Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Label-free concentration of viable neurons, hESCs and cancer cells by means of acoustophoresis†
Marina C.
Zalis
a,
Juan F.
Reyes
b,
Per
Augustsson
c,
Staffan
Holmqvist
d,
Laurent
Roybon
d,
Thomas
Laurell
c and
Tomas
Deierborg
*a
aExperimental Neuroinflammation Laboratory, Department of Experimental Medical Science, Lund University, Sweden. E-mail: tomas.deierborg@med.lu.se
bNeuronal Survival Unit, Department of Experimental Medical Science, Lund University, Sweden
cDepartment of Biomedical Engineering, Lund University, Sweden
dStem Cell Laboratory for CNS Disease Modeling, Wallenberg Neuroscience Center, Department of Experimental Medical Science, BMC A10 and Strategic Research Area MultiPark and Lund Stem Cell Center, Lund University, 22184 Lund, Sweden
First published on 15th February 2016
Abstract
Concentration of viable cell populations in suspension is of interest for several clinical and pre-clinical applications. Here, we report that microfluidic acoustophoresis is an effective method to efficiently concentrate live and viable cells with high target purity without any need for protein fluorescent labeling using antibodies or over-expression. We explored the effect of the acoustic field acoustic energy density and systematically used different protocols to induce apoptosis or cell death and then determined the efficiency of live and dead cell separation. We used the breast cancer cell line MCF-7, the mouse neuroblastoma N2a as well as human embryonic stem cells (hESCs) to demonstrate that this method is gentle and can be applied to different cell populations. First, we induced cell death by means of high osmotic shock using a high concentration of PBS (10×), the protein kinase inhibitor staurosporine, high concentrations of dimethyl sulfoxide (DMSO, 10%), and finally, cell starvation. In all the methods employed, we successfully induced cell death and were able to purify and concentrate the remaining live cells using acoustophoresis. Importantly, the concentration of viable cells was not dependent on a specific cell type. Further, we demonstrate that different death inducing stimuli have different effects on the intrinsic cell properties and therefore affect the efficiency of the acoustophoretic separation.
Insight, innovation, integrationThe concentration of viable cells in suspension is of great interest for both research and clinically oriented applications. Current procedures rely mainly on cell sorting techniques that include fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS). These techniques, however, require immunolabeling and target specific cell proteins. Moreover, these cell-sorting procedures induce cell stress and highly reduce cell viability. Here, we present a microfluidic separation method based on acoustophoresis, which is based on a robust flow-through separation process that employs acoustic radiation forces from ultrasonic standing waves to gently and efficiently discriminate and separate particles (e.g. cells) with high viability within a microchannel. Importantly, acoustophoresis does not require immunolabeling procedures thereby eliminating antibody costs. Here we show that acoustophoresis can be successfully applied to both rodent and several human cell types, including human embryonic stem cells, thereby allowing an efficient concentration of live and viable cells from dead ones. |
Introduction
Many areas in biological, medical research, biotechnology or clinical therapy require an efficient and gentle method for cell separation and sorting.1,2 One such example is the removal of dead cells from cultures that may be detrimental for viable cell populations.3 For instance, improving cell viability enhances the sensitivity of cell-based screening assays and drug selection.4 Moreover, improved cell viability prevents from grafting non-viable cells in animal models, which may trigger a cascade of events that include inflammation within the host and therefore trigger experimental variability, reduce the experimental efficiency of cell engraftment and transplantation.5,6Methods that are currently used such as fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS) require cell labeling, which may damage cell membranes and reduce cell viability or functional activity.7 Methods that are label-free, robust and can be applied to a majority of cell types (prokaryotic and eukaryotic) are therefore currently of great medical need.
Acoustophoresis is a new lab-on-a-chip technology based on the microfluidic technology that offers a persuasive alternative to both FACS and MACS. This microfluidic separation process employs an ultrasound radiation force (ultrasonic standing waves) that gently and efficiently separates particles or cells within a microchannel.8–11 Cells flowing through an acoustic standing wave field are subject to an acoustic radiation force, which deflects their trajectory towards a pressure node located in the channel center.12,13 The sideways acoustic velocity of the cell depends on its size, and its mass density and compressibility relative to the suspending medium. Higher density, lower compressibility (stiffer) and larger size lead to a higher acoustic radiation force on a cell, which makes it go faster into the middle of the separation channel. Therefore, the acoustophoresis method enables a gentle cell separation based on the intrinsic acoustophysical parameters in a non-contact, non-harmful label-free system.14,15
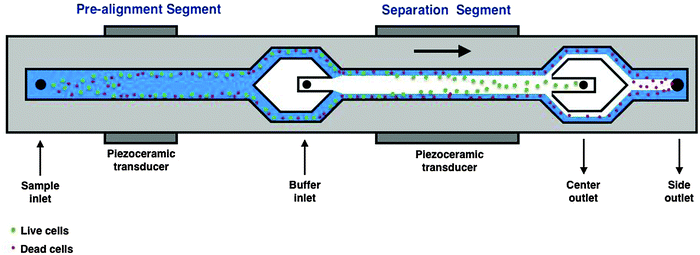
In this study, we investigate whether we can concentrate live cells from a mixed population of live and dead cells using acoustophoresis. Previously, Yang and colleagues demonstrated that microfluidic acoustophoresis could be used to effectively concentrate viable breast cancer cells (MCF-7).16 Osmotically induced cell death reduces the cell size and when mixed with larger viable cells, the authors successfully concentrated viable cells based on their higher acoustophoretic velocity compared to the smaller apoptotic cells.16 In this study, we used a new generation of higher resolution acoustophoresis chip applying a 2D pre-alignment segment that has previously been successfully used to concentrate tumor cells from blood.14 This 2D pre-focusing channel induces the alignment of the cells in the width and height dimensions and therefore ensures that the trajectories of the cells in the separation channel begin from identical positions in the transverse channel-cross section, increasing the separation efficiency.14 Here, we are specifically investigating the effect of the acoustic energy density of the acoustic standing wave and its relationship to separation performance using several different cell types and cell death stimuli. Hence, we explored the possibility to use acoustophoresis to separate viable cells from dead cells that have succumbed under defined death conditions including the natural process of nutrient starvation.
Materials and methods
Materials
We used the human breast tumor cell line MCF-7 obtained from ATCC®, the neuroblastoma cell line N2a (overexpressing α-synuclein, N2a α-Syn, expressing together with mCherry as a marker17) and the H13 human embryonic stem cell line.DMEM Media – GlutaMAX™, DMEM/F12, Fetal Bovine Serum (FBS), KSR, L-glutamine, Dispase, Accutase, Phosphate-Buffered Saline pH 7.4 (1× PBS), SYTOX®Red Dead Cell Stain and Annexin V Alexa Flour® 488 conjugates were purchased from Life Technologies. Counting slides and the trypan blue dye were obtained from BIO-RAD, UK. 10× Phosphate-Buffered Saline (10× PBS), Trypsin, Penicillin–Streptomycin (PS), Staurosporine (STS), and Dimethyl Sulfoxide (DMSO) were purchased from Sigma Aldrich. NEAA and donkey serum were purchased from Millipore and FGF2 was purchased from Peprotech.
Cell culture (cell preparation protocols)
N2a and MCF-7 cells were cultured in T-75 cell culture flasks with 10 mL of growing media (i.e. Dulbecco's Modified Eagle's Medium (DMEM-GlutaMAX) supplemented with 10% FBS, 1% PS) in a humidified 37 °C/5% CO2 incubator.N2a cells were subjected to four different death-inducing methods. N2a cells were washed with 1× PBS and detached using 1 mL trypsin/EDTA solution. Cells were collected with the addition of 4 mL of growth medium and pelleted via centrifugation for 1.5 min at 400g. Cells were re-suspended with medium and divided into two fractions. One fraction was kept at room temperature (RT) overnight while the other fraction with 5 × 106 cells was washed, pelleted and re-suspended in 10 mL of 10× PBS and incubated for 18 h at RT. After incubation, dead and apoptotic cells were centrifuged, washed and re-suspended in a PBS buffer containing 1% FBS and 2 mM EDTA (acoustophoresis buffer). For acoustic cell separation a mixture of approximately 50% live cells and 50% dead cells was prepared at a concentration of approximately 2 × 106 cells per mL.
We then differentiated N2a cells for 2 or 6 days by growing them in the same media as above but without FBS. After 2 days of differentiation, 100 µL of 1 mM staurosporine or 1 mL of DMSO was added to the culture then harvested after 18 h and re-suspended in acoustophoresis buffer. N2a cells that were left to differentiate for 6 days (D6) were collected and re-suspended in the acoustophoresis buffer.
MCF-7 cells at confluence were harvested and then treated in the same way as non-differentiated N2a cells. 10× PBS treated cells were then centrifuged at 500g for 6 min. The cell pellet was re-suspended with 100 µL of FBS and 2 mL of 1× PBS, re-centrifuged and then re-suspended in acoustophoresis buffer. A mixture of approximately 50% live cells/50% dead cells was prepared at a concentration of approximately 2 × 106 cells per mL.
The colonies of H13 hESCs at passage p30–34 were cultured in WiCell medium composed of Advanced DMEM/F12 supplemented with 20% KSR, 2 mM L-glutamine, 1% NEAA and 20 ng mL−1 FGF2. On the day of the experiment, cells were detached by adding dispase (0.5 µg mL−1) and then incubated at 37 °C for 30 min. Colonies were collected and washed 3 times in FGF2-free fresh WiCell medium. Colonies were then mechanically dissociated. Single cells were collected by centrifugation at 300g for 5 min and re-suspended in acoustophoresis buffer (1× PBS, 2% FBS and 4 mM EDTA) at a density of 2 × 106 cells per mL.
Immunohistochemistry
After fixation with 4% paraformaldehyde (PFA), hESC cells were washed and incubated for 1 h in a blocking solution composed of 10% donkey serum and 0.1% Triton-X100 in TBS. Cells were then incubated at 4 °C over night with the mouse anti SSEA4-PE conjugate (Life Technologies A14766, 1:200) and mouse anti Oct3/4 (Millipore MAB4401, 1:200) in combination with the donkey anti mouse AF488 (Life Technologies, 21202, 1:200) secondary antibody. DNA was then stained with DAPI and cultures were imaged using an inverted microscope (Olympus IX73 equipped with a Hamamatsu C11440 Orcaflash 2.8 camera).Acoustophoresis chip, ultrasound actuation and the flow system setup
The acoustophoresis chip used in this study is illustrated in Fig. 1, and similar chips have previously been used for cell sorting experiments14,15 and are described in more detail by Deshmukh et al. (2014).18 Briefly, the chip uses acoustic cell pre-alignment before acoustic cell separation, which is crucial to achieve efficient separation. To generate ultrasound standing waves for pre-alignment and separation, two piezoelectric ceramic transducers were glued to the backside of the chip. The electrical driving signal to the pre-alignment transducer was kept at a constant amplitude of 1.41 V (peak to peak) and at a frequency of 4.93 MHz. The acoustic energy density in the separation channel was varied by changing the amplitude of the electrical signal to the piezoceramic transducer, from 3.5 V to 11.0 V (peak to peak) for the transducer in the separating zone, at a frequency of 2.0 MHz. The inlet and outlet flows were controlled by regulating the pressures within four liquid containers for the cell sample, cell free central inlet liquid and two outlet flow fractions. The total volumetric flow rates were set accordingly; sample inlet, 100 µL min−1, central (buffer) inlet, 400 µL min−1, central outlet, 100 µL min−1 and side outlet 400 µL min−1.Acoustophoresis separation experiments
Prior to acoustophoretic separation, the mixture of live and dead cells (2 × 106 cells per mL) was kept at RT. A small-homogenized volume (300 µL) of this cell suspension was transferred to a 5 mL round-bottom polystyrene tube and connected to the inlet tubing of the device before each acoustophoretic run. One cell fraction at a time was processed repeatedly for each separation voltage (starting from the highest voltage to the lowest) and the center and side outlet fractions were collected and cell concentration in each fraction was measured.Examination of the outlet fractions
After each acoustophoretic cell separation (at specific voltages), cells from the center and side outlet fractions were analyzed using a Bio-Rad TC20™ (Hercules CA, USA) automated cell counter with trypan blue to quantify cell viability. Loading of the sample was performed according to manufacturer’s instructions. For each cell fraction tested using the cell counter, three parameters were obtained: total cell count per mL, viable cell count per mL as well as viability (%) and cell size.Cell preparation for flow cytometry and analysis
MCF-7 cells and N2a cells from all cell-death inducing experiments, and respective controls, were analyzed by flow cytometry. Cells were collected immediately after trypsinization, permeabilized and fixed using the FIX&PERM® Cell Permeabilization Kit (ADG, cat# GAS-002M). Cell populations were further stained with the Annexin-V AF488 conjugated antibody and Sytox®Red to separate out apoptotic cells and dead cells, respectively (according to manufacturer’s instructions, Invitrogen, Life Technologies). The gating strategy used for flow cytometry analysis is as follows: cells were first gated on a forward scatter/side scatter (FSC-A/SSC-A) dot plot to exclude cell debris. These events were further visualized using a FSC-A/FSC-H dot plot and single cells were gated. Single cells were further visualized using a SytoxRed/Annexin-V dot plot and different cell populations were identified on the forward scatter/side scatter (FSC-A/SSC-A) dot plot.Post acoustophoresis hESC fractions were analyzed for pluripotency using the mouse anti SSEA4-PE conjugated antibody. To analyze the cells we first gated them on a forward scatter/side scatter (FSC-A/SSC-A) dot plot to exclude cell debris. These first gated events were further visualized using a FSC-A/FSC-H dot plot and single cells were gated. Single cells were further visualized using the PE-A/% of Max histogram. Fluorescence was quantified using a FACS Aria III flow cytometer and analysis was performed using FlowJo version 9.8.2 for MAC.
Data analysis
Cell recovery, contamination, purity and diameter data were analyzed using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean and standard error of the mean (SEM). Purity was calculated as the number of live cells within the center outlet divided by the total number of cells in the center outlet. The recovery of viable cells was calculated as the number of live cells in the center outlet divided by the sum of live cells in the center outlet and the number of live cells in the side outlet. Cell contamination was calculated as the number of dead cells in the center outlet divided by the sum of dead cells in the center outlet and the dead cells in the side outlet.Results and discussion
Acoustophoresis offers non-contact and label-free cell separation based on size and intrinsic cell properties14 without being detrimental to cells or altering their phenotype.15 Indeed, Yang and colleagues demonstrated that microfluidic acoustophoresis could be used to effectively concentrate viable breast cancer cells (MCF7).16 Osmotically induced cell death reduces the cell size and when mixed with larger, viable cells, the authors successfully concentrated the viable cells based on their higher acoustophoretic velocity compared to smaller apoptotic cells.16In this study, we used a new generation of acoustophoresis chip by applying a 2D pre-alignment that has previously been successfully used to concentrate tumor cells from blood.14 Here, we are specifically investigating the effect of the acoustic energy density of the acoustic standing wave and its relationship to separation performance using several different cell types and cell death stimuli. Hence, we explored the possibility to use acoustophoresis to separate out viable cells from dead cells that have succumbed under defined death conditions including the natural process of nutrient starvation.
To vary the acoustic energy density we applied a sinusoidal electrical signal to the piezoelectric transducer of amplitudes ranging from 3.5 V to 11.0 V (peak to peak) for the transducer in the separation zone. Increasing amplitude leads to a higher acoustic radiation force on the cells and deflects them at a faster rate towards the acoustic pressure node. In order for two different cell populations to be suitable for separation by acoustophoresis, the cells from each population need to have different acoustically induced sideway velocities, either by difference in cell size, mass density or compressibility. It is worth noting that the size of a particle or cell is often a dominant factor since the acoustically induced velocity of a cell scales with the particle's diameter to the power of two.12 Thus, larger cells commonly migrate faster towards the pressure node than smaller cells. However, the density and the compressibility of the cells will also have an impact on their acoustic velocity.
Concentration of viable MCF-7 breast cancer cells following osmotic shock
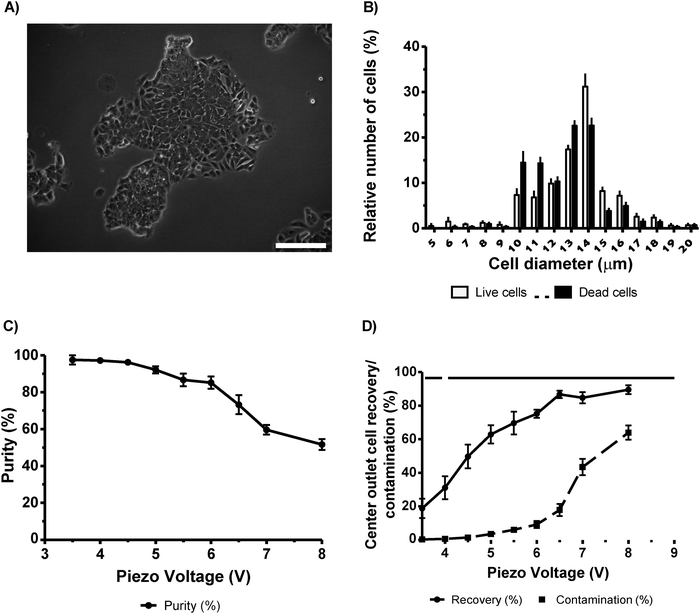
To separate live cells from dead MCF-7 cancer cells, we mixed a population containing equal number of viable cells and cells treated by osmotic shock (10× PBS) for 18 h. By using this method, we expected to retrieve a typical small (in diameter) cell population of dead/dying cells that could be easily sorted as reported by Yang et al. (2012).16 However, in contrast to data reported by Yang and colleagues, we did not observe a clear cell size difference even after 24 h of osmotic shock treatment. Under our conditions, cells treated with 10× PBS regained their cell size when placed under physiological PBS solution (data not shown). The suspension of dead cells was indistinguishable from the viable cells, which range from 10–18 µm in diameter (Fig. 2B). Despite the lack of measurable size differences, we could separate live cells from dead cells using our method. Indeed, by using an actuation voltage of 3.5 V, we successfully separated viable cells at a purity of 97.5 ± 2.5% with a recovery of 18.8 ± 5.8% (Fig. 2C and D, respectively, n = 5). By using a higher voltage (4.5 V), we obtained higher recovery (49.7 ± 7.1%) and yet with high purity (96.2 ± 1.5%). Further increasing the actuator amplitude (6.5 V) yielded a recovery of 85% but reduced the purity to 73.2 ± 5.1% and therefore a higher dead cell contamination (17.9 ± 3.7%).The measured size distributions of live and dead MCF-7 cells do not support a claim that acoustophoretic separation is mainly governed by size differences. Rather, we interpret this as an outcome of changes in cell mass density, which is altered in cells undergoing cell death,19 and/or changed compressibility due to intracellular degradation and that the cell membrane becomes more permeable when cells die.20 Taken together, we demonstrate here that it is possible to effectively separate live MCF-7 cells from dead/dying cells using a 2D acoustophoresis chip. However, we found no support for the separation being solely dependent on cell size and thus we hypothesize that it is likely also due to the differences in compressibility or density.
Separation of viable neuronal cells from cell population undergoing cell death
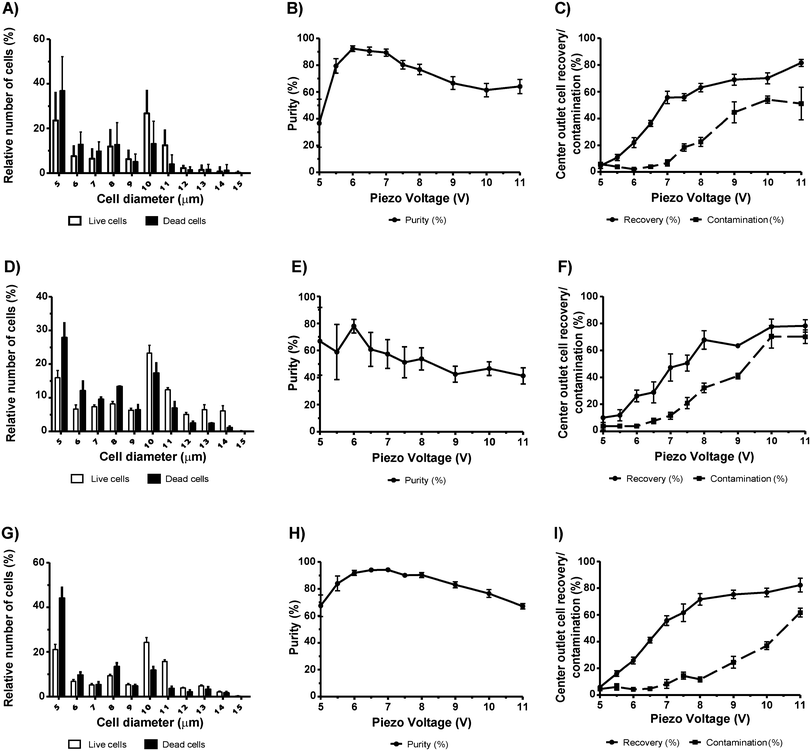
In order to separate live neuronal cells from different death inducing stimuli, we again used the acoustophoresis technique as above. In this instance, we used the neuroblastoma cell line N2a over-expressing alpha-synuclein, a cell line previously reported by Reyes and colleagues to model the cell-to-cell transfer of the protein in the prion-like progression of Parkinson's disease.17 Neuronal cells are highly sensitive and in high concentrations are widely used for cell therapies including grafting, a method of great scientific and clinical interest.21 Our N2a cells were subjected to different death inducing stimuli including 10× PBS (for comparison refer to our MCF-7 data), staurosporine (STS), a well-known apoptosis inducer isolated from Streptomyces staurosporeus22,23 and DMSO, a chemical highly toxic to cells.24First, we induced osmotic shock by treating the N2a cells (Fig. 3A) with 10× PBS for 18 h. We then spiked a viable batch of N2a cells to obtain a similar ratio of viable to non-viable of 50%. After acoustophoresis, we obtained the purity of viable cells between 93.0% ± 2.0% and 94.9% ± 0.8 at piezo voltages between 5.5 V and 6.5 V, respectively (Fig. 3C). At 5.5 V the recovery was of only 31.1% ± 4.9, but at 6.5 V the recovery was more than the double (75.1% ± 3.2) (Fig. 3D). For both voltages, the contamination was less than 3.5% (Fig. 3D). Notably, the majority of cells had a size between 10 µm and 14 µm in diameter, with no apparent size difference between live and dead cells (Fig. 3B). Next, we used STS, a chemical that is less likely to affect cell size compared to 10× PBS. N2a cells were treated with STS for 18 h resulting in 60.6% ± 8.2 (n = 5) viable cells. Live and dead cells had a cell diameter between 5 µm and 11 µm (Fig. 4A) but no apparent difference in cell diameter were observed between the two populations. The maximum purity of viable cells (92.45% ± 2.2) was obtained at a separation voltage of 6.0 V (Fig. 4B). At this voltage, however, the recovery was only 22.1% ± 3.8 (Fig. 4C). At a piezo voltage of 7.0 V, we obtained a high purity (89.5% ± 2.6) with an acceptable recovery of 55.7% ± 4.7 with low dead cell contamination (6.8% ± 2.3).
Next, we examined whether cell death induced by DMSO (10%) could be separated from viable cells. We suspected that the hydrophobic solvent properties of DMSO known to effectively dissolve numerous organic compounds may alter the stiffness of the cells and thereby alter the acoustic contrast factor. Interestingly, we found a somewhat different acoustophoretic characteristic of N2a cells that were killed by 10% DMSO for 18 h. Cells killed by DMSO were not efficiently separated by acoustophoresis and we were not able to reproduce the separation efficiency obtained with STS-treated cells. Instead, we obtained high variability with low purity of viable cells within the center outlet. N2a cells treated with 10% DMSO gave a proportion of live cells of 47.8% ± 8.4 on average. The maximum purity obtained was of 78% ± 5.0 at 6.0 V (Fig. 4E), where recovery and contamination was of only 28.9% ± 4.2 and 7.7% ± 2.0, respectively (Fig. 4F). The cell diameter of both live and dead populations ranged between 5 µm and 14 µm (Fig. 4D). These results indicate that the properties of dead cells may differ depending on different cell death stimuli and thus affect the effectiveness of the acoustophoresis technique.
In our last set of experiments using N2a cells, we induced a slow progressing apoptotic cell death by starvation25 and left the N2a cells in the same differentiation medium over 6 days to induce cell death. When N2a cells were left to differentiate for 6 days, microscopy observations revealed that the cells started shrinking and dying. After 6 days of in vitro starvation, we obtained a proportion of 67.6% ± 4.2 live N2a cells. Fig. 4G shows that both live and dead cells span sizes from 5 µm to 14 µm. At 6.5 V and 7.0 V, a high purity was obtained with 94.2% ± 0.8 and 94.2% ± 1.0, respectively, with recoveries of 41.15% ± 2.14 and 55.8% ± 3.6 respectively (Fig. 4H and I). The successful acoustic separation using starvation/long-term culturing may not be explained solely by the differences in cell size between the two populations. It is well known that starvation induces an unfolded protein response and autophagy.26 These two cellular processes can potentially change both cell stiffness and cell compressibility and thereby alter the acoustic contrast factor.
Altogether, our results show that acoustophoresis can be successfully applied to effectively concentrate viable neurons in suspension from a large proportion of dead cells. We also demonstrate that the acoustophoretic technique of separating dead and live neurons can be accomplished using different cell death stimuli (10× PBS, STS, DMSO and starvation/long-term culturing). Importantly, our data indicate that acoustophoretic separation of dead/live cells can be achieved even when there is no apparent size difference between live and non-living cells. In order to confirm the cell size, we analyzed different cell populations using a coulter counter (data not shown) and flow cytometry, which inversely correlate with the cell size and cell morphology/granularity, respectively, and as expected, our flow cytometry data confirmed the great morphological overlap of live and dead cells (Fig. S1–S4, ESI†).
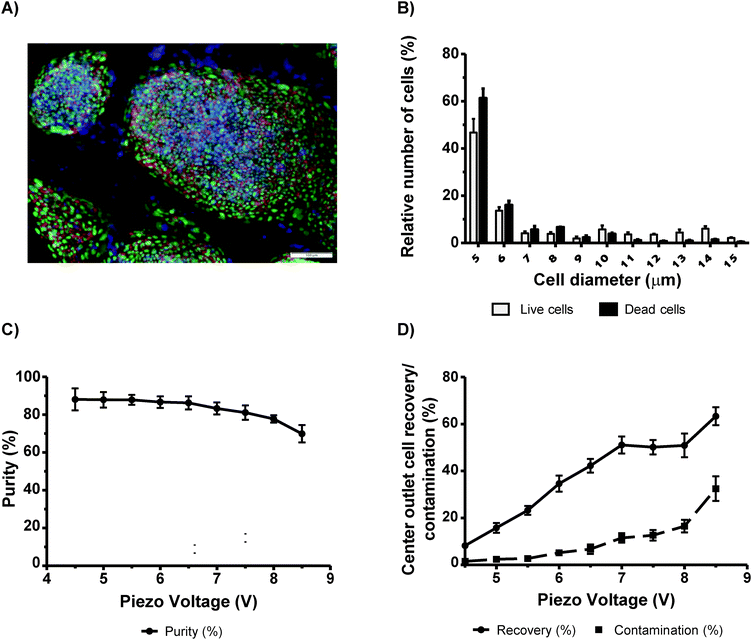
Separation and concentration of viable human embryonic stem cells (hESCs)
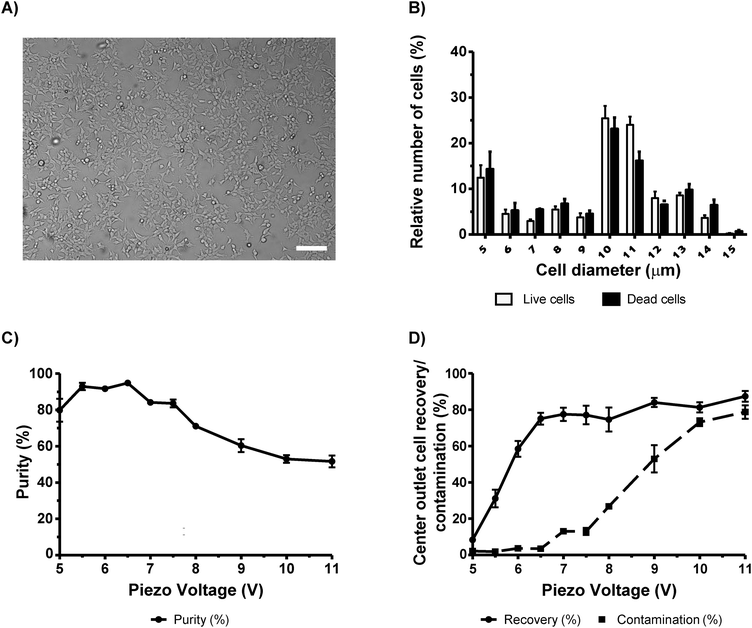
Pluripotent stem cells (PSCs) are considered to be a very promising therapeutic strategy for regenerative medicine and as a platform for drug discovery. Culturing of PSCs and their differentiation into specific cell subtypes is an expensive technique due to the use of growth factors needed for optimal culturing conditions. It is considered that the removal of dead PSCs or contaminants (e.g. feeder cells (irradiated mouse embryo fibroblasts (MEFs) or spontaneously differentiated cells)), at the initial stage, may promote healthier environmental conditions for cell growth and proper cell differentiation.27 Therefore, the concentration of viable cells during cell passages using a sorting system can be applied. Thus, we decided to evaluate the possibility to sort viable human embryonic stem cells (hESCs) from a mixed population of half dead/dying cells by means of acoustophoresis (Fig. 5A). We obtained a maximum purity of 88.1% ± 5.8 at 4.5 V, with similar purities obtained with voltages ranging from 4.5 V to 6.5 V (Fig. 5C). In this voltage range, contamination was below 7% (Fig. 5D). However, we observed big differences in recovery at these voltages (Fig. 5D). At 4.5 V, the recovery was of 8.2% ± 1.4 whereas at 6.5 V the recovery was 42.3% ± 2.9. Thus efficient sorting without compromising cell purity was obtained at an actuation voltage of 6.5 V. We also found that hESCs in suspension were small with diameters ranging from 5 µm to 6 µm (Fig. 5B). In order to confirm that we were sorting a pure population of hESCs (without MEFs and differentiated cells), FACS analysis was performed on cells before and after acoustophoresis (Fig. S7, ESI†). All cells were SSEA4 positive which indicated that they were all hESCs, which were not differentiated. In addition, we also confirm that there was no contamination with MEFs, which would be noted as a distinct population on the side and forward scatter plot using FACS.Immunohistochemical analysis was additionally performed on cultured cells. Cells were stained with SSEA4 (red) and Oct3/4 (green), both stem cells markers, and cell nuclei with Hoechst (blue) (Fig. 5A). It was verified that all of the cells in the colonies were positive, which clearly identified them as undifferentiated stem cells.
To conclude, acoustophoresis has the potential to be used as a flow-through method to concentrate viable PSCs without using anti-apoptotic agents such as ROCK-Y, which are often employed to treat PSC prior to FACS sorting28 for removing spontaneously differentiated cells.
Conclusion
In this study, we report the successful purification of live viable cells from dead non-viable cells using the acoustophoresis technique. We explored the piezo-actuation voltage, i.e. acoustic energy density, on cell sorting by measuring its effect on purity and cell recovery and further examined the effect of different death-inducing stimuli on the acoustophoretic sorting efficiency. We observed that the increase of piezo-actuation voltage is proportional to the increase of recovery at the expense of cell purity. In addition, we found that different death inducing stimuli have different effects on the intrinsic cell properties thereby affecting acoustophoretic cell sorting. We also verified that cell separation may not only depend on the cell size but is likely also an effect of the death stimulus altering their acoustic contrast of the cells relative to the medium. Further work is required to investigate in detail the change in acoustophysical properties of dying cells with respect to size, density and compressibility in relation to different death inducing stimuli. Finally, our work demonstrates that 2D acoustophoresis is a promising cell sorting technique, which gently and efficiently separates viable cells from dying cell populations as it requires no cell immunolabeling techniques.Acknowledgements
We are grateful for the technical support provided by Anna Hammerberg and for her assistance during sorting of cells with FACS, Sameer Deshmukh for his availability and input in the choice of acoustophoresis buffers and Andreas Lenshof for help with the coulter counter. This work was supported by grants from Swedish governmental agency for innovation systems, VINNOVA, CellCARE, Grant No. 2009-00236, the Swedish Research Council grants no. 2012-6708, 2012-2229 and 2010-4389, by the Gyllenstiernska Krapperup, The Royal Physiographic Society, A.E. Berger, Wiberg, Bergvall, G&J Kock, Carl Trygger foundations, the SSF Strategic Research Centre (Create Health), Stroke-riksförbundet the Swedish Parkinson Foundation, and the Strong Research Environment of Multipark (Multidisciplinary research in Parkinson's disease at Lund University).References
- D. Gossett, W. Weaver, A. Mach, S. Hur, H. Tse, W. Lee, H. Amini and D. Di Carlo, Anal. Bioanal. Chem., 2010, 397, 3249–3267 CrossRef CAS PubMed.
- M. J. Tomlinson, S. Tomlinson, X. B. Yang and J. Kirkham, J. Tissue Eng., 2013, 4, 2041731412472690 Search PubMed.
- R. Codina, A. Vanasse, A. Kelekar, V. Vezys and R. Jemmerson, Apoptosis, 2010, 15, 139–152 CrossRef CAS PubMed.
- C. D. Gregory and J. D. Pound, J. Pathol., 2011, 223, 177–194 CrossRef CAS PubMed.
- T. Deierborg, D. Soulet, L. Roybon, V. Hall and P. Brundin, Prog. Neurobiol., 2008, 85, 407–432 CrossRef CAS PubMed.
- A. Y. Wu and D. M. Morrow, J. Transl. Med., 2012, 10, 99 CrossRef CAS PubMed.
- A. P. Gee and A. G. Durett, Cytotherapy, 2002, 4, 91–92 CrossRef CAS PubMed.
- A. Lenshof and T. Laurell, J. Lab. Autom., 2011, 16, 443–449 CrossRef CAS PubMed.
- D. N. Ankrett, D. Carugo, J. Lei, P. Glynne-Jones, P. A. Townsend, X. Zhang and M. Hill, J. Nanobiotechnol., 2013, 11, 20 CrossRef CAS PubMed.
- M. Ohlin, I. Iranmanesh, A. E. Christakou and M. Wiklund, Lab Chip, 2015, 15, 3341–3349 RSC.
- S. Li, P. Glynne-Jones, O. G. Andriotis, K. Y. Ching, U. S. Jonnalagadda, R. O. Oreffo, M. Hill and R. S. Tare, Lab Chip, 2014, 14, 4475–4485 RSC.
- R. Barnkob, P. Augustsson, T. Laurell and H. Bruus, Lab Chip, 2010, 10, 563–570 RSC.
- L. P. Gor'kov, Soviet Physics Doklady, 1962, 6, 773–775 Search PubMed.
- P. Augustsson, C. Magnusson, M. Nordin, H. Lilja and T. Laurell, Anal. Chem., 2012, 84, 7954–7962 CrossRef CAS PubMed.
- M. A. Burguillos, C. Magnusson, M. Nordin, A. Lenshof, P. Augustsson, M. J. Hansson, E. Elmer, H. Lilja, P. Brundin, T. Laurell and T. Deierborg, PLoS One, 2013, 8, e64233 CAS.
- A. H. Yang and H. T. Soh, Anal. Chem., 2012, 84, 10756–10762 CrossRef CAS PubMed.
- J. F. Reyes, T. T. Olsson, J. T. Lamberts, M. J. Devine, T. Kunath and P. Brundin, Neurobiol. Dis., 2015, 77, 266–275 CrossRef CAS PubMed.
- S. Deshmukh, Z. Brzozka, T. Laurell and P. Augustsson, Lab Chip, 2014, 14, 3394–3400 RSC.
- C. L. Lewis, C. C. Craig and A. G. Senecal, Appl. Environ. Microbiol., 2014, 80, 3622–3631 CrossRef CAS PubMed.
- J. J. Lemasters, J. DiGuiseppi, A. L. Nieminen and B. Herman, Nature, 1987, 325, 78–81 CrossRef CAS PubMed.
- G. H. Petit, T. T. Olsson and P. Brundin, Neuropathol. Appl. Neurobiol., 2014, 40, 60–70 CrossRef CAS PubMed.
- L. Michea, D. R. Ferguson, E. M. Peters, P. M. Andrews, M. R. Kirby and M. B. Burg, Am. J. Physiol.: Renal, Physiol., 2000, 278, F209–F218 CAS.
- X. D. Zhang, S. K. Gillespie and P. Hersey, Mol. Cancer Ther., 2004, 3, 187–197 CAS.
- J. L. Hanslick, K. Lau, K. K. Noguchi, J. W. Olney, C. F. Zorumski, S. Mennerick and N. B. Farber, Neurobiol. Dis., 2009, 34, 1–10 CrossRef CAS PubMed.
- G. V. Kulkarni and C. A. McCulloch, J. Cell Sci., 1994, 107(Pt 5), 1169–1179 Search PubMed.
- W. X. Ding and X. M. Yin, Autophagy, 2008, 4, 141–150 CrossRef CAS PubMed.
- M. W. Nestor and S. A. Noggle, Stem Cell Res. Ther., 2013, 4, 37 CrossRef PubMed.
- N. Emre, J. G. Vidal, J. Elia, E. D. O'Connor, R. I. Paramban, M. P. Hefferan, R. Navarro, D. S. Goldberg, N. M. Varki, M. Marsala and C. T. Carson, PLoS One, 2010, 5, e12148 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ib00288e |
| This journal is © The Royal Society of Chemistry 2016 |