Biocompatible fluorinated polyglycerols for droplet microfluidics as an alternative to PEG-based copolymer surfactants†
Olaf
Wagner
a,
Julian
Thiele‡
b,
Marie
Weinhart
a,
Linas
Mazutis
c,
David A.
Weitz
c,
Wilhelm T. S.
Huck
b and
Rainer
Haag
*a
aInstitut für Chemie und Biochemie, Freie Universität Berlin, Takustrasse 3, 14195 Berlin, Germany. E-mail: olaf.wagner@fu-berlin.de; haag@chemie.fu-berlin.de
bRadboud University, Institute for Molecules and Materials (IMM), Physical-Organic Chemistry, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands
cSchool of Engineering and Applied Sciences, Department of Physics, Harvard University, 29 Oxford Street, Cambridge, MA 02138, USA
First published on 24th November 2015
Abstract
In droplet-based microfluidics, non-ionic, high-molecular weight surfactants are required to stabilize droplet interfaces. One of the most common structures that imparts stability as well as biocompatibility to water-in-oil droplets is a triblock copolymer surfactant composed of perfluoropolyether (PFPE) and polyethylene glycol (PEG) blocks. However, the fast growing applications of microdroplets in biology would benefit from a larger choice of specialized surfactants. PEG as a hydrophilic moiety, however, is a very limited tool in surfactant modification as one can only vary the molecular weight and chain-end functionalization. In contrast, linear polyglycerol offers further side-chain functionalization to create custom-tailored, biocompatible droplet interfaces. Herein, we describe the synthesis and characterization of polyglycerol-based triblock surfactants with tailored side-chain composition, and exemplify their application in cell encapsulation and in vitro gene expression studies in droplet-based microfluidics.
Introduction
Droplet-based microfluidics has attracted much attention since the first monodisperse droplets were produced inside microfluidic polyurethane chips in 2001.1 This technology is based on production of pico- to nano-liter volume droplets at high throughput rates (typically 1–10 kHz) and their subsequent manipulation in an automated or semi-automated manner. The small droplet size greatly reduces reagent volumes and provides a powerful tool for single gene, cell, or organism isolation and analysis.2–7To make this technology applicable, cross-contamination between droplets should be minimized or eliminated completely. The use of fluorocarbon oils as a continuous phase is advantageous as it provides hydrophobic and lipophobic properties8 thus significantly reducing the solubility of biochemical compounds and their diffusion between flowing droplets. Furthermore, perfluorinated fluids exhibit high gas solubility, which is important for cell survival in droplets,9 and they cause less swelling of microchannels in PDMS-based microfluidic devices than hydrocarbon oils.10
Since preventing droplet coalescence is crucial for any droplet-based application, surfactants are used to provide droplet stability.11 The degree to which the surfactant organizes at the interface between the immiscible water and fluorous oil phases, such as in a water-in-oil (W/O) emulsion, can be quantified by its reduction of the interfacial tension at the oil water interface, γcmc, where CMC is the critical micellar concentration.12 Efficient surfactants in droplet microfluidics reduce γcmc of a fluorous oil/water mixture below 20 mN m−1.13
In addition to the surfactant's interfacial activity, steric repulsion prevents droplet coalescence.11 Oligomeric perfluoropolyethers (PFPEs), when used as large hydrophobic building blocks of copolymer surfactants, were found to be soluble in fluorocarbon oils and sufficiently large to provide good steric stabilization by forming a dense PFPE layer on the outer droplet surface.14
Poly(perfluoropropylene glycol)-carboxylates, commercially available as “Krytox” by DuPont®, have emerged as a standard PFPE moiety. However, the charged carboxylate group of Krytox interacts with oppositely charged biomolecules, which causes the encapsulated biomacromolecules to lose their activity and agglomerate at the droplet interface.15 Consequently, the carboxylic head group has to be substituted with different nonpolar hydrophilic head groups such as the ammonium salt of carboxy-PFPE, poly-L-lysine, dimorpholinophosphate, and poly(ethylene glycol) (PEG).16 The ammonium salt and poly-L-lysine causes cell lysis, while the latter two performed well. The block copolymer of PEG and perfluoropolyether was further optimized by Holtze et al. who produced a number of PEG–PFPE2 surfactants from PEG and PFPE chains of different lengths. The combination of 600 g mol−1 PEG with 6000 g mol−1 PFPE performed best in droplet formation and long-term droplet stability.17,18
The reduced protein adsorption of this non-ionic surfactant with molecular weights usually ranging from 2000–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 has been used to screen enzymes,19,20 mammalian cells,6,21–23 bacteria,24 and viruses in microdroplets.25 Its biocompatibility is attributed to the PEG-block forming a biologically inert interior surface in the water droplets.
000 g mol−1 has been used to screen enzymes,19,20 mammalian cells,6,21–23 bacteria,24 and viruses in microdroplets.25 Its biocompatibility is attributed to the PEG-block forming a biologically inert interior surface in the water droplets.
PEG–PFPE2 triblock copolymers appear to be the most applied surfactants in fluorous droplet microfluidics nowadays. They are commercially available26 or synthesized from Krytox and amino-functionalized polyethers.
However, the PEG block in between the PFPEs as a hydrophilic moiety offers very limited opportunity for further chemical modification. While custom-made surfactant molecules have been used, for instance, to create droplet interfaces for controlled immobilization27 and to promote chemical reactions28 or protein crystallization by functional hydrophilic moieties, one can only vary the molecular weight of PEG in PEG–PFPE2.29
Polyglycerols represent a class of biocompatible and multihydroxy-functional polymers that may be considered as a multifunctional analog of PEG.30,31
Recently, we introduced a novel class of biocompatible surface coating32,33 as an alternative to PEG. Linear polyglycerol (LPG) derivatives form resistant layers to inhibit the uncontrolled adsorption of fibrinogen, pepsin, albumin, and lysozyme and showed even less adsorption of human plasma protein than a PEG-modified surface. Additionally, cell adhesion experiments on linear polyglycerol LPG(OH) and poly(methyl glycerol) LPG(OMe) modified surfaces showed a similar cell resistance to that of a PEG-modified surface.
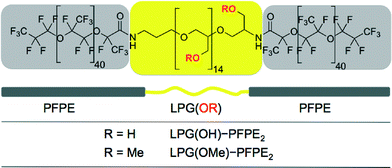
Therefore we employed LPG(OMe) and LPG(OH) as a building block in a triblock copolymer and introduced LPG-based triblock copolymers as a new class of microfluidic fluorosurfactants. The linear polyglycerol block of the LPG-PFPE2 triblock surfactant allows for side-chain functionalization, while maintaining biocompatibility, as shown by performing gene expression and cell encapsulation in surfactant-stabilized W/O microdroplets.
Results
Surfactant characterization
The structure of a nonionic surfactant molecule is defined by its molecular weight, hydrophilic–lipophilic balance (HLB) and geometry. All three were chosen to be the same for the new polyglycerol-based surfactants compared to the optimized PEG-based surfactant for microfluidic applications.19,20 In order to improve the polarity gradient and the biocompatibility we replaced the 1 kDa polyethylene glycol (PEG) hydrophilic center of the triblock copolymer by linear polyglycerol with either a hydroxy (LPG(OH)) or methoxy (LPG(OMe)) side chain as shown in Fig. 1.The degree to which the surfactant organizes at the interface between the water droplet and the fluorous oil in the microfluidic device can be quantified by the reduction of the interfacial tension (γoil–water) between the two phases. The interfacial tension γcmc at the critical micelle concentration (CMC), was determined by the pendant drop method shown in Fig. S2 in the ESI.†
The γ of 54.8 ± 0.4 mN m−1 between de-ionized water and fluorinated oil (HFE7500 3M) was reduced by at least 25 mN m−1 when any of the three surfactants was added to the oil. The PEG triblock copolymer showed the lowest γCMC of 4.2 mN m−1, which is in agreement with previous studies.34 The LPG(OMe) triblock copolymer showed a similar trend with γCMC = 18.8 mN m−1. The determined CMC values were similar for both surfactants (Table 1). We further validated the CMC values using the aggregate count rate of dynamic light scattering measurements. This method can be used to measure the critical aggregation concentration as well as the size of the formed aggregates as shown in Fig. S3.† The two methods showed close agreement of the obtained CMC values in the range of 0.01–0.05 wt% summarized in Table 1. Unfortunately, it was impossible to reliably interpret the surface tension data with LPG(OH) triblock surfactant due to a high variance of measurement points. Compared to LPG(OMe) surfactants, the higher polarity of poly(hydroxyl glycerol) in the LPG(OH) surfactant seems to cause uncontrolled agglomeration induced by hydrogen bonds, which leads to a lower free surfactant concentration and therefore a lower surface activity. The uncontrolled agglomeration at different time points and sample concentrations may be the reason for the high variances of the interfacial tension measurement of LPG(OH)–PFPE2.
| Name | γ CMC [mN m−1] | CMCIFT [wt%] | CMCDLS [wt%] | Diameter [nm] |
|---|---|---|---|---|
| PEG–PFPE2 | 4.2 | 0.05 | 0.04 | 398 ± 98 |
| LPG(OMe)–PFPE2 | 18.8 | 0.02 | 0.01 | 200 ± 23 |
| LPG(OH)–PFPE2 | 12–24 | — | 0.02 | 284 ± 88 |
High molecular weight surfactants generally provide steric stabilization at the interface and exhibit very low CMC. Therefore a very low surfactant concentration is required for the targeted reduction of the interfacial tension. However, the molecular weight of the surfactant and the resulting low CMC must be balanced against the demand to achieve stable droplets as fast as possible after formation inside the device. The rate at which the droplet is stabilized by the surfactant depends upon the rate at which surfactant is adsorbed at the interface, which is again dependent upon the amount of surfactant present in the system.34
Droplet stability and permeability
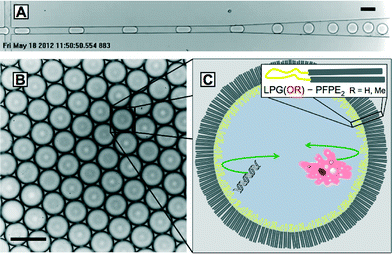
The droplet formation inside a microfluidic channel is shown in Fig. 2A. During droplet formation both polyglycerol-based surfactants prevented the droplets from coalescencing upon contact with each other. Fig. 2B shows the stored droplets in a hexagonal arrangement indicating their monodisperse size distribution and therefore the absence of coalesced bigger droplets on a longer time scale.To further test the mechanical stability of our emulsions, samples of surfactant stabilized droplets were collected on a glass slide under a brightfield microscope and deformed by a microneedle. An emulsion was considered to be stable if it remained monodisperse and deformed droplets did not coalesce. While LPG(OH) surfactant coalesced under the mechanical stress of the needle tip, LPG(OMe) surfactant produced mechanically stable emulsions shown in the image sequence in Fig. S5 in the ESI.† The instability of the LPG(OH) surfactant emulsions may derive from the aggregation behavior, which was observed in the surface tension and DLS measurements. Although LPG(OH)–PFPE2 exhibited a regular CMC in the DLS results, its aggregation at higher concentrations resulted in the inconsistent interfacial tension values shown in Fig. S2† and could be the reason for the resulting droplet instability. To avoid this and to offer droplet stability as well as functionalizable hydroxyl groups, a copolymerization of glycidol and methyl glycidol monomer forming a poly(glycerol-co-methyl glycerol) is possible. Further experiments were conducted with the poly(methoxy glycerol) surfactant, LPG(OMe)–PFPE2, because of its high droplet stability.
While surfactants are a key ingredient for providing droplet stability, they do not necessarily form an impenetrable barrier. Molecules can diffuse across the surfactant layer, if it is porous enough. This diffusion is molecular weight-dependent and can be tested by FITC-labelled dextranes.35 Moreover, micelles formed from excess surfactant in the oil phase can promote cargo transport from one droplet into another droplet.36–38
To study if microemulsions exhibit enhanced partitioning of droplet cargo into the surrounding oil phase when stabilized with our surfactants, we microfluidically fabricated water droplets loaded with fluorescein isothiocyanate (FITC)-labeled dextran (500 kDa) in fluorinated oil (HFE7500, 3M). Regarding the application of our surfactants for encapsulating and stabilizing complex biological processes in microemulsions such as the below-described in vitro transcription/translation (IVTT), the probe size reflects typical molecular weights of plasmid DNA and other key ingredients of the IVTT machinery.39
As a proof of principle, we stabilized the FITC dextran-emulsion with LPG(OMe)–PFPE2 at a concentration far above the CMC of 2 wt%. The emulsion was then loaded into a microfluidic chamber fabricated in PDMS that enabled fluorescence imaging over several hours without significant droplet shrinkage due to evaporation. As shown in Fig. S6A,† the droplets were monodisperse and aligned into a hexagonal package. The fluorescence image taken after four hours also showed that the fluorescence was homogenous throughout the droplet volume without any noticeable accumulation of the FITC dextran at the surfactant-stabilized droplet interface. Both the fluorescence of the microdroplets as well as the oil phase did not change significantly over the course of the experiment, which indicated that the surfactant layer was stable enough to prevent leakage of the fluorescent cargo, as shown in Fig. S6B.†
Biocompatibility with DNA and cells
A key application of microdroplets is the encapsulation and analysis of simple biological samples like proteins, enzymes and DNA as well as the compartmentalization of complex biological functions including microtubule formation, biochemical oscillators, and gene expression machinery.27,40–42The spatial barrier for encapsulated samples is created by the surfactant ordered at the droplet interface as shown in Fig. 2C. Previous studies have indicated that the surfactant chemistry influences biocompatibility and non-specific biomolecule adsorption at the droplet interface, in particular.15,16
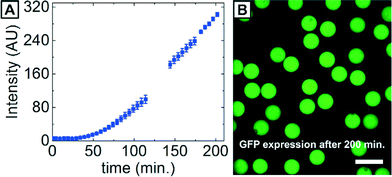
The biochemical process of in vitro transcription/translation (IVTT) expressing proteins from DNA was chosen to test our surfactants. An operating gene expression machinery contains DNA, mRNA, proteins, cell lysate extract, buffer, energy regeneration system, amino acids, and nucleoside triphosphates, which makes it ideally suited for investigating the biocompatibility of our surfactants as well as adsorption characteristics of biomolecules. We encapsulated a commercial IVTT kit (5Prime) in surfactant-stabilized W/O microdroplets at 2 wt% LPG(OMe)–PFPE2 at 4 °C, collected the emulsion in a PDMS-based collection chamber, and warmed up the sample to room temperature on a confocal microscope to control the onset of mRNA production. The IVTT mixture contained plasmid encoding a green fluorescent protein (GFP), which allowed us to follow gene expression via fluorescence, as shown in Fig. 3A. After approximately 200 min, the IVTT-containing droplets showed significant increase in fluorescence due to the production of GFP, proving that the IVTT machinery was working. Moreover, all droplets exhibited homogeneous fluorescence and no protein accumulation at the droplet interface occurred, as shown in Fig. 3B. These results show the applicability of LPG(OMe)–PFPE2 surfactant for IVTT in droplets.
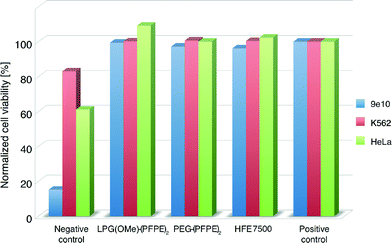
To test the effect on cell survival of surfactant stabilized interfaces, we used three types of mammalian cell lines: 9e10, K562, and HeLa cells. Approximately 500–1000 cells were incubated overnight on fluorinated oil (HFE7500, 3M) containing 2 wt% of surfactant inside a 96-well microtiter plate at 37 °C and 5% CO2. In addition, we tested a surfactant mix containing traces of triethylamine or pyridine (originating from the synthesis step) that is supposed to enhance the toxicity of the cells. This negative control was included to verify the sensitivity of the cells to biological hazards. Cells grown in a cell culture flask served as a positive control. After incubation, the ratio of live and dead cells was determined using an Invitrogen viability assay. The results summarized in Fig. 4 show that the LPG(OMe)–PFPE2 and PEG–PFPE2 surfactants, as well as HFE-7500 alone, showed excellent biocompatibility with all three cell lines tested, thus proving that these two surfactants are equally well suited for cell-based assays.
Conclusions
We have developed a novel, non-ionic, polyglycerol-based fluorosurfactant that stabilizes aqueous droplets in fluorocarbon oil. The presented poly(methyl glycerol)–perfluoropolyether triblock surfactant stabilizes picoliter-scale droplets of water-in-oil emulsions. These individual compartments exhibit a polyglycerol interface created by the surfactant that keeps FITC-labeled dextran, as model cargo, inside without noticeable accumulation at the interface.Furthermore this new highly bioinert surfactant supported the in vitro expression of GFP inside droplets and proved to be biocompatible with mammalian cell lines, enabling IVTT and living cell assays for high-throughput analysis.
A polyglycerol center in the triblock copolymer surfactant bearing methoxy as well as hydroxyl groups enables further side chain functionalization that could be used to create functional inner droplet surfaces in the future.
Acknowledgements
This work was supported by the Freie Universität Berlin graduate school “Fluorine as a Key Element” (GRK 1582) funded by the German Science Foundation (DFG). JT was funded as a Feodor-Lynen fellow by the Humboldt Foundation from 2012 to 2014. LM acknowledges the support by Marie Curie International Outgoing Fellowship (300121). The work at Harvard University was supported by the NSF (DMR-1310266) the Harvard MRSEC (DMR-1420570) and DARPA (HR0011-11-C-0093). We thank our colleagues Dr. Ralph Sperling, Torsten Rossow and Sabine Paffenholz for their scientific support and Dr. Pamela Winchester for proofreading.Notes and references
- T. Thorsen, R. W. Roberts, F. H. Arnold and S. R. Quake, Phys. Rev. Lett., 2001, 86, 4163–4166 CrossRef CAS PubMed.
- N. R. Beer, E. K. Wheeler, L. Lee-Houghton, N. Watkins, S. Nasarabadi, N. Hebert, P. Leung, D. W. Arnold, C. G. Bailey and B. W. Colston, Anal. Chem., 2008, 80, 1854 CrossRef CAS PubMed.
- N. R. Beer, B. J. Hindson, E. K. Wheeler, S. B. Hall, K. A. Rose, I. M. Kennedy and B. W. Colston, Anal. Chem., 2007, 79, 8471 CrossRef CAS PubMed.
- M. M. Kiss, L. Ortoleva-Donnelly, N. R. Beer, J. Warner, C. G. Bailey, B. W. Colston, J. M. Rothberg, D. R. Link and J. H. Leamon, Anal. Chem., 2008, 80, 8975 CrossRef CAS PubMed.
- Y. Schaerli, R. C. Wootton, T. Robinson, V. Stein, C. Dunsby, M. A. A. Neil, P. M. W. French, A. J. deMello, C. Abell and F. Hollfelder, Anal. Chem., 2009, 81, 302 CrossRef CAS PubMed.
- L. Mazutis, J. Gilbert, W. L. Ung, D. A. Weitz, A. D. Griffiths and J. A. Heyman, Nat. Protoc., 2013, 8(5), 870–891 CrossRef CAS PubMed.
- A. B. Theberge, F. Courtois, Y. Schaerli, M. Fischlechner, C. Abell, F. Hollfelder and W. T. S. Huck, Angew. Chem., Int. Ed., 2010, 49(34), 5846–5868 CrossRef CAS PubMed.
- J. A. Gladysz, D. P. Curran and I. T. Horváth, Handbook of Fluorous Chemistry, John Wiley & Sons, 2006 Search PubMed.
- K. C. Lowe, J. Fluorine Chem., 2001, 109(1), 59–65 CrossRef CAS.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS PubMed.
- J. C. Baret, Surfactants in droplet-based microfluidics, Lab Chip, 2012, 12, 422–433 RSC.
- M. J. Rosen, Surfactants and Interfacial Phenomena, 3rd edn, Wiley Interscience, 2004 Search PubMed.
- D. J. Holt, R. J. Payne, W. Y. Chow and C. Abell, J. Colloid Interface Sci., 2010, 350(1), 205–211 CrossRef CAS PubMed.
- T. F. Tadros, Applied Surfactants - Principles and Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005 Search PubMed.
- L. S. Roach, H. Song and R. F. Ismagilov, Anal. Chem., 2005, 77, 785–796 CrossRef CAS PubMed.
- J. Clausell-Tormos, D. Lieber, J. C. Baret, A. El-Harrak, O. J. Miller, L. Frenz, J. Blouwolff, K. J. Humphry, S. Koster, H. Duan, C. Holtze, D. A. Weitz, A. D. Griffiths and C. A. Merten, Chem. Biol., 2008, 15, 427–437 CrossRef CAS PubMed.
- C. Holtze, A. C. Rowat, J. J. Agresti, J. B. Hutchison, F. E. Angile, C. H. J. Schmitz, S. Koster, H. Duan, K. J. Humphry, R. A. Scanga, J. S. Johnson, D. Pisignano and D. A. Weitz, Lab Chip, 2008, 8, 1632–1639 RSC.
- C. Holtze, J. J. Agresti, D. A. Weitz, K. Ahn, J. B. Hutchison, A. Gifiths, A. El Harrak, O. J. Miller, J.-C. Barat, V. Taly, M. Ryckelynck and C. Merton, Fluorocarbon emulsion stabilizing surfactants, WO/2008/021123A1, 2008.
- J. J. Agresti, E. Antipov, A. R. Abate, K. Ahn, A. C. Rowat, J.-C. Baret, M. Marquez, A. M. Klibanov, A. D. Griffiths and D. A. Weitz, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4004–4009 CrossRef CAS PubMed.
- L. Granieri, J.-C. Baret, A. D. Griffiths and C. A. Merten, Chem. Biol., 2010, 17, 229–235 CrossRef CAS PubMed.
- J.-C. Baret, O. J. Miller, V. Taly, M. Ryckelynck, A. El-Harrak, L. Frenz, C. Rick, M. L. Samuels, J. B. Hutchison, J. J. Agresti, D. R. Link, D. A. Weitz and A. D. Griffiths, Lab Chip, 2009, 9, 1850–1858 RSC.
- J.-C. Baret, Y. Beck, I. Billas-Massobrio, D. Moras and A. D. Griffiths, Chem. Biol., 2010, 17, 528–536 CrossRef CAS PubMed.
- E. Brouzes, M. Medkova, N. Savenelli, D. Marran, M. Twardowski, J. B. Hutchison, J. M. Rothberg, D. R. Link, N. Perrimon and M. L. Samuels, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 14195–14200 CrossRef CAS PubMed.
- N. Q. Balaban, J. Merrin, R. Chait, L. Kowalik and S. Leibler, Science, 2004, 305, 1622–1625 CrossRef CAS PubMed.
- J. E. Kreutz, L. Li, L. S. Roach, T. Hatakeyama and R. F. Ismagilov, J. Am. Chem. Soc., 2009, 131(17), 6042–6043 CrossRef CAS PubMed.
- RainDance Technologies Inc. at http://raindancetech.com/purchase and PicosurfTM at http://www.dolomite-microfluidics.com/webshop.
- I. Platzman, J.-W. Janiesch and J. P. Spatz, J. Am. Chem. Soc., 2013, 135, 3339–3342 CrossRef CAS PubMed.
- A. B. Theberge, G. Whyte, M. Frenzel, L. M. Fidalgo, R. C. R. Wootton and W. T. S. Huck, Chem. Commun., 2009, 6225–6227 RSC.
- M. Green and P. M. Loewenstein, Current Protocols in Microbiology, 2006, Ch. 14, 14C 11 Search PubMed.
- R. K. Kainthan, J. Janzen, E. Levin, D. V. Devine and D. E. Brooks, Biomacromolecules, 2006, 7(3), 703–709 CrossRef CAS PubMed.
- A. Thomas, S. Müller and H. Frey, Biomacromolecules, 2014, 15(6), 1935–1954 CrossRef CAS PubMed.
- M. Weinhart, I. Grunwald, M. Wyszogrodzka, L. Gaetjen, A. Hartwig and R. Haag, Chemistry – An Asian Journal, 2010, 5(9), 1992–2000 CrossRef CAS PubMed.
- T. Becherer, M. Vieira Nascimento, J. Sindram, P.-L. M. Noeske, Q. Wei, R. Haag and I. Grunwald, Prog. Org. Coat., 2015, 87, 146–154 CrossRef CAS.
- J.-C. Baret, F. Kleinschmidt, A. El Harrak and A. D. Griffiths, Langmuir, 2009, 25(11), 6088–6093 CrossRef CAS PubMed.
- H. F. Chan, Y. Zhang, Y.-P. Ho, Y.-L. Chiu, Y. Jung and K. W. Leong, Sci. Rep., 2013, 3, 3461 Search PubMed.
- L. Mazutis and A. D. Griffiths, Lab Chip, 2012, 12(10), 1800–1806 RSC.
- Y. Skhiri, P. Gruner, B. Semin, Q. Brosseau, D. Pekin, L. Mazutis, V. Goust, F. Kleinschmidt, A. El Harrak, J. B. Hutchison, E. Mayot, J.-F. Bartolo, A. D. Griffiths, V. Taly and J.-C. Baret, Soft Matter, 2012, 8, 10618–10627 RSC.
- F. Courtois, L. F. Olguin, G. Whyte, A. B. Theberge, W. T. S. Huck, F. Hollfelder and C. Abell, Anal. Chem., 2009, 81, 3008–3016 CrossRef CAS PubMed.
- S. R. McGuffee and A. H. Elcock, PLoS Comput. Biol., 2010, 6, e1000694 Search PubMed.
- S. Mellouli, B. Monterroso, H. R. Vutukuri, E. te Brinke, V. Chokkalingam, G. Rivas and W. T. S. Huck, Soft Matter, 2013, 9, 10493–10500 RSC.
- M. Weitz, J. Kim, K. Kapsner, E. Winfree, E. Franco and F. C. Simmel, Nat. Chem., 2014, 6, 295–302 CrossRef CAS PubMed.
- J. Thiele, Y. Ma, D. Foschepoth, M. M. K. Hansen, C. Steffen, H. A. Heus and W. T. S. Huck, Lab Chip, 2014, 14, 2651–2656 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, used materials, surfactants synthesis and fabrication of microfluidic devices. See DOI: 10.1039/c5lc00823a |
| ‡ Current address: Dr. Julian Thiele, Department of Nanostructured Materials and Leibniz Research Cluster (LRC), Leibniz-Institut für Polymerforschung Dresden e. V., 01069 Dresden, Germany. E-mail: thiele@ipfdd.de |
| This journal is © The Royal Society of Chemistry 2016 |