Microfluidic PDMS on paper (POP) devices†
Jin-Wen
Shangguan
,
Yu
Liu
,
Jian-Bin
Pan
,
Bi-Yi
Xu
*,
Jing-Juan
Xu
* and
Hong-Yuan
Chen
State Key Laboratory of Analytical Chemistry for Life Science and Collaborative Innovation Center of Chemistry for Life Sciences, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: xujj@nju.edu.cn; Fax: +86 25 89687294; Tel: +86 25 89687294
First published on 9th November 2016
Abstract
In this paper, we propose a generalized concept of microfluidic polydimethylsiloxane (PDMS) on paper (POP) devices, which combines well the merits of paper chips and PDMS chips. First, we optimized the conditions for accurate PDMS spatial patterning on paper, based on screen printing and a high temperature enabled superfast curing technique, which enables PDMS patterning to an accuracy of tens of microns in less than ten seconds. This, in turn, makes it available for seamless, reversible and reliable integration of the resulting paper layer with other PDMS channel structures. The integrated POP devices allow for both porous paper and smooth channels to be spatially defined on the devices, greatly extending the flexibility for designers to be able to construct powerful functional structures. To demonstrate the versatility of this design, a prototype POP device for the colorimetric analysis of liver function markers, serum protein, alkaline phosphatase (ALP) and aspartate aminotransferase (AST), was constructed. On this POP device, quantitative sample loading, mixing and multiplex analysis have all been realized.
Introduction
Paper based microfluidics made its debut in 2007 from Whitesides' group.1 It has witnessed booming development over the past decade as low-cost disposable microfluidic paper analytical devices (μPADs) have been used for a wide range of applications in healthcare, environmental monitoring and food safety.2–5 Compared with traditional materials paper is structurally special, which makes μPADs easy to fabricate and especially suitable for point of care (POC) applications. With their special porous structures, μPADs have both advantages of easy surface functional patterning and automatic flow control, which distinguishes them from commercialized testing papers. Thus they are growing into a branch of microfluidics that is among the most promising for future commercialization.4However, also because of this special porous structure, fabrication techniques for μPADs are quite different from those for traditional microfluidic chips.6 Paper has to be regionally impregnated with materials like wax,7–9 polydimethylsiloxane (PDMS),10,11 polystyrene12,13 and photoresists1,14 or to be treated with reactive gases15,16 to delineate hydrophilic and hydrophobic regions, so that liquid can flow only in the hydrophilic region.17,18 A channel defined in this way is filled with porous or fibrous structures, which usually generate large resistance to liquid flow and lead to inevitable nonspecific surface absorption of samples,19,20 making it difficult to transport macromolecules any length.21 In addition, many valuable functions rely on convective flow like liquid pinning, liquid mixing22 and gradient generation and thus cannot be easily realized. These all have limited the applications of μPADs.
Many efforts have been made to accelerate the flow by changing the flow mode on the paper. Jahanshahi-Anbuhi et al. first pointed out this and solved it by sandwiching a paper chip with two flexible films, and the gaps between the films greatly accelerated the speed of the liquid.20 Dou et al. placed chromatography paper pads into the PDMS reservoirs for 3D reagent immobilization, so that the merits of easy sample storage of the paper and good hydrodynamic controllability of the flows in PDMS channels are both maintained.23 More intimate combination of the paper and channels comes from the introduction of channels to the μPADs. Crooks' group proposed the concept of a hollow channel which is generated by sandwiching an engraved hollow channel layer with two other layers by wax printing and clamp sealing.24,25 Later on, Martinez' group devised two-ply channels which were formed by putting two layers of wax patterned paper together and generating channels between the two layers.19 These are all valuable contributions. However, limits still remain. Further considerations like flexibility for designing the topology and geometry of channels, reliability of interlayer bonding, throughput for tests and chip reusability should be taken into account.
PDMS is one of the most widely applied materials for microfluidics and it was first devised by Whitesides’ group, the same as μPADs.26 It is highly biocompatible, transparent, stretchable and cheap,27 and thus is the most typical material for channel based microfluidics. However, for a long time its influence was limited in the microfluidic field due to difficulties in surface modification. Interestingly, paper has complementary characteristics for easy surface modification.5 Thus, if PDMS and paper were combined properly, they might complement each other perfectly and overcome their own weak points. The combination is not only limited to the individual characteristics, but the combining of the two areas: channel based microfluidics and paper based microfluidics. PDMS patterning on paper can be an ideal glue for paper to bond with PDMS channel structures. Their combination might initiate a new hot topic in microfluidics, considering all the attractive characteristics of both paper chips and traditional channel based chips.
Fortunately, some early pioneering research studies have paved the way to patterning PDMS on paper. It was first realized using a home-made injector based printing system10 the year after the birth of μPADs. Later on, flexographic printing,11 ink printing11 and screen printing28 based PDMS patterning on paper were all proposed. However, despite the technological advancements, PDMS patterning on paper has neither gained wide popularity for μPADs, nor been successfully combined with PDMS channel structures. Basically, this is because the patterned PDMS is not delicate enough compared with existing techniques like that for wax. The final resolution of the pattern is limited by two characteristics of the uncured PDMS: high viscosity and high penetration ability. The high viscosity of the PDMS makes it difficult for it to be directly printed unless diluters like xylene or toluene are applied. Direct printing causes large pixel dots, while diluted ink printing is linked with poisonous reagents. The high penetration ability of PDMS on paper causes PDMS to quickly exude outside the predefined boundary within tens of seconds upon touching the material, leading to a deformed geometry with low linear resolution. It also results in surfaces that are too rough to allow for further sealing with other layers.
In this paper we are reporting our recent progress in preparing microfluidic PDMS on paper (POP) devices based on a screen printing technique and temperature accelerated PDMS curing. By adopting this technique, the PDMS can cure within seconds on paper, and the exuding phenomenon can be greatly inhibited. By tuning the printing times and the sitting time for pre-curing, the PDMS penetrating depth can be controlled delicately from semi-penetration to thorough penetration. The PDMS pattern on polyether sulphonyl (PES) paper with a line width of 60 μm can be realized in single layer mode, and is near 150 μm for thoroughly penetrated lines. This not only helps us to overcome the barriers to PDMS-paper hybridization, but also opens the door to a novel concept for POP devices: the integration of microfluidic PDMS channels with PDMS patterned paper. Consequently, distinctive regions for porous paper and smooth channels can be generated on the same chip, which eventually leads to the functional extension of traditional μPADs. As an example to demonstrate this general concept, POP device based multiplex colorimetric analysis was carried out for typical liver function markers: serum protein, alkaline phosphatase (ALP) and aspartate aminotransferase (AST). The three markers can give the first indications for deteriorating liver function. Based on the special structure of the POP device, we proposed a shooting star sampling mechanism that enabled quantitative and fast reagent loading and sampling for a matrix of discrete testing points that requires no equipment, only manual operation by hand.
Experimental
Materials and reagents
The types of paper applied in the experiments for PDMS patterning are PES-80 and PES-22 paper, printing paper and filter paper. The printing paper was from Paper One Co., Ltd. The filter paper used is Whatman 202 quantitative filter paper. The polyether sulphonyl (PES) paper was from DeLv technology, with a pore size of 0.80 μm for PES-80 paper and 0.22 μm for PES-22 paper. Silicon elastomer (PDMS precursor and curing agent mixture) was bought from Dow Corning, namely Sylgard 184 dual component suit. A screen stencil T-350 nylon mesh with ∼35 μm pore size was applied for screen printing. Trehalose, ethylene diamine-tetraacetic acid (EDTA), Tris, polyvinyl alcohol (PVA), methyl green, Triton X-100, sodium citrate, citrate and tetrabromophenol blue (TBPB), all analytical grade, were bought from Sino-Pharm Chemical Reagent Corp. A BCIP/NBT alkaline Phosphatase Colour Development Kit, bovine serum albumin (BSA) and phosphate buffer saline (X1 PBS) were from Keygen Biotech. Cysteine sulfinic acid (CSA) was bought from Huixing Biochem Reagent Co., Ltd. α-Ketoglutarate was from Aladdin Industrial Corporation. Rhodamine B was from Tianjin Research Institute of Chemical Reagent. Alkaline phosphatase (ALP) & aspartate aminotransferase (AST) were provided by Shanghai Yuanye Biotech Co., Ltd.Equipment and settings
The hot plate for fast PDMS curing was homemade by assembling an aluminium heating module, pt-100 sensor, solid state relay (AC mode), OMRON E5CC-QX2ASM-800 temperature controller and Atmel atmega328p micro-controlling unit. A cell phone camera was used for chip imaging and movie recording. A Leica DMR2 reverse optical microscope mounted with Olympus DP-71 CCD was also used for chip imaging. The optical data was analysed further using image pro plus (IPP) 6.0 software. Delicate microstructure characterization was carried out using S-4800 scanning electron microscopy (SEM), JEOL Japan.Characterization of PDMS curing with temperature control
Throughout all the experiments, the uncured mixture of PDMS was prepared by mixing the precursor and curing agent in a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The PDMS was used after degassing at room temperature for 10 minutes. To analyse the curing speed of the PDMS thin layer, a freshly prepared uncured PDMS was screen printed at room temperature onto the sample paper. Then the sample paper was quickly moved to a hot plate with a predefined temperature. After a predefined time, it was removed from the hot plate to room temperature. The shortest time required for curing was recorded. After that, the paper with a cured thin layer of PDMS was characterized geometrically.
1. The PDMS was used after degassing at room temperature for 10 minutes. To analyse the curing speed of the PDMS thin layer, a freshly prepared uncured PDMS was screen printed at room temperature onto the sample paper. Then the sample paper was quickly moved to a hot plate with a predefined temperature. After a predefined time, it was removed from the hot plate to room temperature. The shortest time required for curing was recorded. After that, the paper with a cured thin layer of PDMS was characterized geometrically.
Prototype POP device design and fabrication
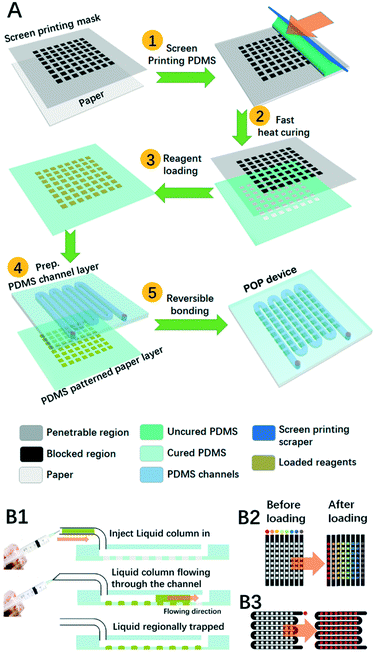
The prototype POP device is composed of at least two layers: the PDMS patterned paper layer and the PDMS channel layer. Scheme 1A demonstrates the typical fabrication procedures for POP devices. First, freshly prepared uncured PDMS was spread out on a stencil as a screen printing mask to generate a thin membrane of pattern on the paper, and this was quickly moved onto a hot plate at 150 °C for 10 s before it was cooled down at room temperature. After it was loaded with reagents in testing points, this PDMS-patterned paper layer was hybridized with a PDMS layer where microfluidic channels were patterned. The layer was fabricated using typical PDMS soft lithography. In short, a mixture of uncured PDMS was poured onto the mould, degassed and heated until cured. Then we peeled the cured PDMS off the mould and punched it with inlets and outlets before use. To combine it with the microfluidic PDMS patterned paper, we just put them together by reversible bonding.Shooting-star liquid loading process
On the proposed POP device, both reagent loading and sampling are realized by a shooting-star liquid loading process as is demonstrated in Scheme 1B1. First, a short column of liquid was sucked into a small tube, then a syringe connected with the tube was pushed by hand to quickly cause the liquid to shoot along the channel until the liquid was totally pushed out of the outlet. During the process, the liquid would soak the hydrophilic paper region, but not the hydrophobic PDMS region.As depicted in Scheme 1B2 and B3, two typical PDMS channel structures were adopted in our experiment: the channel array and the zigzag channel. For reagent loading, the PDMS channel array was applied in which each column needs to be loaded with different reagents, while the zigzag channel was chosen when all the testing points were to be modified with the same reagent. After that the loaded reagents were dried with a nitrogen flow. For multistep reagent loading, we just repeat the loading-drying steps. For sample loading, the PDMS channel array was also adopted for the analysis of several samples, or a zigzag channel was adopted when only one sample was to be analysed.
Colorimetric analysis for liver function markers on the POP device
Total serum protein, AST and ALP are three typical liver function markers, which as a whole can give a first indication of liver function deterioration. In our experiment, the three markers were analysed by typical colorimetry. The preparation of colorimetric reagents followed the same methods as reported before.29 The preparation of the reagents is detailed in the ESI.†To measure the volume of sample trapped on each testing point, the length of the short liquid column was measured constantly. The shortened length of the liquid column was translated to volume information by multiplying with the cross-sectional area of the channel.
To generate the standard curve for serum protein, AST and ALP, the PDMS patterned PES-80 testing point array was preloaded with the corresponding colorimetric testing reagents. Before usage, the PDMS channel array layer (with 8 parallel channels) was mounted onto the surface of the PDMS patterned PES paper layer, so that the colorimetric testing could be repeated 8 times for each concentration.
To apply the chip for liver function analysis, the PDMS channel layer depicted in Scheme 1B was used to combine with the PDMS patterned PES paper. After sampling with the shooting star mode, a cell phone camera was used to monitor the colour change on the arrayed testing regions. Images were taken over 10 minutes of the reaction. The readings were extracted instantly using colour analysis.
Results and discussion
High temperature accelerated curing for PDMS droplets
PDMS-paper hybridization is a highly plausible marriage because it provides a way to combine all the advantages of the two most popular materials for microfluidics. PDMS is smooth, hydrophobic, transparent, biocompatible, soft, elastic, and easily structured using soft lithography, while paper is rough, hydrophilic and rich with fibers and thus it is easy to immobilize reagents, making them available for capillary flow. Most of their characteristics are complementary, and thus their marriage will double the power of the hybridized chip.Though their combination can be in different modes, all of them can be broken down into two unit steps: surface patterning and inter-layer bonding. Since PDMS can be reliably sealed with another piece of PDMS simply by reversible bonding (just align and put them together), we only need to focus on patterning the PDMS surface that is smooth enough. The major difficulty in their combination lies in the surface patterning step. Until now, delicate control over PDMS patterning has been challenging. Control over factors like penetration depth, linear resolution and surface smoothness all need to be considered.
In a typical heat triggered polymerization process like that for PDMS, curing temperature usually determines the curing speed, and a faster curing speed usually leads to better resolution because it reduces the time for pre-polymers to spread over the predefined boundaries. Thus we first tried to screen for better curing temperatures. Fig. 1 is an image of a series of droplets cured under different temperatures on a filter paper. The diameters of the cured droplets decrease with the increase of temperature, which implies that the spread of the PDMS precursors can be well inhibited by increasing the curing temperature. We also quantified the time for curing. As is shown in Fig. S1A,† in the tested range the curing time decreases significantly with the increase of temperature. In addition, Fig. S1B† details the geometries of the cured droplets, and these indicate that the penetration depth of the droplet also decreases with the increase of temperature. As a whole, these data prove that at higher temperatures we may get PDMS patterns with faster curing speeds and better resolution.
 | ||
| Fig. 1 PDMS droplet curing over different temperatures. The numbers indicate the curing temperature (°C) corresponding to each droplet. | ||
Curing of a screen printed PDMS thin layer on paper
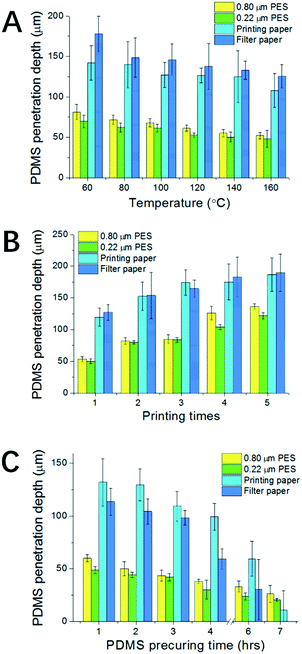
After testing the phenomenon, condition screening experiments were carried out for curing a thin layer of PDMS on paper. Because the geometry of the final patterned PDMS is closely linked with accurate timing, techniques that can generate a pattern in seconds are preferred, like flexography, stamping, spraying and screen printing. Here the screen printing technique is adopted because it can generate patterns with ideal accuracy, repeatability, controllability and flexibility. In addition, the technology itself is accessible for most labs and inexpensive. Combining screen printing and temperature control, we aim to get delicate control over both the spatial distribution and penetration depth for the resulting patterns.For condition screening, three factors were examined: the curing temperature, the number of printing times and the viscosity of PDMS. These factors would influence the final geometry of the pattern. To test their influence, the PDMS penetration depth was used as the index, which can reflect well the delicacy of the patterning technique for both semi-penetrated patterns and thoroughly penetrated patterns.
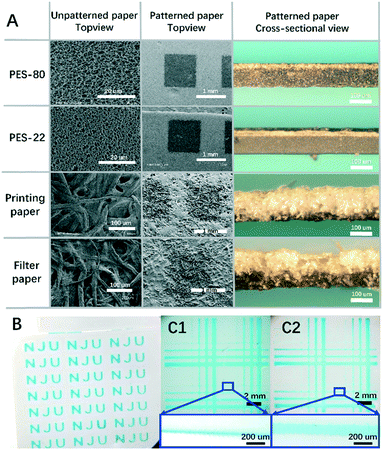
To examine the influence of curing temperature, a single layer of screen printed PDMS was applied on each paper. As shown in Fig. 2A, for all four types of paper, the PDMS penetration depth decreases with the increase of temperature. However, compared with the results for PDMS droplets in Fig. S1B,† the inhibition of the penetration is not significant. This is mainly because of the use of screen printing, where the amount of PDMS applied to a unit area is constant and is much less than in the droplet case. Thus the decrease of the penetration depth here is totally attributed to the increase of the temperature. Besides the general tendency, there are also differences between the four types of paper. At 160 °C, the percentages of penetration are 40% for PES-80, 33% for PES-22, 80% for filter paper and 80% for printing paper. The results imply that the penetration depth is much smaller for the PES papers than for filter paper and printing paper. The differences come from the porosity which defines the amount of PDMS required to fill up a unit volume of paper. For PES papers, though the pores are small, they are highly dense, while for filter paper and printing paper they are the opposite. In addition, the corresponding error is also much smaller for PES papers due to their better structure uniformity. Thus patterning on PES papers is more controllable.
Besides temperature, Fig. 2B shows the relationship between the number of printing times and penetration depth. The results imply that by increasing the number of printing times we can effectively increase the penetration depth of the resulting PDMS pattern. Penetrated patterning throughout the paper can be achieved for filter paper, printing paper and PES-80 paper for 5 times of repeated screen printing. However, penetrated patterning can hardly be achieved for the PES-22 paper after 5 times.
To achieve better control over the penetration depth we further explored the relationship between penetration depth and PDMS viscosity. To generate PDMS with different viscosities, PDMS samples have been precured at room temperature for different predefined times. It is well known that at room temperature, the degree of polymerization for PDMS will gradually increase with time, until it ultimately forms a uniform piece of polymerized PDMS. As expected, the experiment data in Fig. 2C show that PDMS with a longer precuring time can lead to a lower penetration depth after curing. It is also noticeable that the errors associated with the depth increase with precuring time, which become prominent over 6 h. Thus there's a balance between better controllability of penetration depth and the error. PDMS pre-curing over less than 6 h is recommended. The resulting thinner layer of patterned PDMS is especially valuable for defining 3D microchannels on a single layer mode. The better we can control the penetration depth, the more delicate 3D structures on a single layer of paper can be constructed in the future. This is one of the most valuable developing directions for 3D paper chips, and is thus a good measurement for the delicacy of the proposed patterning techniques. It can also contribute to increasing the flexibility of chip design and thus more powerful functions.
Colorimetric analysis for liver function markers
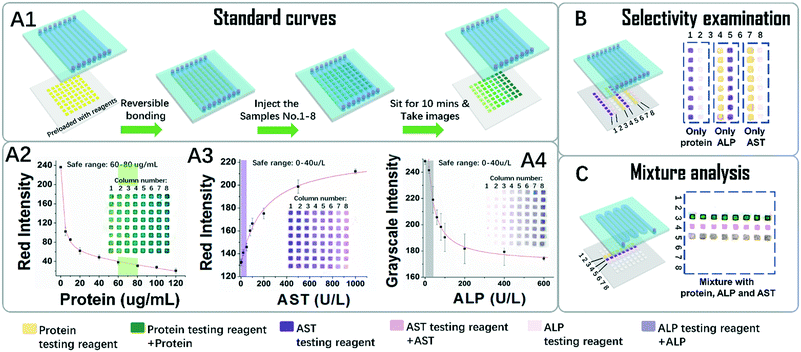
As a conceptual demonstration of POP devices, a dual layered device for the detection of colorimetric liver function markers was developed. Three key markers, the whole blood serum protein (here modelled by BSA) and liver function related proteases AST and ALP, were tested for liver function evaluation.First, we tried to generate standard curves for the three markers. As depicted in Fig. 4A1, a typical POP device was composed of an arrayed PDMS channel layer and a piece of PDMS patterned paper preloaded with testing reagents was applied. A series of standard sample solutions was tested using a shooting star mode. It is so named because in this mode an isolated short column of liquid is quickly flowed throughout the channel like a star shooting across the sky. Because PES is hydrophilic and PDMS is hydrophobic, after liquid was flowed through the region, discrete testing points were loaded with sample solutions through one injection. More interestingly, the amount of liquid trapped on the testing points is generally the same because it is defined by the geometry and surface tension of the testing point region. In our case, the amount of liquid trapped on each testing point reads as 0.15 μL. This result also implies that we can do serial quantitative sampling in the submicroliter range on this platform, simply by hand. This is a big advantage for in situ applications, where equipment and apparatus are limited. In addition, since the liquid is propelled by a hand controlled syringe, the whole process for one injection takes only seconds to accomplish, and the whole process for sample injections that are needed for a standard curve takes less than two minutes, which greatly reduced the time error for colour development between each testing point.
The standard curves and the original colorimetric data visualized in Fig. 4A2–4 supported the above conclusions. The inserted images, which depict the resulting colours of the testing point array of the papers, were taken after ten minutes of colour development. They showed that in each column the colours for each testing points are homogeneous. Meanwhile in each row, the colour changed gradually. Quantitative analysis of the colours in the figures further implies that the data in each column are in good consistency from point to point, with errors in an acceptable range. The effective testing ranges sufficiently covered the range for healthy people as indicated in the shadowed bars, which proved that this test can distinguish abnormal samples from healthy ones.
To check possible crosstalk between the sample and the reagents on the testing points, the selectivity was examined. As shown in Fig. 4B, columns 1–2 were pre-loaded with AST and the ALP testing reagent, and after contact with the solution of protein, no colour change was observed, indicating no crosstalk between them. The results are the same for ALP and AST, so it can be deduced that the colour changes for the reagent are highly selective for their targets.
The analysis results for mixtures of the three markers were tested, as shown in Fig. 4C. In this case, a test with 8 repeat units for the three markers was carried out over one shooting star process. The results from each repeat unit show good consistency with each other. Although only three markers were tested, the system with 8 × 8 testing points actually can support tests for 8 samples with 8 multiplex markers. The above data, in general, indicate that combining the PDMS channels and the PDMS patterned paper has provided the resulting POP device with many advantages. Besides easy reagent immobilization, which is typical for μPADs, the POP device is accustomed to the shooting star liquid loading mechanism, which makes fast, quantitative, high-throughput and homogeneous sampling and analysis possible.
Conclusions
In summary, we have successfully combined PDMS with paper by screen printing and high temperature curing. The material is cheap and convenient, and the process is fast, reliable and easy to carry out. By tuning the curing temperature, printing times and the precuring times, the penetration depth of PDMS on paper can be well controlled. In addition, linear resolution down to tens of micrometres has also been achieved. This versatility has made this patterning technique competitive with the most popular patterning techniques like wax and toner. More importantly, the PDMS pattern on the paper has made it easy to assemble this layer with a microfluidic PDMS layer for POP devices, which can provide the paper system with additional laminar flow, thus combining the merits of a paper chip and PDMS chip. As an example to demonstrate its strength, a POP device for the colorimetric analysis of liver function markers was designed. On this device, a shooting star liquid loading mechanism was proposed, which has enabled fast and quantitative sample loading, mixing and reliable analysis. We hope that the POP devices can enrich the concept of μPADs and contribute to their wide application in the near future.Acknowledgements
This work was supported by the Ministry of Science and Technology Program of China (2016YFA0201200), the National Natural Science Foundation of China (21327902, 21535003, 21505069), and Natural Science Foundation of Jiangsu Province (BK20140597).Notes and references
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef CAS PubMed.
- J. C. Cunningham, P. R. DeGregory and R. M. Crooks, in Annu. Rev. Anal. Chem., Vol 9, ed. P. W. Bohn and J. E. Pemberton, 2016, vol. 9, pp. 183–202 Search PubMed.
- S. K. Mahadeva, K. Walus and B. Stoeber, ACS Appl. Mater. Interfaces, 2015, 7, 8345–8362 CAS.
- D. M. Cate, J. A. Adkins, J. Mettakoonpitak and C. S. Henry, Anal. Chem., 2015, 87, 19–41 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3–10 CrossRef CAS PubMed.
- Y. He, Y. Wu, J.-Z. Fu and W.-B. Wu, RSC Adv., 2015, 5, 78109–78127 RSC.
- A. W. Martinez, S. T. Phillips, E. Carrilho, S. W. Thomas III, H. Sindi and G. M. Whitesides, Anal. Chem., 2008, 80, 3699–3707 CrossRef CAS PubMed.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 7091–7095 CrossRef CAS PubMed.
- S.-H. Yeh, K.-H. Chou and R.-J. Yang, Lab Chip, 2016, 16, 925–931 RSC.
- D. A. Bruzewicz, M. Reches and G. M. Whitesides, Anal. Chem., 2008, 80, 3387–3392 CrossRef CAS PubMed.
- A. Maattanen, D. Fors, S. Wang, D. Valtakari, P. Ihalainen and J. Peltonen, Sens. Actuators, B, 2011, 160, 1404–1412 CrossRef.
- Y. Sameenoi, P. N. Nongkai, S. Nouanthavong, C. S. Henry and D. Nacapricha, Analyst, 2014, 139, 6580–6588 RSC.
- J. Olkkonen, K. Lehtinen and T. Erho, Anal. Chem., 2010, 82, 10246–10250 CrossRef CAS PubMed.
- E. Carrilho, S. T. Phillips, S. J. Vella, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 5990–5998 CrossRef CAS PubMed.
- P.-K. Kao and C.-C. Hsu, Anal. Chem., 2014, 86, 8757–8762 CrossRef CAS PubMed.
- Q. He, C. Ma, X. Hu and H. Chen, Anal. Chem., 2013, 85, 1327–1331 CrossRef CAS PubMed.
- J. Li, F. Rossignol and J. Macdonald, Lab Chip, 2015, 15, 2538–2558 RSC.
- K. Yamada, T. G. Henares, K. Suzuki and D. Citterio, Angew. Chem., Int. Ed., 2015, 54, 5294–5310 CrossRef CAS PubMed.
- C. K. Camplisson, K. M. Schilling, W. L. Pedrotti, H. A. Stone and A. W. Martinez, Lab Chip, 2015, 15, 4461–4466 RSC.
- S. Jahanshahi-Anbuhi, P. Chavan, C. Sicard, V. Leung, S. M. Z. Hossain, R. Pelton, J. D. Brennan and C. D. M. Filipe, Lab Chip, 2012, 12, 5079–5085 RSC.
- X. Deng, N. M. B. Smeets, C. Sicard, J. Wang, J. D. Brennan, C. D. M. Filipe and T. Hoare, J. Am. Chem. Soc., 2014, 136, 12852–12855 CrossRef CAS PubMed.
- A. R. Rezk, A. Qi, J. R. Friend, W. H. Li and L. Y. Yeo, Lab Chip, 2012, 12, 773–779 RSC.
- M. Dou, D. C. Dominguez, X. Li, J. Sanchez and G. Scott, Anal. Chem., 2014, 86, 7978–7986 CrossRef CAS PubMed.
- C. Renault, X. Li, S. E. Fosdick and R. M. Crooks, Anal. Chem., 2013, 85, 7976–7979 CrossRef CAS PubMed.
- C. Renault, M. J. Anderson and R. M. Crooks, J. Am. Chem. Soc., 2014, 136, 4616–4623 CrossRef CAS PubMed.
- D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 4974–4984 CrossRef CAS PubMed.
- J. C. McDonald and G. M. Whitesides, Acc. Chem. Res., 2002, 35, 491–499 CrossRef CAS PubMed.
- S. Mohammadi, M. Maeki, R. M. Mohamadi, A. Ishida, H. Tani and M. Tokeshi, Analyst, 2015, 140, 6493–6499 RSC.
- S. J. Vella, P. Beattie, R. Cademartiri, A. Laromaine, A. W. Martinez, S. T. Phillips, K. A. Mirica and G. M. Whitesides, Anal. Chem., 2012, 84, 2883–2891 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6lc01250g |
| This journal is © The Royal Society of Chemistry 2017 |