Element labeling of antibody fragments for ICP-MS based immunoassays
Abstract
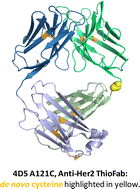
This work introduces and evaluates the use of recombinantly produced antigen binding fragments (Fab) for ICP-MS based immunoassays. A labeling procedure for a genetically modified anti HER2-specific Fab, so-called ThioFab, with rare earth element labeled macrocycles, and its characterization via liquid chromatography inductively coupled plasma mass spectrometry is presented. The employment of this engineered ThioFab, which carries a recombinantly introduced cysteine allows accurate control of the labeling stoichiometry. The labeling procedure was performed after coordination of selected lanthanides by the complexing DOTA–maleimide-moiety and subsequent linking of the complex to the ThioFab. With the introduced cysteine residue, only a mild reduction step was necessary prior to the attachment of the lanthanide–DOTA–maleimide preventing fragmentation of the Fab-molecule. Pre-optimization steps were performed with human serum albumin as the model protein. By applying the optimized labeling procedure the labeling degree of the genetically modified ThioFab was increased to 90.5 ± 0.5%. The formation of the final ThioFab/antigen conjugate employing Anti-HER2/HER2 as the model system was successfully demonstrated indicating the high potential of the new approach for immunoassays in imaging and flow-cytometry based assays.
- This article is part of the themed collection: Speciation Analysis

 Please wait while we load your content...
Please wait while we load your content...