Precisely parameterized experimental and computational models of tissue organization†
Abstract
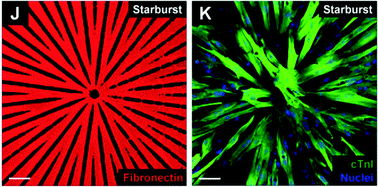
Patterns of cellular organization in diverse tissues frequently display a complex geometry and topology tightly related to the tissue function. Progressive disorganization of tissue morphology can lead to pathologic remodeling, necessitating the development of experimental and theoretical methods of analysis of the tolerance of normal tissue function to structural alterations. A systematic way to investigate the relationship of diverse cell organization to tissue function is to engineer two-dimensional cell monolayers replicating key aspects of the in vivo tissue architecture. However, it is still not clear how this can be accomplished on a tissue level scale in a parameterized fashion, allowing for a mathematically precise definition of the model tissue organization and properties down to a cellular scale with a parameter dependent gradual change in model tissue organization. Here, we describe and use a method of designing precisely parameterized, geometrically complex patterns that are then used to control cell alignment and communication of model tissues. We demonstrate direct application of this method to guiding the growth of cardiac cell cultures and developing mathematical models of cell function that correspond to the underlying experimental patterns. Several anisotropic patterned cultures spanning a broad range of multicellular organization, mimicking the cardiac tissue organization of different regions of the heart, were found to be similar to each other and to isotropic cell monolayers in terms of local cell–cell interactions, reflected in similar confluency, morphology and connexin-43 expression. However, in agreement with the model predictions, different anisotropic patterns of cell organization, paralleling in vivo alterations of cardiac tissue morphology, resulted in variable and novel functional responses with important implications for the initiation and maintenance of cardiac arrhythmias. We conclude that variations of tissue geometry and topology can dramatically affect cardiac tissue function even if the constituent cells are themselves similar, and that the proposed method can provide a general strategy to experimentally and computationally investigate when such variation can lead to impaired tissue function.

 Please wait while we load your content...
Please wait while we load your content...