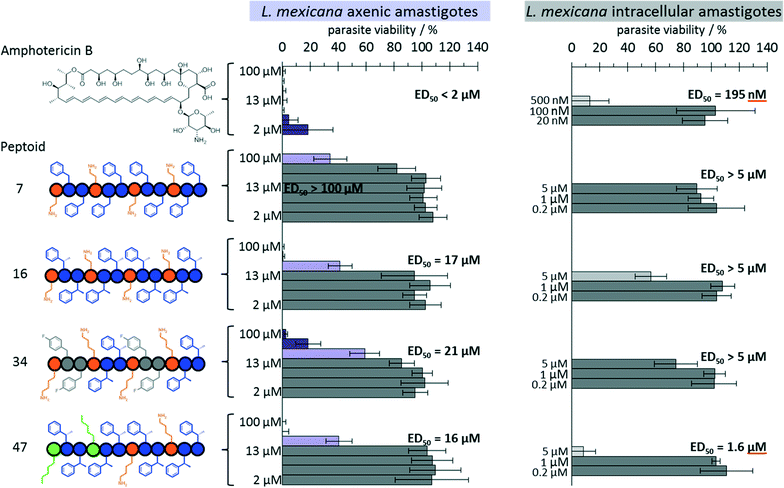
All aforementioned results discuss assays against axenic, i.e. extracellular, cultured amastigotes. In the clinical stage of leishmaniasis the amastigotes reside within macrophages. Triage from these axenic assays highlighted four promising sequences from our library that were screened against L. mexicana infected RAW264.7 murine macrophages. The results were obtained using an assay system recently described for screening compounds against L. donovani infected cells,21–23 with some modifications to suit our cell lines, and are summarised in Table 2/Fig. 2.
| Compound | Peptoid sequence | ED50 (μM) | |||
|---|---|---|---|---|---|
| Promastigotes | Axenic amastigotes | Intracellular amastigotes | RAW 264.7 | ||
| Amphotericin B | <2 | <2 | 0.195 | >5 | |
| 7 | (NaeNpheNphe)4 | 21 | >100 | >5 | >5 |
| 16 | (NaeNspeNspe)4 | 7 | 17 | >5 | 3 |
| 34 | [(NLysNpfbNpfb)(NLysNspeNspe)]2 | 6 | 21 | >5 | 5 |
| 47 | (NamyNspeNspe)2(NLysNspeNspe)2 | 11 | 16 | 1.6 | 1.7 |
Peptoid 7 and 16 were chosen from our earlier study. Peptoid 16 (NaeNspeNspe)4 was the most potent peptoid from the previous library and showed low μM activity against both promastigote and axenic amastigote forms of the parasite. Peptoid 7 is an analogue of peptoid 16 and only showed activity against promastigotes so was used as negative control in our assay. From this study, peptoid 34 was chosen to investigate the mixed use of the aromatic building blocks Nspe and Npfb. Peptoid 47, was chosen to examine the effect of a reduced overall positive charge and greater hydrophobicity of the Namy residue.
The results show that not all peptoids with activity against axenic amastigotes can retain their activity against the intracellular amastigotes. Encouragingly, peptoid 16 showed some activity against the intracellular parasites at 5 μM, the highest concentration measured, whilst peptoid 34 was only moderately active at this concentration. However, peptoid 47, which had the lowest ED50 value against axenic amastigotes (16 μM), was also the most active against the intracellular amastigotes with an ED50 of 1.6 μM. Peptoid 7 was, as expected, non-active under the concentrations tested indicating that peptoids with little activity against the axenic amastigotes are likely to have poor activity against intra-macrophage amastigotes.
However, it is noteworthy, and consistent with studies from other groups,16 that peptoids with increased antimicrobial activity also demonstrated increased host cell cytotoxicity (Table 2), limiting the range of concentrations studied in the infection assays to ≤5 μM. For example, peptoids 16 and 34 demonstrated greater toxicity against the RAW264.7 macrophages than the intracellular (and axenic) amastigotes (ED50 > 5 vs. the intracellular parasite against ED50 of 3 μM and 5 μM respectively vs. the macrophages); and peptoid 47 is equivalently cytotoxic to both host and parasite (ED50 of 1.7 and 1.6 respectively). Whilst further screening determine the toxicity of the complete library against the host cell may have some value for future consideration of the cytotoxicity and mode of action of peptoids, this study sought to establish the selective nature of peptoids identified as having significant potency against L. mexicana axenic amastigotes.
Clearly overcoming host cell cytotoxicity is a general problem for peptoids, however the fact that peptoid 47 demonstrated low micromolar activity against L. mexciana infected macrophages may provide a starting for studies of the antimicrobial and cytotoxic effects of peptoids. Despite the host cell toxicity the data gathered from the peptoid library (and specifically peptoids 16, 34 and 47) will help to further elucidate the key characteristics required for anti-leishmanial compound development.
Conclusions
In conclusion within this study we have significantly enlarged the peptoid activity data available for the development of potential anti-leishmanials. Peptoid 47 (NamyNspeNspe)2(NLysNspeNspe)2 is the first peptoid published to have activity in the low μM range against L. mexicana promastigotes and axenic amastigotes, and also retain activity against the clinically significant intra-macrophage amastigotes. Despite evident host macrophage cytotoxicity, 47 is a promising starting point for peptoid-based anti-leishmanials.Since any potential treatments for cutaneous leishmaniasis could be formulated for topical application, a certain level of toxicity to host cells may be acceptable. To overcome or reduce the toxicity against macrophages future medicinal chemistry investigations will need to be undertaken, perhaps to maximise delivery of the peptoid to the parasite by optimising host cell penetration. Given that the predicted mode of action of peptoids is plasma membrane pore-formation or disruption17 rather than inhibition of a protein target, the risk of development of parasite resistance is low, conferring an advantage to this approach in treatment.
In summary, the results described provide further evidence that peptoids are a promising new class of anti-infectives, particularly in the search for improved topical therapeutics for the treatment of CL.
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (Durham University DTG, HLB) and the Swiss National Science Foundation (GAE) for financial support.Notes and references
- S. Y. Chon, H. Q. Doan, R. M. Mays, S. M. Singh, R. A. Gordon and S. K. Tyring, Antibiotic overuse and resistance in dermatology, Dermatol. Ther., 2012, 25, 55–69 CrossRef PubMed.
- J. Alvar, I. D. Velez, C. Bern, M. Herrero, P. Desjeux, J. Cano, J. Jannin and M. den Boer, Leishmaniasis worldwide and global estimates of its incidence, PLoS One, 2012, 7, e35671 CAS.
- L. Kedzierski, Leishmaniasis Vaccine: Where are We Today?, J. Global Infect. Dis., 2010, 2, 177–185 CrossRef PubMed.
- S. L. Croft, S. Sundar and A. H. Fairlamb, Drug resistance in leishmaniasis, Clin. Microbiol. Rev., 2006, 19, 111–126 CrossRef CAS PubMed.
- N. Singh, M. Kumar and R. K. Singh, Leishmaniasis: current status of available drugs and new potential drug targets, Asian Pac. J. Trop. Med., 2012, 5, 485–4974.6 CrossRef CAS PubMed.
- M. Ephros, E. Waldman and D. Zilberstein, Pentostam induces resistance to antimony and the preservative chlorocresol in Leishmania donovani promastigotes and axenically grown amastigotes, Antimicrob. Agents Chemother., 1997, 41, 1064–1068 CAS.
- London Declaration on Neglected Tropical Diseases, www.dndi.org/images/stories/press_kit/PressRoom/NTDs/NTD_Event_London_Declaration.pdf Search PubMed.
- F. L. Chadbourne, C. Raleigh, H. Z. Ali, P. W. Denny and S. L. Cobb, Studies on the antileishmanial properties of the antimicrobial peptides temporin A, B and 1Sa, J. Pept. Sci., 2011, 17, 751–755 CrossRef CAS PubMed.
- S. L. Cobb and P. W. Denny, Antimicrobial peptides for leishmaniasis, Curr. Opin. Invest. Drugs, 2010, 11, 868–875 CAS.
- M. L. Mangoni, J. M. Saugar, M. Dellisanti, D. Barra, M. Simmaco and L. Rivas, Temporins, small antimicrobial peptides with leishmanicidal activity, J. Biol. Chem., 2005, 280, 984–990 CrossRef CAS PubMed.
- S. Dabirian, Y. Taslimi, F. Zahedifard, E. Gholami, F. Doustdari, M. Motamedirad, S. Khatami, K. Azadmanesh, S. Nylen and S. Rafati, Human Neutrophil Peptide-1 (HNP-1): A New Anti-Leishmanial Drug Candidate, PLoS Neglected Trop. Dis., 2013, 7, e2491 Search PubMed.
- D. K. Mercer and D. A. O'Neil, Peptides as the next generation of anti-infectives, Future Med. Chem., 2013, 5, 315–337 CrossRef CAS PubMed.
- M. T. Dohm, R. Kapoor and A. E. Barron, Peptoids: bio-inspired polymers as potential pharmaceuticals, Curr. Pharm. Des., 2011, 17, 2732–2747 CrossRef CAS PubMed.
- M. L. Huang, M. A. Benson, S. B. Y. Shin, V. J. Torres and K. Kirshenbaum, Amphiphilic Cyclic Peptoids That Exhibit Antimicrobial Activity by Disrupting Staphylococcus aureus Membranes, Eur. J. Org. Chem., 2013, 3560–3566 CrossRef CAS.
- R. Kapoor, M. W. Wadman, M. T. Dohm, A. M. Czyzewski, A. M. Spormann and A. E. Barron, Antimicrobial peptoids are effective against Pseudomonas aeruginosa biofilms, Antimicrob. Agents Chemother., 2011, 55, 3054–3057 CrossRef CAS PubMed.
- B. Mojsoska, R. N. Zuckermann and H. Jenssen, Structure–activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides, Antimicrob. Agents Chemother., 2015, 59, 4112–4120 CrossRef CAS PubMed.
- N. P. Chongsiriwatana, J. A. Patch, A. M. Czyzewski, M. T. Dohm, A. Ivankin, D. Gidalevitz, R. N. Zuckermann and A. E. Barron, Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2794–2799 CrossRef CAS PubMed.
- G. A. Eggimann, H. L. Bolt, P. W. Denny and S. L. Cobb, Investigating the Anti-leishmanial Effects of Linear Peptoids, ChemMedChem, 2015, 10, 233–237 CrossRef CAS PubMed.
- G. A. Eggimann, K. Sweeney, H. L. Bolt, N. Rozatian, S. L. Cobb and P. W. Denny, The Role of Phosphoglycans in the Susceptibility of Leishmania mexicana to the Temporin Family of Anti-Microbial Peptides, Molecules, 2015, 20, 2775–2785 CrossRef PubMed.
- M. Torrent, D. Pulido, L. Rivas and D. Andreu, Antimicrobial peptide action on parasites, Curr. Drug Targets, 2012, 13, 1138–1147 CrossRef CAS PubMed.
- S. K. Jain, R. Sahu, L. A. Walker and B. L. Tekwani, A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line, J. Visualized Exp., 2012, 30, 4054 Search PubMed.
- D. Paape, A. S. Bell, W. P. Heal, J. A. Hutton, R. J. Leatherbarrow, E. W. Tate and D. F. Smith, Using a non-image-based medium-throughput assay for screening compounds targeting N-myristoylation in intracellular Leishmania amastigotes, PLoS Neglected Trop. Dis., 2014, 8, e3363 Search PubMed.
- P. A. Bates, Complete developmental cycle of Leishmania mexicana in axenic culture, Parasitology, 1994, 108, 1–9 CrossRef PubMed.
Footnotes |
| † The authors declare no competing interests. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6md00060f |
| § These authors contributed equally to the project. |
| This journal is © The Royal Society of Chemistry 2016 |