Microfluidic fuel cells for energy generation
M.
Safdar
a,
J.
Jänis
*a and
S.
Sánchez
*bcd
aDepartment of Chemistry, University of Eastern Finland, FI-80101 Joensuu, Finland. E-mail: janne.janis@uef.fi
bMax Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart, Germany. E-mail: sanchez@is.mpg.de
cSmart nano-bio-devices Laboratory, Institute for Bioengineering of Catalonia (IBEC), Baldiri Reixac, 10-12, Barcelona 08028, Spain. E-mail: ssanchez@ibecbarcelona.eu
dInstitució Catalana de Recerca i Estudis Avançats (ICREA), Psg. Lluís Companys, 23, 08010 Barcelona, Spain
First published on 1st July 2016
Abstract
Sustainable energy generation is of recent interest due to a growing energy demand across the globe and increasing environmental issues caused by conventional non-renewable means of power generation. In the context of microsystems, portable electronics and lab-on-a-chip based (bio)chemical sensors would essentially require fully integrated, reliable means of power generation. Microfluidic-based fuel cells can offer unique advantages compared to conventional fuel cells such as high surface area-to-volume ratio, ease of integration, cost effectiveness and portability. Here, we summarize recent developments which utilize the potential of microfluidic devices for energy generation.
Rapidly growing global energy consumption and increasing environmental concerns due to fossil fuel combustion mean that sustainable and environmentally friendly energy resources need to be discovered and implemented. Commonly adopted renewable energy resources, such as wind power and photovoltaics, suffer from low efficiency. Different fuel cells, which convert the chemical energy of a fuel into electric power with an efficiency up to 60%, have emerged as an alternative means for energy production.1 Miniaturized fuel cells offer several advantages over conventional methods, such as portability, faster mass transfer, and hence, a fast start up for rapid power generation, and higher power density. Potential application areas of these miniaturized fuel cells include portable communication and transportation systems, lab-on-a-chip diagnostic devices and integrated (bio)chemical sensors. A typical fuel cell consists of an anode and a cathode decorated with a catalyst to trigger an electrochemical reaction. A fuel undergoes oxidation at the anode to produce positive ions and electrons. The released electrons flow towards the cathode through an external circuit, whereas the positive ions are transported to the cathode through an electrolyte membrane.
Microfluidics involves structures where one of the dimensions is in the range of 1–1000 μm to control the fluid flow at low Reynold's number, i.e. laminar flow. This means that mixing by turbulence is minimized and the only remaining mechanism for mixing is diffusion. A microfluidic fuel cell refers to a miniaturized energy generator with integrated electrodes, an interconnected network of channels for the fluid flow, and inlets/outlets for the delivery and removal of a fuel and an oxidant. The fuel is typically an aqueous solution of either glucose, methanol, formic acid or dissolved hydrogen. An oxidant solution may contain dissolved oxygen, hydrogen peroxide or permanganate. An electrolyte is added to the fuel and oxidant streams to facilitate transport of ions across the co-flowing streams, thereby eliminating the need for a separating membrane. In this way, the issues arising from membrane degradation and fuel crossover can be avoided. However, microfluidic fuel cells containing a proton exchange membrane (PEM) do exist. In addition, the independent control over the composition of the fuel and oxidant streams, the possibility to tune the diffusion/depletion zones and the higher surface-to-volume ratio of the microsystem can all be exploited to enhance the overall reaction kinetics and cell performance. In this Focus article, we discuss recent developments in the field of microfluidic devices for the purpose of energy generation. We will present microfluidic fuel cells categorized into three groups, i.e. biofuel cells, photocatalytic fuel cells and electrochemical fuel cells. This classification is based on the type of catalysts used to trigger anodic reactions.
Microfluidic biofuel cells
Biofuel cells make use of enzymes or microorganisms for the conversion of the chemical energy of a fuel (usually alcohol or carbohydrate) into electric power. Conventional biofuel cells are based on macroscale diffusion chambers separated by large Nafion membranes, which lead to mass transfer limitations and restricted portability. In contrast, microfluidic devices enable introduction of single or multiple fluid streams containing a biocatalyst, fuel and supporting electrolytes, into a conductive, confined environment. Zebda et al. reported an enzyme-based Y-shaped microfluidic fuel cell with co-flowing fuel and oxidant streams.2 The electrodes were placed in parallel along the catholyte and anolyte streams. As the cathode performed reduction of O2 by laccase, the anode enabled oxidation of glucose by glucose oxidase. The resulting power density of the fuel cell was 110 μW cm−2 at 0.3 V.Enzymatic fuel cells, with enzyme molecules in the solution phase suffer from limited lifetimes and higher costs. This issue can be overcome by immobilizing enzyme molecules to the electrode surface. Noh et al. developed an enzymatic fuel cell by covalently immobilizing glucose oxidases on the carboxylated polyterthiophene-assembled layers of nanoparticles.3 The cell operation involved conversion of glucose into gluconic acid and hydrogen peroxide, which was subsequently reduced at the cathode. A power density of 0.78 mW cm−2 and an open circuit voltage of 0.48 V were observed, with lifetime of 16 days due to chemical bonding of the enzyme to the support material. A flexible, air-breathing microfluidic fuel cell powered by glucose from human serum and blood was reported by Dector et al.4 The anode was made of crosslinked glucose oxidase, Vulcan carbon and carbon nanotubes, whereas Pt/C was used as the cathode. A power density of 0.20 mW cm−2 at 0.52 V was produced when human blood was directly injected into the cell.
The other class of biofuel cells can be referred to as microbial fuel cells, which typically utilize electrogenic bacteria or algae for the production of electricity. These fuel cells can operate under ambient conditions for longer periods of time, and are low cost. Qian et al. developed a miniaturized microbial fuel cell with a PEM sandwiched between a carbon cloth cathode and a gold anode.5Shewanella oneidensis bacterium consumed the supplied organic fuel (lactate) to produce electrons and protons which were transferred to the cathode through an external circuit and the PEM, respectively. As a result, a maximum power density of 0.15 μW cm−2 was achieved (Fig. 1A). The low performance of the microbial fuel cell can be attributed to the use of a bacterium which requires extracellular electron transfer mediators, the presence of electron sinks or the high internal resistance of the cell. A microbial fuel cell with high specific surface area, an O2 scavenger and Geobacter-enriched mixed bacterial culture capable of forming a conductive biofilm matrix for high current density, was reported by Choi et al.6 The resulting power density of the cell was 4.7 μW cm−2. Even higher power output and longer operational lifetime can be achieved through an integrated array of fuel cells on a single platform with the possibility to periodically replenish both the fuel and the oxidant solutions.7 Such a device can be used to perform power generation with various combinations of microbes and fuels (Fig. 1B).
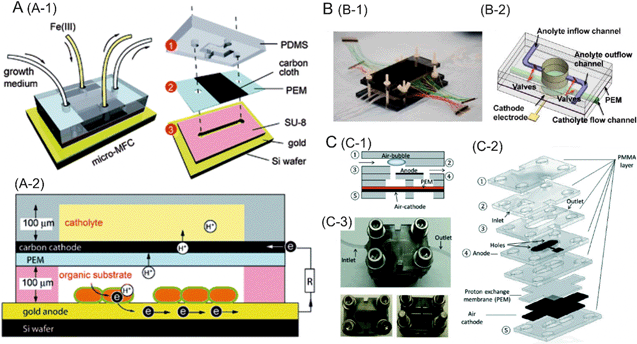 | ||
| Fig. 1 A. Schematic representation of the microbial fuel cell. (A-1) design and (A-2) principle of operation of a microfluidic biofuel cell consisting of Shewanella oneidensis strain MR-1 inoculated on a gold anode. B. Microfluidic microbial fuel cell array. B-1 shows a photograph of the fully assembled microfluidic fuel cell array. B-2 is the graphical illustration of a single functional microfluidic fuel cell unit. A PEM sandwiched anode and cathode chamber layers, an anode and a cathode electrode. C. A microfluidic bio-solar cell. C-1 and C-2 are schematic representations of the bio-solar cell showing the device architecture. C-3 is a photograph of the fully assembled bio-solar cell with top and bottom views. Reprinted with permission from ref. 5 (A), ref. 7 (B) and ref. 8 (C). | ||
Bio-solar cells employ photosynthetic microorganisms, which do not require an external fuel, and can produce power throughout a day–night cycle. For example, cyanobacteria can convert H2O and CO2 into carbohydrates under light, thus generating electrons which are transferred to the cathode to produce an electromotive force. A microfluidic bio-solar cell based on this principle generated a power density of 0.9 mW m−2 (Fig. 1C).8
Paper-based lateral flow devices offer unique features over microfluidic platforms made from plastics or glass, such as capillarity-assisted fluid transport, high surface area-to-volume ratio and availability of techniques to produce flexible, transparent nanostructured paper. A paper-based multi-layered energy generator driven by Shewanella oneidensis was recently introduced by Fraiwan et al.9 The device architecture enabled realization of an array of microbial fuel cells connected in series to power up an LED lamp for more than 30 minutes. The bacteria in the anodic chamber caused oxidation of the organic matter to produce electrons which were transferred to the cathode through an external resistor.
Microfluidic photocatalytic fuel cells
Photocatalytic fuel cells (PFCs) realize light absorption and charge separation/transfer processes by the association of a light absorbing sensitizer layer (typically a dye) onto a nanocrystalline wide band gap semiconductor material. A layer of TiO2 nanoparticles is applied and sintered onto a transparent conducting oxide layer. The resulting nanocrystalline TiO2 is then treated with a photosensitizer, such as an organic dye. The dyes used in most PFCs are susceptible to degradation caused by light or temperature, which may lead to the decreased long term performance of the device. Thus, techniques ensuring sustained performance of the cell in spite of light or temperature fluctuations are needed. Microfluidics possesses enormous potential in this regard. As an example, Koo et al. have developed a microfluidic PFC which can achieve photocurrent recovery after damage of the adsorbed dye by intense UV illumination.10 The device consists of agarose hydrogel with an embedded network of microfluidic channels sandwiched between a TiO2 photoanode and a platinum counter electrode. By controlling the pH-dependent desorption/adsorption of Eosin Y dye from the TiO2 anode, the performance of the PFC was successfully recovered (Fig. 2A).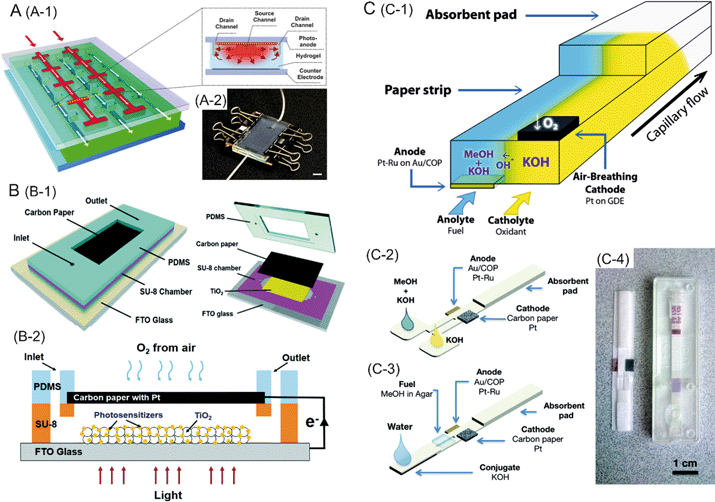 | ||
| Fig. 2 A (A-1) Schematic of the hydrogel photovoltaic device. Inset shows a cross sectional view along the dotted yellow line. The dye and electrolyte solutions first flow through the source channels, followed by penetration into the gel to reach the drain channel. Red arrows depict lateral diffusive/convective transport between the source and drain channels. A-2 is the photograph of the prototype device. B (B-1) shows the schematic layout of the photocatalytic fuel cell with a CdS–ZnS composite photosensitive layer. B-2 represents the working principle of the fuel cell. C. Paper-based microfluidic fuel cell. C-1 shows a schematic of the working principle of the fuel cell. C-2 is a graphical representation of the fuel cell. C-3 shows the concept of a paper-based fuel cell activated with water, resembling a lateral flow test strip. C-4 is the comparison of a paper-based microfluidic fuel cell and a commercial lateral flow diagnostic strip. Reprinted with permission from ref. 10 (A), ref. 11 (B) and ref. 13 (C). | ||
Photocatalytic fuel cells may enable energy generation by transforming the chemical energy of organic pollutants into electricity through wastewater remediation. The absorption of light by a semiconductor photoanode produces electron–hole pairs. While the electrons lead to a flow of current, the holes oxidize pollutant molecules for water remediation. Li et al. have recently developed a microdevice based on a TiO2 photoanode treated with a CdS–ZnS composite as the photosensitive layer.11 The fuel cell performed the degradation of glucose and methylene blue as model wastewater contaminants, with a maximum output power density of 0.58 mW cm−2 for glucose (Fig. 2B).
Microfluidic electrochemical fuel cells
Though the mechanism of operation of the fuel cells discussed in the previous sections is based on redox reactions, they either employ biocatalysts or are based on photocatalytic reactions. In contrast, the microfluidic electrochemical fuel cells often utilize carbon electrodes, metals or metal alloys as the catalyst. Jayashree et al. developed an air-breathing microfluidic fuel cell composed of a Pt cathode and palladium-based anode.12 Using formic acid as a fuel and a co-flowing stream of aqueous sulfuric acid solution, the cell utilized oxygen delivery directly from the environment to the cathode, enabling a power density of 26 mW cm−2.A microfluidic paper-based fuel cell developed by Esquivel et al. was capable of generating power densities in the range of 1–5 mW cm−2.13 The device consists of a conjugated pad to store an electrolyte and a fuel, and integrated electrodes. The fuel cell is operated by simply soaking the sample pad in water. An electric current could be observed when the diluted fuel underwent oxidation at the Pt–Ru anode (Fig. 2C). Another example of a capillarity-mediated microfluidic fuel cell based on Y-shaped filter paper strip was recently presented by Arun et al.14 The device was constructed using graphite electrodes bound to conductive wires via silver adhesive paste. When the inlet channels were soaked in formic acid (fuel) and sulfuric acid (oxidant) solutions, the anode caused decomposition of formic acid into CO2 and electrons, which were transferred to the cathode by the external circuit. The device produced 32 mW cm−2 of power for about 1000 minutes using 1 mL of fuel.
Reversed electrodialysis is a method of retrieving energy from two solutions with a difference in the salt concentrations (i.e. the salinity gradient) by means of an asymmetric ion transport through ion selective membranes. The salinity difference generates a potential difference across the membrane, which leads to the generation of power. Based on this principle, a multi-layered paper-based energy harvesting device was designed by Chang et al.15 The electrodes were made of Ag/AgCl ink painted onto filter paper. An ion selective membrane was sandwiched in between two layers of patterned, wax printed filter paper for use as flow channels. By applying a concentration gradient of potassium chloride (0.1 mM/100 mM) across the membrane, the device produced a power density of 275 nW cm−2.
Microfluidic lab-on-a-chip systems require several components to function for real world applications, such as a power source, a fluid pumping system and a necessary electronic circuitry. Towards this end, a microfluidic fuel cell has been developed to serve a dual function, i.e. to act as a power source and a fluid pump.16 The fuel cell generates electricity by decomposing methanol molecules to produce electrons and CO2. The accumulation of CO2 in the inlet chamber gradually builds up a pressure on the sample fluid to displace it towards the analysis chamber (Fig. 3). A linear relation between the sample flow rate and the produced current was observed, achieving an available power of 1–4 mW at flow rates in the range of 4–18 μL min−1. An extension of this work resulted in an autonomous platform with power and pumping sources, an integrated electronics module, an amperometric sensor and a display for chemical sensing of ferrocyanide redox species.17 The fuel cell could produce 4 mW of power, whereas the power consumption of the electronics and display was only 1 mW, hence the remaining 75% of the generated electric power remained available to perform other operations.
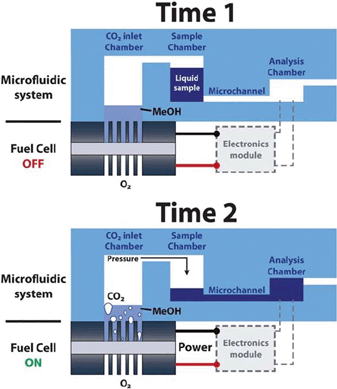 | ||
| Fig. 3 Schematic illustration depicting the operation of a fuel cell powered microfluidic platform. Reprinted with permission from ref. 16. | ||
Conclusions
In this Focus article, we have summarized the potential of microfluidics for energy generation and some of the proof-of-concept applications of these devices for lab-on-a-chip operations. Clearly, microfluidics is an enabling tool towards the development of miniaturized fuel cells for portable applications in the field of point-of-care diagnostics and (bio)chemical sensing. There remains a need for further development of the existing manufacturing technologies of microfluidic fuel cells, capable of generating higher power densities and sustainable energy production, with minimal fabrication and operational cost. Efforts should also be made to discover new but low-cost catalysts for energy conversion, printing technologies for large scale production, and integration of energy storage components to preserve the excess charge.Acknowledgements
This work was supported by the Spanish MINECO under grants CTQ2015-68879-R (MICRODIA) and CTQ2015-72471-EXP (Enzwim) and from the European Community ERC StG Grant Agreement 311529 (LT-NRBS) and ERC PoC Grant Agreement 713608 (MICROCLEANERS). Funding from The Doctoral School of the University of Eastern Finland to MS is gratefully acknowledged.Notes and references
- A. Kirubakaran, S. Jain and R. K. Nema, Renewable Sustainable Energy Rev., 2009, 13, 2430–2440 CrossRef CAS
.
- A. Zebda, L. Renaud, M. Cretin, F. Pichot, C. Innocent, R. Ferrigno and S. Tingry, Electrochem. Commun., 2009, 11, 592–595 CrossRef CAS
.
- H.-B. Noh and Y.-B. Shim, J. Mater. Chem. A, 2016, 4, 2720–2728 RSC
.
- A. Dector, R. A. Escalona-Villalpando, D. Dector, V. Vallejo-Becerra, A. U. Chávez-Ramírez, L. G. Arriaga and J. Ledesma-García, J. Power Sources, 2015, 288, 70–75 CrossRef CAS
.
- F. Qian, M. Baum, Q. Gu and D. E. Morse, Lab Chip, 2009, 9, 3076–3081 RSC
.
- S. Choi, H.-S. Lee, Y. Yang, P. Parameswaran, C. I. Torres, B. E. Rittmann and J. Chae, Lab Chip, 2011, 11, 1110–1117 RSC
.
- H. Hou, L. Li, C. Ü. Ceylan, A. Haynes, J. Cope, H. H. Wilkinson, C. Erbay, P. De Figueiredo and A. Han, Lab Chip, 2012, 12, 4151–4159 RSC
.
- H. Lee and S. Choi, Lab Chip, 2015, 15, 391–398 RSC
.
- A. Fraiwan and S. Choi, Phys. Chem. Chem. Phys., 2014, 16, 26288–26293 RSC
.
- H.-J. Koo and O. D. Velev, Sci. Rep., 2013, 3, 2357 CrossRef PubMed
.
- L. Li, G. Wang, R. Chen, X. Zhu, H. Wang, Q. Liao and Y. Yu, Lab Chip, 2014, 14, 3368–3375 RSC
.
- R. S. Jayashree, L. Gancs, E. R. Choban, A. Primak, D. Natarajan, L. J. Markoski and P. J. A. Kenis, J. Am. Chem. Soc., 2005, 127, 16758–16759 CrossRef CAS PubMed
.
- J. P. Esquivel, F. J. Del Campo, J. L. Gómez de la Fuente, S. Rojas and N. Sabaté, Energy Environ. Sci., 2014, 7, 1744–1749 Search PubMed
.
- R. K. Arun, S. Halder, N. Chanda and S. Chakraborty, Lab Chip, 2014, 14, 1661–1664 RSC
.
- H.-K. Chang, E. Choi and J. Park, Lab Chip, 2016, 16, 700–708 RSC
.
- J. P. Esquivel, M. Castellarnau, T. Senn, B. Löchel, J. Samitier and N. Sabaté, Lab Chip, 2012, 12, 74–79 RSC
.
- J. P. Esquivel, M. Castellarnau, T. Senn, B. Löchel, J. Samitier and N. Sabaté, Lab Chip, 2012, 12, 4232–4235 RSC
.
| This journal is © The Royal Society of Chemistry 2016 |
