Development of a biomimetic microfluidic oxygen transfer device
A. A.
Gimbel†
a,
E.
Flores†
a,
A.
Koo
b,
G.
García-Cardeña
b and
J. T.
Borenstein
*a
aDepartment of Biomedical Engineering, The Charles Stark Draper Laboratory, Inc., Cambridge, MA 02139, USA. E-mail: jborenstein@draper.com
bLaboratory for Systems Biology, Center for Excellence in Vascular Biology, Department of Pathology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA
First published on 5th July 2016
Abstract
Blood oxygenators provide crucial life support for patients suffering from respiratory failure, but their use is severely limited by the complex nature of the blood circuit and by complications including bleeding and clotting. We have fabricated and tested a multilayer microfluidic blood oxygenation prototype designed to have a lower blood prime volume and improved blood circulation relative to current hollow fiber cartridge oxygenators. Here we address processes for scaling the device toward clinically relevant oxygen transfer rates while maintaining a low prime volume of blood in the device, which is required for clinical applications in cardiopulmonary support and ultimately for chronic use. Approaches for scaling the device toward clinically relevant gas transfer rates, both by expanding the active surface area of the network of blood microchannels in a planar layer and by increasing the number of microfluidic layers stacked together in a three-dimensional device are addressed. In addition to reducing prime volume and enhancing gas transfer efficiency, the geometric properties of the microchannel networks are designed to increase device safety by providing a biomimetic and physiologically realistic flow path for the blood. Safety and hemocompatibility are also influenced by blood-surface interactions within the device. In order to further enhance device safety and hemocompatibility, we have demonstrated successful coating of the blood flow pathways with human endothelial cells, in order to confer the ability of the endothelium to inhibit coagulation and thrombus formation. Blood testing results provide confirmation of fibrin clot formation in non-endothelialized devices, while negligible clot formation was documented in cell-coated devices. Gas transfer testing demonstrates that the endothelial lining does not reduce the transfer efficiency relative to acellular devices. This process of scaling the microfluidic architecture and utilizing autologous cells to line the channels and mitigate coagulation represents a promising avenue for therapy for patients suffering from a range of acute and chronic lung diseases.
Introduction
Mortality for lung disease continues to rise in the U.S., while rates for most other leading causes of death, such as cancer, heart disease and stroke are declining.1 This is largely due to a rise in the number of patients with chronic obstructive pulmonary disease (COPD) and other chronic lung diseases.2 Consequently, the demand for lung transplantation is increasing, but donor organ availability is severely limited, and lung transplants are associated with poorer outcomes compared to other solid organ transplantations. In spite of these limitations, lung transplantation remains the best option for patients suffering from severe lung disease. Currently, approximately 5000 people are registered on a waiting list for lung transplantation, while only 1783 lung transplants were performed in the United States in 2012, due largely to the severe shortage of donor organs and poor survival of recipients on the waiting list. Approximately 10% of these potential recipients will die waiting for an organ this year.3For respiratory failure, highly invasive interventions remain as the standard of care to provide oxygen support and carbon dioxide removal for critically ill patients. Invasive techniques, including mechanical ventilation, are associated with increased mortality rates in patients awaiting lung transplantation.4–6 Mechanical ventilation is administered to supplement the function of the lungs during episodes of acute respiratory failure, both for oxygenation and for carbon dioxide removal. However, numerous studies show that mechanical ventilation is associated with a number of severe complications, including barotrauma (high pressures), hyperextension of the lung parenchyma, a high risk of ventilator-induced pneumonia, and oxygen toxicity when administered at high concentrations and for extended periods.7 Together these insults can lead to chronic and irreversible lung injury and often death. As an alternative to ventilator support, extracorporeal membrane oxygenator (ECMO) therapy in a critical care setting is available in some advanced medical centers, but the technology is very complex and extended use is severely limited.
Studies addressing the benefits of ECMO technologies as a bridge to lung transplantation compared to the standard of care remain inconclusive.8–12 Conventional ECMO devices have a number of technical limitations related to the safety and complexity of the blood circuit. Generally, a significant volume of blood (referred to as the prime volume) is in circulation in the device and the circuitry components during operation. A large prime volume is undesirable because it may necessitate blood transfusions to avoid hemodilution. Additionally, blood interactions with synthetic surfaces in the oxygenator can lead to thrombus formation, resulting in a reliance on high concentrations of anticoagulants. Blood flow patterns in hollow fiber oxygenators are another area of concern, because non-physiological flow patterns lead to regions of excessively high or low shear, potentially causing hemolysis and thrombus formation in various regions of the circuit. These deleterious interactions with the blood lead to a clinical dilemma: an increased need for anticoagulants with a concomitant higher risk of bleeding.13 Current ECMO technologies are also less efficient at gas exchange than the natural lungs, due to limitations in gas exchange rates and in the surface area available for exchange. Surface area-to-volume ratios in standard ECMO cartridges are ten times lower than in the lungs, and gas diffusion distances are far greater than physiological values.14
Standard oxygenator technology comprises twisted bundles or sheets of hollow porous fibers housed in a solid plastic cartridge.15 Oxygen travels through hollow porous fiber bundles and blood flows outside the fiber membrane, allowing exchange of oxygen and carbon dioxide at the blood-membrane interface by diffusion. Although the hollow fiber oxygenator has undergone significant optimization in terms of gas exchange over the past several decades, efficacious gas transfer rates remain a challenge. Oxygenation in the hollow fiber bundle is limited by the relatively low surface area-to-volume ratio and non-physiologic and potentially damaging blood flow patterns in hollow fiber oxygenators (HFO) can lead to thromboembolic events and, in some HFO configurations, plasma protein leakage through membrane pores.16 Due to limitations in hollow fiber membrane technology, currently available ECMO cartridges are limited in gas exchange and are associated with significant complications associated with bleeding and clotting.
The fundamental appeal of ECMO is that it bypasses the damaged lungs and allows them to rest, thus avoiding the trauma and injury associated with mechanical ventilation while providing efficacious rates of blood gas transfer. To capture these benefits of oxygenator technology while addressing the shortcomings of current lung assist devices, we have applied microfluidic techniques toward the design of an ECMO device. Previously we reported on the design, fabrication and characterization of a multi-layered microfluidic oxygenator capable of clinically relevant oxygen volume fraction rates at blood flows of up to 4 mL min−1.17,18 Here we report on further scaling of the device up to 25 mL min−1, via expansion of the size of the blood flow network pattern and an increase in the number of gas transfer modules in a device stack. We also show for the first time that scaling in both of these directions increases gas transfer in a predicted manner. This scaling process retains the fundamental advantages in blood health conferred by the biomimetic design of the blood flow networks. However, these designs are still subject to deleterious blood-surface interactions with the silicone membrane and channel walls. In order to address this aspect of hemocompatibility, we have explored the use of endothelial linings on channel walls as a means to eliminate blood-surface interactions and to allow the endothelium to confer the same anti-thrombotic properties that it does in vivo. We report on the establishment of functional endothelial cell linings, and for the first time evaluate the influence of this lining on gas transfer and on blood clotting under physiologically relevant rates of blood flow. Ultimately we envision that this technology can be scaled for human clinical use in both intensive care settings, for ambulatory use in treating chronic lung disease and as a bridge to lung transplantation.
Methods
Design
Two different device configurations were designed, built and tested in order to analyse scaling of oxygen transfer rates in the microfluidic oxygenators. The first design, Rev1, was reported previously,18 and comprised a stack of 10 transfer modules each with an active membrane surface area of 85 mm2. This Rev1 design was shown to be capable of supporting 5 volume% oxygen transfer at a blood flow rate of 4 mL min−1. The goal of this new design process was to scale the device up to a blood flow rate of 25 mL min−1 while maintaining the clinically targeted 5 volume% oxygen transfer rate. The design approach used here was to scale both the active membrane surface area and the number of transfer modules in order to reach this targeted performance level. We chose to increase the number of transfer modules to 14, and to increase the active membrane surface area to 330 mm2 by expanding the existing Rev1 vascular design (Table 1).| Rev1 | Rev2 | |
|---|---|---|
| Surface area (SA) | 85.2 mm2 | 330.2 mm2 |
| Volume (V) | 8.5 mm3 | 16.5 mm3 |
| SA/V | 100 cm−1 | 200 cm−1 |
Fabrication
Microfluidic ECMO devices were fabricated out of multi-layered PDMS (poly(dimethylsiloxane)), excluding the inlet and outlet ports. Both the Rev1 and Rev2 designs were built using the same fabrication process other than the expansion of the vascular and oxygen layer design as previously mentioned. Transfer modules, comprising a non-porous PDMS membrane sandwiched between a PDMS blood layer and a PDMS oxygen layer, were bonded together and routed by internal distribution manifolds. An image of the Rev1 and Rev2 devices is shown in Fig. 1. Blood and gas layers were dry-bonded using an oxygen plasma treatment in a plasma asher (March PX-250 Plasma Etcher, Nordson, Concord, CA) set at 100 W, 280 mTorr, and a 10 second process time. Once plasma-treated, the layers were aligned and then bonded together permanently. Ports for tubing connections were formed using biopsy punches at the blood and oxygen inlets and outlets. Microchannel networks in the blood and oxygen layers were fabricated by casting uncured PDMS polymer (Sylgard 184, Dow Corning, Midland, MI) over a patterned SU-8 molded silicon wafer, and then peeling the cured PDMS layer from the silicon master.19 The network pattern in the blood layer comprised a branched network of microchannels, while the oxygen layer pattern was a large open chamber with an array of staggered posts to provide mechanical support and maintain the chamber height. Uncured PDMS polymer was spun on a blank wafer using a spin coater for a selected spin speed and duration (Cee200, Brewer Science, Rolla, MO) to yield PDMS membranes with a specific targeted thickness. The blank wafer was coated to reduce adhesion to PDMS by depositing 60 μL of silane (trichlorosilane, Sigma-Aldrich, St. Louis MO) in a vacuum chamber.Multiple transfer modules (TM) were bonded together to expand the capacity of the device in a parallel fashion. Individual transfer modules were exposed to oxygen plasma at 100 W and 280 mTorr for 45 s, and then bonded together with a water interface to facilitate alignment of the blood layer ports. Plasma exposure time increased to 45 s with water bonding compared to the 10 s dry bond to optimize bond integrity. For the Rev2 devices containing 14 TM, 2 transfer unit pairs with the larger surface area were bonded at a time. Each set of two pairs of transfer units was bonded with the same plasma process and secured between a foam-layered C-clamp to apply uniform pressure and improve contact between the bonding surfaces. Dowels were slipped into each port to ensure proper alignment under the pressure of the clamping. The process of adding a pair of bonded transfer units to the stack was repeated until 12 transfer unit layers were built. Finally, top and bottom transfer units with side inlet ports were bonded to the stack using plasma bonding to complete the 14 TM stack. Tubing ports were positioned in the oxygen and blood ports for fluid and gas access.
Blood testing
After priming the device with deionized water to facilitate blood flow and remove bubbles, heparinized bovine blood (Lampire Biological Laboratories, Pipersville PA) was used to test oxygen transfer in devices. In order to simulate in vivo oxygenation by the lungs, the gas concentration of blood entering device was standardized at venous conditions. Prior to the experiments, blood-conditioning procedure was implemented to standardize inlet conditions. Blood was pumped through an off the shelf ECMO device (Affinity Pixie, Medtronic, Minneapolis MN) at 5–10 mL min−1 and nitrogen gas through the hollow fibers at 20–100 mL min−1 to achieve oxygenation at a standard range of 65% ± 2%. Pure oxygen gas (Middlesex Gases & Technologies, Everett, MA) was passed through the oxygen chambers, while the heparinized bovine blood was pumped through the blood chambers using a syringe pump (PHD 2000 Harvard Apparatus, Holliston, MA). Oxygen flow rates were kept constant for the tests (60–70 mL min−1), while blood flows were varied to determine the oxygen transfer as a function of blood flow rate.Blood was analyzed with clinical test equipment at the inlet and outlet locations of the device, to assess the change in oxygen content of the blood and determine the total oxygen transfer across the device. Total hemoglobin concentration and the fraction of hemoglobin bonded to oxygen were determined using a hemoximeter (Avoximeter 4000, International Technidyne Corp, Edison, NJ), and a blood gas analyzer (GEM Premier3000, Instrumentation Lab, Bedford, MA) was used to measure the dissolved oxygen level in a blood sample. The total oxygen content of the blood was determined by taking the sum of the hemoglobin-bound oxygen and dissolved oxygen content.18 The difference in oxygen content after and before each run determined the total oxygen transferred for that test.
Endothelial cell seeding
Prior to cell seeding, microfluidic Rev1 ECMO devices were primed with deionized water inside a vacuum chamber. In the chamber, the inlets and outlets of the devices were submerged into water-filled beakers, and then the system was exposed to vacuum for 1 hour. The vacuum was then released to permit water to fill the devices for 15 minutes. Remaining bubbles inside the system were forced out using a water-filled syringe. After the priming step, fibronectin (Sigma-Aldrich, St. Louis, MO) at a concentration of 50 μg mL−1 was injected into the system using a syringe and incubated at 37 °C for 2 hours. Following this incubation, the devices were washed twice with DPBS+, then replaced with culture media. For cell seeding, 200 μL HUVEC media with suspended human umbilical vein endothelial cells (HUVEC, cell density: 6000 per μL) was added directly through the inlet, and the cell-filled devices were incubated at 37 °C and 5% CO2 for 1 hour. The unattached cells were subsequently washed out by injecting the devices with fresh HUVEC media. Complete HUVEC media was replaced every 12 hours for incubation under static (no flow) conditions.Hemocompatibility testing
In order to test the endothelialized and non-endothelialized devices for their hemocompatibility, brief initial studies were conducted on both device types (cellular and acellular), each of which comprised the oxygen chamber/post pattern of a Rev1 device. For hemocompatibility experiments, the inlet and outlet of the device were connected to a tubing system with flow driven by an Ismatec 78017-00 pump (Cole-Parmer, Vernon Hills, IL) at a flow rate of 9.37 μL min−1. For these studies, cell culture media was flowed through both endothelialized and non-endothelialized devices to prime them for the subsequent blood testing. After flow for 72 hours, each device type was flowed with heparinized bovine blood as described earlier in the blood testing section. Heparinization was carried out at the collection point of the blood (vendor site at Lampire) prior to shipping. The blood ACT was not measured. For the hemocompatibility studies, devices were monitored for visible signs of clot formation for periods of 1–10 minutes, to obtain an initial evaluation of thrombotic response. The blood flow rates were similar to the flow rates for oxygen transfer, roughly 2 mL min−1, with shear rates in the range of 1000 s−1 in the predominant transfer channels. These short-term experiments were conducted because the geometry of the test device (oxygen layer post pattern) was highly prone to clotting, enabling a very rapid response and distinction between the endothelialized and non-endothelialized devices.Results and discussion
Device scaling
Pioneering work by Federspiel20 and others21,22 has paved the way toward microchannel-based designs for blood oxygenators. However, one of the most significant challenges for microfluidic approaches to respiratory assist applications is the requirement for scaling to very high levels of blood flow and oxygen transfer for clinical applications.23 Hollow fiber oxygenators are capable of supporting large blood flows over the fiber mats or bundles, but this advantage is offset by the damage incurred by the blood during this process. For microfluidic designs, the blood flow patterns are far more physiologic in nature, but scaling becomes a challenge due to limitations in the rate of blood flow that narrow and shallow microchannels can support without creating unacceptably high pressure drops. Challenges associated with scaling of microfluidic oxygenator devices have been addressed in a recent review,23 and have been debated in subsequent communications.24,25 Most reports of microfluidic oxygenators have been limited to blood flow rates of 5 mL min−1 or less;17,18,21,26–29 one has reported blood flow rates as high as 40 mL min−1, but the oxygen content of the blood appeared to drop below commercial oxygenator oxygen transfer levels (5 volume percent oxygen relative to the total blood volume) above 10 mL min−1 blood flow rates.30 Another recent report from Rieper et al. has demonstrated blood oxygen transfer at blood flow rates as high as 60 mL min−1, clearly a major advance over most of the microfluidic literature [T. Rieper, C. Muller and H. Reinecke]. However, this report does not describe an approach to ensure that blood flow patterns in the channel structures are designed in a manner to avoid sharp corners and non-physiologic and potentially deleterious flow paths. This highlights the need to develop strategies for larger scale devices while retaining the advantages of microfluidic devices in achieving and maintaining blood stability in the flow networks.31Oxygen transfer testing of the stacked devices reported here demonstrates that 5 volume percent of oxygen transfer to the blood flow rate can be achieved at blood flow rates as high as 25 mL min−1, as shown in Fig. 2. In these instances, the starting oxygen saturation in the blood is 65 ± 5%, due to the blood conditioning before the start of the transfer studies. Oxygen saturation levels at the 5 volume percent transfer rates are typically in the range of 99%, as measured by the blood gas analyzer.
The chart in Fig. 2 shows the oxygen transfer rates for a Rev1 layer pattern device comprising 10 transfer modules (10-TM Rev1) device, reported previously18 along with the oxygen transfer rates for a scaled device with a larger footprint (Rev2 layer pattern) and 14 transfer modules (14-TM Rev2) device, and the predicted gas transfer rates for the Rev2-based device. The predicted gas transfer rate was based on a multiplicative scaling of the Rev1 data by active membrane surface area and number of transfer modules. Note that the oxygen transfer rate of the 10-TM Rev1 device at a blood flow rate of 4 mL min−1 is roughly 0.2 mL min−1, which correspond to a volume percent transfer of 5%. For the 14-TM Rev2 device, at a blood flow rate of 25 mL min−1, the oxygen transfer rate is 1.2 mL min−1, which corresponds to a volume percent transfer of 4.8%. The predicted scaling factor for the 14-TM Rev2 devices versus the 10-TM Rev1 devices is 5.2×, based on the ratio of transfer modules (14/10) and the ratio of the active channel area of the Rev2 versus the Rev1 layer (3.7×). The predicted curve (dashed line) was generated by fitting the Rev1 data to a logarithmic curve and then multiplying both the blood flow rate and the oxygen transfer rate by 5.2×. The excellent match between the actual Rev2 oxygen transfer data and the predicted scaling from the Rev1 data demonstrates that scaling of the devices in both active channel area and in number of transfer modules results in highly predictable increases in oxygen transfer capacity.
Endothelialization
Realization of the full potential of microfluidic technologies for extracorporeal oxygenators will require significant increases in scaling while maintaining blood safety and achieving a high level of hemocompatibility. We have previously reported on the advantages that biomimetic surface coating can confer upon mitigation of thrombosis.32 In that study the surfaces of a PDMS device were treated with a hemocompatible coating to prevent surface-induced clot formation during device operation. Here we explore another avenue toward achieving high levels of hemocompatibility, namely establishment of an endothelial lining on the blood-contacting surfaces of the microfluidic device. This approach was first envisioned and microchannels lined with cultured endothelium by Burgess et al. in 2008,33 but they were not exposed to blood or tested for gas transfer in the presence of blood flow.Ultimately we envision that endothelial lining of all critical surfaces in the oxygenator will confer properties that deter thrombus formation in the blood without the need for anticoagulants. Here we have conducted initial testing of this concept in a prototype microfluidic design. Due to the relatively large scale and multiple layers in the 14-TM Rev2 device, we first attempted the endothelialization of a microfluidic test device using a single microfluidic transfer module. Further, we selected a layer design that would be expected to produce rapid clotting due to flow disturbances, in order to obtain a rapid and clear response regarding the impact of the endothelial coating on blood interactions. Ultimately, we plan on integrating the large-scale microfluidic devices with full endothelialization of the multi-layer networks, based on the success of the initial demonstration achieved here. The microfluidic structure was similar to the Rev1/Rev2 gas layer and was comprised of a large open area supported by an array of staggered posts to mechanically stabilize the construct. This design will support blood flow from end to end, but is expected to result in rapid clotting due to the disrupting effect of the staggered post array. Complete endothelialization of the surfaces was easily accomplished due to the relatively open design of the channels, and microscopic observations showed endothelial cells lining the outer walls, posts, and the floor and ceiling of the blood layer. For this demonstration, HUVECs were chosen for seeding and expansion, due to their well-established behavior in microfluidic devices. Any adverse interactions (e.g., immunologic) between the bovine blood and the HUVECs were not expected to be significant, and no such observations were made in these experiments of short duration.
For the endothelialization studies, HUVECs were seeded under perfusion for periods of 48 hours. Images of the devices are shown in the various panels in Fig. 3, with phase contrast images at low and higher magnification in panels A and B, and staining to confirm cell viability in panels C and D. These results were confirmed in multiple devices in preparation for gas transfer testing to assess the effect of the endothelial lining on oxygen transfer.
Gas transfer testing of endothelialized devices
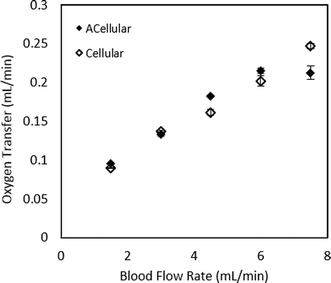
Potential attenuation of gas transfer resulting from the intervening layer of endothelial cells on the PDMS gas transfer membrane was investigated by oxygen transfer measurements of devices with and without the cellular coating under blood flow. As an experimental design, a total of 3 acellular devices and 3 devices seeded with HUVECs on the membrane and on other surfaces within the blood layer were tested using the procedures described in the methods section. All devices were tested using the same batch of bovine blood on the same test day. Blood flows were swept from 0.3–2.7 mL min−1 while oxygen levels were monitored using the techniques described above. Oxygen transfer levels are plotted in Fig. 4, in terms of actual transfer rates in mL min−1. Comparison of the average values for oxygen transfer for endothelialized and acellular devices reveals no discernible difference between the two curves, suggesting that the endothelial lining does not measurably attenuate oxygen transfer.Clotting properties
Devices were exposed to heparinized bovine blood at flow rates similar to those used for gas transfer testing, in order to assess and compare the propensity for thrombus formation between the two groups. As mentioned above, short-term testing of the thrombogenicity of a blood circuit, such as the microfluidic devices reported here, is challenging due to the biomimetic nature of the flow patterns and the use of heparinized blood. Therefore, we chose to utilize a blood flow pattern that would be expected to lead to rapid clot formation even when tested with heparinized blood, in order to achieve a rapid and clear indication of how well surface endothelialization might mitigate coagulation in future studies with larger and more complex blood circuits and using non-anticoagulated blood.As an initial readout for thrombus formation, visual assessment of fiber aggregation was used both before and after washing the blood from the devices that had been exposed to flow. Images of the various devices are shown in Fig. 5.
Observations from the images in Fig. 5 indicate that thrombi formed across more than half of the total area of the acellular device, while minimal fiber formation was seen in the endothelialized devices (Fig. 5c.) To confirm the presence of fibers, acellular devices were rinsed to remove the blood, and inspected for the residual presence of fiber aggregates. Across all three devices from each group, a consistent response of heavy fiber formation in the acellular devices and negligible fiber formation in the cellularized devices was seen. This first experiment suggests that the anti-thrombogenic properties may be conferred by an endothelial lining, although further studies with non-anticoagulated blood over extended periods of operation would be required to determine whether this is the case.
Finally, cell surface expression analysis using fluorescence-activated cell sorting (FACS) was conducted on the endothelial cells following exposure to blood, to determine whether the culture on the devices and/or blood contact had altered the properties of the cells. Fig. 6 shows expression of E-selectin, VCAM-1 and thrombomodulin after the initial 48 h of exposure to perfusion flow, and then after the subsequent exposure to the blood. The absence of E-selectin and VCAM-1 expression at the end of the experiment indicates that the cells did not experienced measurable pro-inflammatory activation. Importantly, the increased expression of the thrombomodulin at the endothelial surface indicates that the cells display a vasoprotective, anti-thrombotic phenotype, characteristic of the vascular endothelium in vivo.
Conclusions
Microfluidic oxygenators have the potential to significantly improve both the efficacy and the safety of ECMO procedures, thereby enabling blood gas transfer to become available to larger patient populations requiring respiratory support due to a range of acute and chronic lung diseases. In this work, we have addressed two of the major barriers to the development of enhanced therapy: 1) scaling of devices toward clinically relevant blood flow and gas transfer rates, and 2) reduction in deleterious blood-surface interactions that may cause coagulation. Toward the former goal, we have successfully scaled our device by a factor of nearly 6-fold, using a combination of expansion of the blood flow patterns and an increase in the number of bonded gas transfer modules in a stack. Oxygen transfer data obtained from these studies exhibits an excellent correlation between the expected and obtained levels of gas transfer based on surface area. Further expansion of these dimensions will ultimately yield devices that can achieve clinical rates of transfer for both oxygenation and carbon dioxide removal.Toward the second goal, we have cultured continuous monolayers of human endothelial cells within a microfluidic architecture to explore the effect that the endothelial lining has on coagulation. In order to demonstrate a clear difference in coagulation, we utilized a microfluidic design with flow properties expected to result in rapid and severe clotting, and showed that when these devices were lined with endothelial cells, coagulation was substantially mitigated. Expression of thrombomodulin confirmed that the endothelial cells maintained their anti-thrombotic phenotype, while an absence of E-selectin and VCAM-1 expression indicated that the cells were not activated during the duration of the experiment. Moreover, we documented that the presence of the endothelial lining did not attenuate the level of oxygen transfer occurring in the devices, an encouraging finding that suggests that this avenue toward improving the hemocompatibility of microfluidic blood oxygenators can be leveraged toward safer and more efficacious therapy. Future studies will integrate endothelial linings in larger-scale microfluidic oxygenators and will evaluate the antithrombogenicity conferred by this process in non-anticoagulated blood.
Ultimately, the full potential of this technology may be realized by the convergence of advances in microfluidic fabrication technologies and stem cell biology toward a device that utilizes autologous cells in a compact blood circuit suitable for ambulatory use. Endothelial cell populations derived from induced pluripotent stem cells (iPSCs) obtained from patient blood samples could be expanded to enable lining of critical elements of the blood circuit, conferring hemocompatible properties to the device for chronic use. In combination with microfabrication and manufacturing techniques that scale the microfluidic architecture while minimizing blood prime volume and device complexity, this strategy could result in a therapeutic device for long-term use by patients suffering from COPD and other diseases. Simplification of the overall blood circuit, perhaps with low-resistance blood pathways that mitigate the need for an external blood pump, could lower the cost, weight and complexity of the overall device and improve safety. Together, these technological advances have the potential for addressing the urgent need for improved therapies for the large patient populations suffering from chronic and debilitating respiratory diseases.
Acknowledgements
We gratefully acknowledge support for this research from NIH NHLBI grant 1 R21 HL106585-01 and from Draper. We would also like to thank Medtronic, Inc. for invaluable technical support.References
- National Heart, Lung, and Blood Institute Fact Book, http://www.nhlbi.nih.gov/files/docs/factbook/FactBook2012.pdf, (accessed January 2016) Search PubMed.
- R. D. Yusen, T. H. Shearon, Y. Qian, R. Kotloff, M. L. Barr, S. Sweet, D. B. Dyke and S. Murray, Am. J. Transplant., 2010, 10, 1047 CrossRef CAS PubMed.
- M. Valapour, M. A. Skeans, B. M. Heubner, J. M. Smith, M. A. Schnitzler, M. I. Hertz, L. B. Edwards, J. J. Snyder, A. K. Israni and B. L. Kasiske, Am. J. Transplant., 2013, 14, 139 CrossRef PubMed.
- J. D. Christie, L. B. Edwards, A. Y. Kucheryavaya, C. Benden, F. Dobbels, R. Kirk, A. O. Rahmel, J. Stehlik and M. I. Hertz, J. Heart Lung Transplant., 2011, 30, 1104 CrossRef PubMed.
- M. A. Baz, S. M. Palmer, E. D. Staples, D. G. Greer, V. F. Tapson and D. D. Davis, Chest, 2001, 119, 224 CrossRef CAS PubMed.
- G. O'Brien and G. J. Criner, J. Heart Lung Transplant., 1999, 18, 255 CrossRef.
- D. Dreyfuss and G. Saumon, Am. J. Respir. Crit. Care Med., 1998, 157, 294 CrossRef CAS PubMed.
- G. J. Peek, M. Mugford, R. Tiruvoipati, A. Wilson, E. Allen, M. M. Thalanany, C. L. Hibbert, A. Truesdale, F. Clemens, N. Cooper, R. K. Firmin and D. Elbourne, CESAR trial collaboration, The Lancet, 2009, vol. 374, p. 1351 Search PubMed.
- D. P. Mason, L. Thuita, E. R. Nowicki, S. C. Murthy, G. B. Pettersson and E. H. Blackstone, J. Thorac. Cardiovasc. Surg., 2010, 139, 765 CrossRef PubMed.
- G. Lang, S. Taghavi, C. Aigner, F. Rényi-Vámos, P. Jaksch, V. Augustin, K. Nagayama, B. Ghanim and W. Klepetko, Transplantation, 2012, 93, 729 CrossRef PubMed.
- C. A. Bermudez, R. V. Rocha, D. Zaldonis, J. K. Bhama, M. M. Crepo, N. Shigemura, J. M. Pilewski, P. L. Sappington, A. J. Boujoukos and Y. Toyoda, Ann. Thorac. Surg., 2011, 92, 1226 CrossRef PubMed.
- C. W. Hoopes, J. Kulreja, J. Golden, D. L. Davenport, E. Diaz-Guzman and J. B. Zwishcenberger, J. Thorac. Cardiovasc. Surg., 2013, 145, 862 CrossRef PubMed.
- M. C. Yang and C. C. Lin, ASAIO J., 2000, 46, 293 CrossRef CAS PubMed.
- P. W. Dierickx, D. S. De Wachter, F. De Somer, G. Van Nooten and P. R. Verdonck, ASAIO J., 2001, 47, 628 CrossRef CAS PubMed.
- L. Lequier, S. B. Horton, D. M. McMullan and R. H. Bartlett, Pediatr. Crit. Care Med., 2013, 14, S7 CrossRef PubMed.
- J. P. Montoya, C. J. Shanley, S. I. Merz and R. H. Bartlett, ASAIO J., 1992, 38, M399 CrossRef CAS PubMed.
- T. Kniazeva, J. C. Hsiao, J. L. Charest and J. T. Borenstein, Biomed. Microdevices, 2011, 13, 315 CrossRef CAS PubMed.
- T. Kniazeva, A. A. Epshteyn, J. C. Hsiao, E. S. Kim, V. B. Kolachalama, J. L. Charest and J. T. Borenstein, Lab Chip, 2012, 12, 1686 RSC.
- J. T. Borenstein, H. Terai and K. R. King, Biomed. Microdevices, 2002, 3, 167 CrossRef.
- W. J. Federspiel and R. G. Svitek, in Encyclopedia of Biomaterials and Biomedical Engineering, Marcel Dekker, Inc., New York, NY, USA, 2004, pp. 922–931 Search PubMed.
- J. Lee, M. Kung, H. H. Kung and L. F. Mockros, ASAIO J., 2008, 54(4), 390–395 CrossRef PubMed.
- T. C. Page, W. R. Light and J. D. Hellums, in Oxygen transport to tissue XXI, ed. A. Eke and D. Deply, Plenum, New York, 1st edn, 1999, pp. 715–730 Search PubMed.
- J. A. Potkay, Lab Chip, 2014, 14(21), 4122–4138 RSC.
- G. Wagner, A. Kaesler, U. Steinseifer, T. Rode-Schmitz and J. Arens, Lab Chip, 2016, 16, 1272–1273 RSC.
- J. A. Potkay, Lab Chip, 2016, 16, 1274–1277 RSC.
- J. Potkay, M. Magnetta, A. Vinson and B. Cmolik, Lab Chip, 2011, 11(17), 2901–2909 RSC.
- D. M. Hoganson, J. L. Anderson, E. F. Weinberg, E. Swart, B. K. Orrick, J. T. Borenstein and J. P. Vacanti, J. Thorac. Cardiovasc. Surg., 2010, 140(5), 990–995 CrossRef PubMed.
- D. M. Hoganson, H. I. Pryor II, E. K. Bassett, I. D. Spool and J. P. Vacanti, Lab Chip, 2011, 11(4), 700–707 RSC.
- W. Wu, N. Rochow, E. Chan, G. Fusch, A. Manan, D. Nagpal, P. R. Selvaganapathy and C. Fusch, Lab Chip, 2013, 13, 2641–2650 RSC.
- N. Rochow, A. Manan, W. I. Wu, G. Fusch, S. Monkman, J. Leung, E. Chan, D. Nagpal, D. Predescu, J. Brash, P. R. Selvaganapathy and C. Fusch, Artif. Organs, 2014, 38(10), 856–866 CrossRef CAS PubMed.
- T. Rieper, C. Muller and H. Reinecke, Biomed. Microdevices, 2015, 17(5), 86 CrossRef PubMed.
- Z. Zhang, J. T. Borenstein, L. Guiney, R. Miller, S. Sukavaneshvar and C. Loose, Lab Chip, 2013, 13(10), 1963–1968 RSC.
- K. A. Burgess, H. H. Hu, W. R. Wagner and W. J. Federspiel, Biomed. Microdevices, 2009, 11, 117–127 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |