Actomyosin contractility and RhoGTPases affect cell-polarity and directional migration during haptotaxis†
Abstract
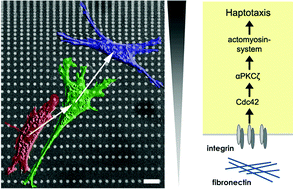
Although much is known about chemotaxis- induced by gradients of soluble chemical cues – the molecular mechanisms involved in haptotaxis (migration induced by substrate-bound protein gradients) are largely unknown. We used micropatterning to produce discontinuous gradients consisting of μm-sized fibronectin-dots arranged at constant lateral but continuously decreasing axial spacing. Parameters like gradient slope, protein concentration and size or shape of the fibronectin dots were modified to determine optimal conditions for directional cell migration in gradient patterns. We demonstrate that fibroblasts predominantly migrate uphill towards a higher fibronectin density in gradients with a dot size of 2 × 2 μm, a 2% and 6% slope, and a low fibronectin concentration of 1 μg ml−1. Increasing dot size to 3.5 × 3.5 μm resulted in stationary cells, whereas rectangular dots (2 × 3 μm) orientated perpendicular to the gradient axis preferentially induce lateral migration. During haptotaxis, the Golgi apparatus reorients to a posterior position between the nucleus and the trailing edge. Using pharmacological inhibitors, we demonstrate that actomyosin contractility and microtubule dynamics are a prerequisite for gradient recognition indicating that asymmetric intracellular forces are necessary to read the axis of adhesive gradients. In the haptotaxis signalling cascade, RhoA and Cdc42, and the atypical protein kinase C zeta (aPKCζ), but not Rac, are located upstream of actomyosin contractility.

 Please wait while we load your content...
Please wait while we load your content...