Smart biosensors for multiplexed and fully integrated point-of-care diagnostics
A.
Romeo
a,
T. S.
Leung
a and
S.
Sánchez
*abc
aInstitute for Bioengineering of Catalonia (IBEC), Baldiri Reixac, 10-12, 08028 Barcelona, Spain. E-mail: ssanchez@ibecbarcelona.eu; Tel: +34 93 402 0558
bMax Planck Institute for Intelligent Systems, Heisenbergstrasse 3, 70569 Stuttgart, Germany. E-mail: sanchez@is.mpg.de; Tel: +49 711 689 1846
cInstitució Catalana de Recerca i Estudis Avançats (ICREA), Psg. Lluís Companys 23, 08010 Barcelona, Spain
First published on 5th May 2016
Abstract
Point-of-care diagnostics (PoC) and personalised medicine are highly valuable for the improvement of world health. Smartphone PoC platforms which precisely diagnose diseases and track their development through the detection of several bioanalytes represent one of the newest and most exciting advancements towards mass-screening applications. Here we focus on recent advances in both multiplexed and smartphone integrated PoC sensors.
Introduction
Point-of-care (PoC) diagnostics is commonly based on portable, inexpensive, and user-friendly sensor platforms that allow sensitive, robust, and real-time detection of biotargets. The possibility to easily and frequently perform diagnostic assays opens up opportunities in clinical and food safety applications. In particular, personalised medicine benefits from the advantages of PoC diagnostics since sensitive and cost-effective detection of biomarkers is required for early disease diagnostics and treatment monitoring. PoC technologies have even greater value in developing countries and resource-limited environments where the diffusion of infectious diseases can be curbed using timely and personalised diagnostic procedures. Two aspects that strengthen the value of PoC diagnostics are the possibility to detect multiple bioanalytes in a single device and the integration of PoC biosensors with smartphone technology. On one hand, multiplex biosensing allows for finely monitoring the evolution of diseases, which often involve a complex panel of biotargets.1 On the other hand, integration of PoC biosensors with smartphones unifies the advantages of portable diagnostics with ubiquitous devices such as smartphones used to collect and elaborate data, and give real-time feedback to the patient.2,3 In this Focus article we discuss recent advances in both multiplexed biosensing and the full integration of PoC diagnostics with smartphones.Multiplexing
The accuracy and reliability of disease diagnostic protocols can be improved by analyzing multiple biomarkers and using multiplexed assays. Multiplexing is a decisive technological breakthrough since it allows the multivariate analysis of large numbers of samples from different patients. This, in turn, results in improved prognostic determination of the role of biomarkers in specific diseases. The great diagnostic potential of multiplexed point-of-care biosensing systems has generated a strong interest in the scientific community during the last few years. This has resulted in portable, cheap, and user-friendly, but still highly sensitive and reliable, devices for multiplexed sensing of both disease-related biomarkers and microorganisms (i.e., viruses, bacteria, and cells).One of the examples of multiplexed PoC devices was reported by Song et al.4 who developed a multiplexed volumetric bar-chart chip (V-Chip) for rapid quantification of cancer protein biomarkers (Fig. 1Ai). The authors reported a glass chip where bar charts consisted of microfluidic channels. Each channel contained an enzyme linked immunosorbent assay (ELISA) with captured antibodies covalently attached to the glass surface of the wells, pre-loaded hydrogen peroxide, and a dye. The working principle of the V-Chip relied on the volumetric measurement of oxygen generated on-chip by the reaction between hydrogen peroxide and an ELISA probe (catalase), whose concentration is proportional to that of the target analyte. The resulting advancement of the inked bar result was proportional to the concentration of the corresponding ELISA target in each well. Full integration of the device allowed for the results to be displayed without the need for optical instruments or data processing steps. A six-plexed V-Chip was successfully tested for breast cancer cell classification, by distinguishing the expression profile of several biomarkers (oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2)) in different breast cancer cell lines (Fig. 1Aii). Finally, the possibility to run ten parallel measurements of carcinoenmbryonic antigen from multiple serum samples was also demonstrated.
 | ||
| Fig. 1 A i) Ink advancement images from volumetric bar-chart chips to distinguish the expression profile of biomarkers in different breast cancer cell lines (SKBR-3 and MCF-7). C, E, P and H indexes stand for control, oestrogen receptor (ER), progesterone receptor (PR) and human hepidermal growth factor receptor 2 (HER2), respectively. ii) V-Chip readouts of different cell lysates.4 B Schematic representation of the working principle of the LSPR microarray chip described in ref. 5. C i) Schematic operation principle of the electrochemical enzyme-linked magnetic beads immunoassay. ii) Results of the assay in diagnosing Chagas disease. D i) Sketch of the proteinticle probe-based LFA system for detection of viral infectious diseases. ii) Multiplex tests for AIDS, hepatitis C and hepatitis A infections using the proteinticle probe-based LFA. E i) Nanoparticle aggregation concept for bacteria detection on a cellulose paper and ii) its integration with a mobile phone camera. Adapted from ref. 4–8 with permission from Nature Publishing Group,4,8 the American Chemical Society,5 and Elsevier.6,7 | ||
Multiplexed detection of biomarkers in serum was also reported by Chen et al.5 who used a localized surface plasmon resonance (LSPR)-based biosensor microarray for the multiplex immunoassay of cytokines (Fig. 1B). The authors exploited the nanoplasmonic detection of gold nanorods (AuNRs) functionalized with antibodies against six specific cytokines (interleukin-2, -4, -6, 10, interferon-gamma and tumor-necrosis-factor alpha) that are involved in several immune response processes. Excellent correlation was observed between the results obtained by LSPR microfluidic immunoassay and ELISA assay, with highly sensitive cytokine detection down to 5–20 pg ml−1 with the LSPR chip. The short time required for the whole assays (40 min) and small volume of sample required (1 μL serum) demonstrated the low invasiveness of this technique. The technique reliably monitored the evolution of cytokine levels in infant patients before and after undergoing cardiopulmonary bypass surgery.
Advancements in the multiplexed detection of biomarkers are also highly relevant for the diagnosis of infectious diseases. In this case, the capability of finely detecting different microorganisms is required. Cortina et al.6 used superparamagnetic microbeads in an electrochemical enzymatic biosensor for point-of-care serodiagnosis of both human and animal infections. The magnetic microbeads were functionalized with antigens specific towards the targets investigated (parasitic protozoa, bacteria, and viruses), and incubated with the samples and horseradish peroxidase-conjugated antibodies. Upon multiple washing steps, the system was able to electrochemically detect the catalytic activity of the enzyme, once the target-bound microbeads were magnetically collected onto an electrode (Fig. 1Ci and ii). The multiplexed configuration allowed up to 8 readings in less than 3 min (20 s per reading) using a portable potentiostat, resulting in a highly compact, portable, and fast PoC system.
Lee et al.7 reported more specifically on the multiplexed detection of viral infectious diseases (AIDS, hepatitis C, and hepatitis A) using a lateral flow assay (LFA) based on engineered nano-scale protein particles (proteinticles) used as LFA probes (Fig. 1D). Proteinticles were genetically engineered to display viral antigens on their outer surface, with homogeneous orientation and native conformation that guarantee extremely high sensitivity. Indeed, the assay based on proteinticles probes showed outstanding performance compared to peptide-only probes, with 100% sensitivity (no false negative cases in patients with viral disease) and 100% specificity (i.e., no false positive signals in healthy patients). The results obtained demonstrate the feasibility of the proteinticle-probe-based LFA for qualitative assay of viral disease, although more investigations are needed for quantitative assessment of the viral infection progress.
The use of inexpensive materials and smart designs can achieve low-cost and user-friendly PoC diagnostic tools. Paper, among other materials, has recently experienced a considerable growth as a flexible substrate for biosensing devices. The recent work from Shafiee et al.8 highlights the potential of cellulose paper for cheap and easy-to-use PoC diagnostics. The authors developed different biosensing platforms based on paper and flexible polyester films for the detection of multiple biotargets, namely human immunodeficiency virus-1, Escherichia coli and Staphylococcus aureus, and CD4+ T lymphocytes, in complex biological samples (Fig. 1Ei and Eii). Both electrical and optical sensing mechanisms were exploited, using antibodies and peptides as recognition elements. For instance, pathogen detection was achieved by exploiting nanoparticles aggregation strategy where gold nanoparticles were functionalized with specific recognition elements and transferred to a disposable paper. The authors demonstrated that the morphology and structure of the nanoparticles aggregation was closely related to the presence of E. coli in the sample solution, as determined via optical measurements.
Smartphone integration
Today's smartphone is a multifaceted device which can be used as a camera, sensor for health and activity tracking applications, and social networking client which provides the perfect platform for developing point-of-care devices. The papers presented here take advantage of standard smartphone features such as the camera and wireless connectivity with surrounding instruments that help make truly integrated biosensors for personalised medicine and medical devices possible.Using a smartphone camera, Zhu et al.9 created miniaturized microscopy techniques, where a 3-part opto-mechanical system measured red and white blood cell (RBC & WBC) densities and hemoglobin concentration from a 10 μm sample (Fig. 2Ai). A blood analysis application provides a user friendly interface where the user chooses the measurement type and places the correct attachment and the pre-processed blood sample together. A combination of coloured LEDs and a plano-convex lens are used to record fluorescent and bright field micrographs for WBCs and RBCs, respectively, and data is retrieved within 10 seconds (Fig. 2Aii). Image processing for both RBCs and WBCs converts RAW image red, blue, green (RBG) values into hue, saturation values (HSV) where saturation is used to measure concentration. Light absorbance of lysed blood in a standard cuvette was used to establish hemoglobin concentration. Here, a substitute pinhole camera notes and analyses the transmission intensity. The RAW image is then transformed into a grayscale image from which the absorbance value is calculated using a calibration curve. Results were compared against a standard hematology analyser where WBC, RBC, and hemoglobin exhibited a 7%, 5%, and <5% error respectively proving its usability for rapid blood analysis.
 | ||
| Fig. 2 A Point-of-care diagnostic tools and their respective mobile user interfaces. i) Schematic design of the cellphone-based cytometer for blood analysis, ii) displayed results after hemoglobin measurement. B i) Picture of the smartcard smartphone cholesterol accessory with attachments, ii) calculated cholesterol readout following analysis. Reproduced from ref. 9 and 10, respectively with permission from the Royal Society of Chemistry. C i) ELISA colorimetric reader model, ii) top down view of the microplate and iii) its resulting image as taken by the camera, iv) screenshot of quantitative test results. Reprinted from ref. 11 with permission from the American Chemical Society. | ||
Using a similar method, Oncescu et al.10 were able to monitor cholesterol levels with the help of enzymatic colorimetric test strips. This was achieved with an attachable smartcard accessory comprised of a black PDMS flash diffuser and an optical path (Fig. 2Bi). When dry reagent test strips were placed into the device, the colorimetric color change is recorded 3 times with flash, analysed, and compared against reference data. In the captured image, its average RGB values are converted into hue, saturation, lightness (HSL) values which can be easily quantified into cholesterol levels. Results were displayed within 60 seconds on a user friendly interface (Fig. 2Bii). This system boasts 1.8% accuracy within the physiological range and variability of 5.8% between readings, enabling home cholesterol testing.
Smartphone camera-based microscopy is even applicable to diagnostic tools for identifying contagious diseases, as demonstrated by Berg et al.11 Here, a 3D printed ELISA colormetric microplate reader (Fig. 2Ci) serves as a 3-part system where the top is a smartphone holder, the middle allows for the insertion of 96-well plate, and the bottom part holds 96 optical fibers. Once an ELISA plate is loaded, the 24 blue LED array light illuminates the well which is then transmitted through the fiber optic array to a collection lens where the smartphone's camera is able to capture an image at 3 different exposure times (Fig. 2Cii and iii). Using the custom-made application, images are loaded and processed by calculating the average intensities of each well and measuring transmittance values on a scale of 0 to 1 and thusly converted into a quantified clinical index value (Fig. 2Civ). The accuracy of this device was improved using a machine learning algorithm to facilitate a blind diagnostic decision based on gathered statistics. This test was applicable for the testing of measles, HSV-1, HSV-2, and mumps and obtained accuracies of 98.6% and above compared against FDA approved reference methods. The reader can be powered by 6 AAA batteries allowing for use in resource-limited settings.
Another common feature on smartphones today is their wireless connectivity to wearable technologies. Electrolyte-based sensing (in terms of hydration and heat-stress monitoring) can help monitor athletes and military personnel condition in extreme conditions.12 The bodily fluid targeted here is sweat as it contains a number of analytes including glucose, lactate, and various salts. Tattoo sensors have been developed but only recently have wirelessly integrated systems become available, as demonstrated by Rose et al.12 and Gao et al.13 with the use of radio frequency identification (RFID) and Bluetooth connectivity, respectively.
Inspired by a Band-Aid form factor, Rose et al.12 fabricated an adhesive potentiometric sensor patch that determines Na+ concentration which achieved 96% accuracy in the 20–70 mM range with a 30 second response time. The bandage sensor is comprised of sensor electrodes for detecting the Na+ concentration, a commercially sold RFID transponder chip that operates at 13.56 MHz, external capacitors, a diode, and a coil loop antenna. Electromagnetic waves generated by the reader were harvested into usable energy by the transponder chip allowing for battery-free operation. Together, the tuned RFID circuit and antenna provided 0.5 W, enough to power the device. The generated voltage is then delivered to the sensor to begin measurements. The sensor exhibited a sensitivity of 0.3 mV mW−1, excellent stability, and repeatability as low as 0.1%. However, it must be noted that a customizable application was not made for the sensor but could be interfaced with RFID readers and smartphones.
The potential for a multianalyte system discussed by Rose et al.12 was realized by Gao et al.13 who presented a truly interconnected multiplexed sweat sensor (measuring Na+ and K+ ions, glucose, lactate, and temperature) with a flexible and wireless PCB (Fig. 3Ai) and battery module combined into a smart wristband or headband (Fig. 3Aii). Launching the Perspiration Analysis App establishes a Bluetooth connection between the wristband and the phone, where data are received and displayed. Once the subject engages in exercise and starts to sweat, a stabilized input of +5 V for the microcontroller and +3.3 V for Bluetooth are generated. This voltage is delivered to the lactate and glucose sensors which produces an electrical current signal. This is then converted back into a voltage within the input voltage range of the analogue to digital converter. As for the K+ and Na+ sensors, a differential voltage is obtained, filtered, and then translated back into the microcontroller and transmitted through the Bluetooth receiver to the android interface. This continuous feedback loop provides real time monitoring once a sufficient amount of sweat is obtained to display a result (Fig. 3Aiii).
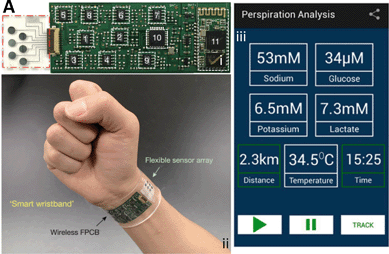 | ||
| Fig. 3 A Wirelessly-enabled point-of-care device as developed by Gao et al.13 i) Sensor array noted in the red dashed box with integrated circuit layout. ii) Photo of the flexible sensor wristband. iii) User interface with real time monitoring for sweat analysis. Adapted from ref. 13 with permission from Nature Publishing Group. | ||
Conclusions
It is evident that inexpensive, portable, and rapid PoC diagnostics for detecting multiple biomarkers related to infectious diseases and microorganisms is feasible nowadays. When combined with smartphone technology, it becomes a powerful, user-friendly, and versatile tool, especially for those in poverty stricken environments. However, the major challenges of the devices examined here include their need for end user training in terms of sample preparation and technical knowledge. This is only beginning to be addressed as demonstrated in sweat-based sensors where full integration was actualized. With this emerging trend, smart and fully integrated multiplexed biosensors are sure to revolutionize the medical and smartphone industries alike.Acknowledgements
The authors would like to thank the European Research Council (ERC) Starting Grant “Lab-in-a-tube and Nanorobotics biosensors; LT-NRBS” [no. 311529] for financial support.References
- W. Su, X. Gao, L. Jiang and J. Qin, J. Chromatogr. A, 2015, 1377, 13–26 CrossRef CAS PubMed.
- A. Roda, E. Michelini, M. Zangheri, M. Di Fusco, D. Calabria and P. Simoni, TrAC, Trends Anal. Chem., 2015 DOI:10.1016/j.trac.2015.10.019.
- X. Xu, A. Akay, H. Wei, S. Wang, B. Pingguan-Murphy, B.-E. Erlandsson, X. Li, W. Lee, J. Hu, L. Wang and F. Xu, Proc. IEEE, 2015, 103, 236–247 CrossRef CAS.
- Y. Song, Y. Zhang, P. E. Bernard, J. M. Reuben, N. T. Ueno, R. B. Arlinghaus, Y. Zu and L. Qin, Nat. Commun., 2012, 3, 1283 CrossRef PubMed.
- P. Chen, M. T. Chung, W. McHugh, R. Nidetz, Y. Li, J. Fu, T. T. Cornell, T. P. Shanley and K. Kurabayashi, ACS Nano, 2015, 9, 4173–4181 CrossRef CAS PubMed.
- M. E. Cortina, L. J. Melli, M. Roberti, M. Mass, G. Longinotti, S. Tropea, P. Lloret, D. A. R. Serantes, F. Salomón, M. Lloret, A. J. Caillava, S. Restuccia, J. Altcheh, C. A. Buscaglia, L. Malatto, J. E. Ugalde, L. Fraigi, C. Moina, G. Ybarra, A. E. Ciocchini and D. J. Comerci, Biosens. Bioelectron., 2016, 80, 24–33 CrossRef CAS PubMed.
- J. H. Lee, H. S. Seo, J. H. Kwon, H. T. Kim, K. C. Kwon, S. J. Sim, Y. J. Cha and J. Lee, Biosens. Bioelectron., 2015, 69, 213–225 CrossRef CAS PubMed.
- H. Shafiee, W. Asghar, F. Inci, M. Yuksekkaya, M. Jahangir, M. H. Zhang, N. G. Durmus, U. A. Gurkan, D. R. Kuritzkes and U. Demirci, Sci. Rep., 2015, 5, 8719 CrossRef PubMed.
- H. Zhu, I. Sencan, J. Wong, S. Dimitrov, D. Tseng, K. Nagashima and A. Ozcan, Lab Chip, 2013, 13, 1282–1288 RSC.
- V. Oncescu, M. Mancuso and D. Erickson, Lab Chip, 2014, 14, 759–763 RSC.
- B. Berg, B. Cortazar, D. Tseng, H. Ozkan, S. Feng, Q. Wei, R. Y. L. Chan, J. Burbano, Q. Farooqui, M. Lewinski, D. Di Carlo, O. B. Garner and A. Ozcan, ACS Nano, 2015, 9, 7857–7866 CrossRef CAS PubMed.
- D. P. Rose, M. E. Ratterman, D. K. Griffin, L. Hou, N. Kelley-Loughnane, R. R. Naik, J. A. Hagen, I. Papautsky and J. C. Heikenfeld, IEEE Trans. Biomed. Eng., 2015, 62, 1457–1465 CrossRef PubMed.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
