Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Syntheses and biological evaluations of highly functionalized hydroxamate containing and N-methylthio monobactams as anti-tuberculosis and β-lactamase inhibitory agents
Mark W.
Majewski
a,
Kyle D.
Watson
a,
Sanghyun
Cho
b,
Patricia A.
Miller
a,
Scott G.
Franzblau
b and
Marvin J.
Miller
*a
aDepartment of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN 46556, USA. E-mail: mmiller1@nd.edu; Fax: +1 574 631 6652; Tel: +1 574 631 7571
bInstitute for Tuberculosis Research, College of Pharmacy, University of Illinois at Chicago, MIC 964, Rm. 412, IL 60612, USA
First published on 6th November 2015
Abstract
Correction for ‘Syntheses and biological evaluations of highly functionalized hydroxamate containing and N-methylthio monobactams as anti-tuberculosis and β-lactamase inhibitory agents.
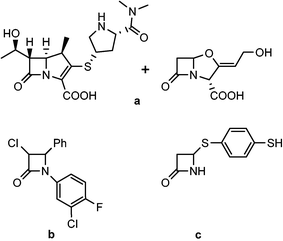
The authors regret that compound number 1 was used for two different compounds in the manuscript. In Fig. 2 compound numbers 1, 2 and 3 should be corrected to show a, b and c. The corrected figure is shown below.
And consequently, the text on page 2, referring to Fig. 2, should be corrected to read: Reports of β-lactam compounds with potent anti-TB activity, however, have been scarce. Certain classic β-lactams can exhibit anti-TB activity when administered in combination with clavulanate, a β-lactamase inhibitor (Fig. 2, a).9,10 Furthermore, monobactam alkylthiols and halogen substituted aromatic monobactams have also demonstrated intrinsic activity (Fig. 2, b–c).11,12 In general, β-lactams have not been widely used in TB therapy for three major reasons: issues with permeability of the cell wall of M. tb, the persistent threat of inactivation by β-lactamases, and poor activity in vivo.13
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2016 |