Renewability – a principle of utmost importance!
Audrey
Llevot
* and
Michael A. R.
Meier
*
Karlsruhe Institute of Technology (KIT), Institute of Organic Chemistry (IOC), Materialwissenschaftliches Zentrum MZE, Building 30.48, Straße am Forum 7, 76131 Karlsruhe, Germany. E-mail: audrey.llevot@kit.edu; m.a.r.meier@kit.edu; Web: http://www.meier-michael.com
The sustainability evaluation of a product’s manufacture starts from the analysis of the employed feedstock and its extraction.1 This consideration highlights the importance of the 7th principle of green chemistry: “a raw material or feedstock should be renewable rather than depleting, wherever technically and economically practicable”. The use of renewable resources in the chemical industry is an old concept that has regained attention in the past 25 years with the raise of Green Chemistry and the formulation of the twelve principles of green chemistry by Anastas and Warner.2 Although, until the end of the 19th century, human needs were mainly fulfilled by exploiting biomass resources, the petrochemical revolution of the 20th century announced the beginning of an era of fossil resource dependency.3 Fossil oil is consumed both in supplying energy as well as in the production of chemicals and polymers. Its extensive exploitation over the last 60 years has led to the cost-effective easy manufacture of our daily life products. The increase in the world population and economic development, along with the decrease of the economically available amount of fossil oil, highlights the issue of its finite availability.4 Coal and natural gas constitute alternatives to fossil oil, but are also expected to deplete in the future. Indeed, with a regeneration time of several million years, fossil resources are faster extracted and consumed than they are produced and are thus considered as non-renewable.5 Additionally, environmental concerns related to their production and use, such as greenhouse gas emission, motivate researchers to develop sustainable solutions. All these disadvantages have led scientists to take a step from fossil resources to renewable resources. Biomass, with a regeneration time measured in decades, constitutes the only source of available renewable carbon. The progressive substitution of fossil resources by bio-based resources for the production of chemicals and energy, indispensable in our daily lives, is of both academic and industrial concern. For a more sustainable future, the concept of a facility that integrates biomass conversion processes and equipment to produce fuels, heat and value-enhanced chemicals from biomass was imagined with respect to the twelve principles of green chemistry and is called the biorefinery.6 Along with their abundance and renewability, the diversity of biomass constitutes an advantage for the manufacture of chemicals and synthetic materials. In nature, aliphatic structures can be found in vegetable oils, cycloaliphatic molecules in carbohydrates and terpenes and aromatics in lignin. Moreover, all these renewable resources display manifold functional groups, which make them easily suitable for chemical modifications or polymerization.7,8 However, for a more sustainable future, researchers have to keep in mind that renewability is not enough. The applied chemical procedures and industrial processes must be sustainable and respect at least the basic principles of green chemistry, but should preferentially go far beyond this simple concept.9,10 With a worldwide plastic production of over 300 million metric tons per year, polymer science represents a very active field in the use of renewable feedstocks.11 Biomass derived chemicals can be either converted into monomers with unique structures, leading to materials with novel properties, or modified in order to mimic commercial petroleum-based key molecules and monomers.
Although a large number of publications report the synthesis of novel molecules and polymers from biomass, industrial success stories are still limited. However, considering the versatility of biomass structures, this approach is very interesting and could also enable the synthesis of molecules or materials with similar or better properties that are potentially less toxic than their commercial petroleum-based counterparts. For instance, bisphenol A (BPA), widely used for the synthesis of polycarbonates and epoxy resins, is both an estrogen receptor and an androgen receptor antagonist, and is known to be toxic for living organisms.12 Current petroleum-based substitutes, such as bisphenol S, are also questionable in terms of toxicity.13 This field could tremendously benefit from the available biomass structures, especially aromatic molecules, and several studies related to the substitution of BPA by bio-based and non-toxic molecules were recently published.14
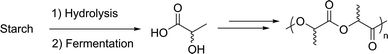
The interest of multinational companies in sustainability has increased over the last few years. One of their major actions consists in the incorporation of renewable materials in product fabrication. For instance, 3 M advertises a range of greener products partially made of plant-based adhesives (up to 67%) and/or recycled materials (packaging or dispenser).15 Another commercially available example of a new material developed from biomass derived compounds is polylactic acid (PLA). Indeed, as its chemical synthesis from fossil resources is unviable, large scale PLA production has become possible only with the development of new fermentation processes, which nowadays constitute efficient biotransformations of starch to lactic acid (Scheme 1).16 NatureWork opened the first production facility in 2002 with a capacity of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year and remains the biggest producer worldwide.17 The progress in biotechnology allows decreasing the price of PLA, but the brittleness of the crude material still limits its applications. Therefore, researchers intensively investigate the use of additives, blends and composites in order to broaden the accessible properties of this bio-based and biodegradable material.18
000 t per year and remains the biggest producer worldwide.17 The progress in biotechnology allows decreasing the price of PLA, but the brittleness of the crude material still limits its applications. Therefore, researchers intensively investigate the use of additives, blends and composites in order to broaden the accessible properties of this bio-based and biodegradable material.18
Alternatively, synthesizing already existing monomers and polymers from biomass has been intensively investigated, but generally involves several reaction steps to produce a known molecule, the production of which is already well implemented and economically viable. However, over the past 10 years, some companies have successfully developed clean sustainable processes to convert biomass into monomers or polymers chemically similar to petroleum-based equivalents. In this context, a few examples will be described in order to highlight the technical and economic feasibility of biorefineries and discussed in terms of sustainability in order to give a framework for future green developments.
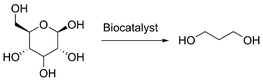
Dupont has developed a bio-based process for the production of 1,3-propanediol, offering lower manufacturing costs and improved environmental impact compared to the petroleum-based process (Scheme 2). Indeed, the recent development of biocatalysis has enabled the production of 1,3-propanediol by the fermentation of glucose. Dupont went even one step further by not only implementing the production of the monomer, but also of the thereof derived high performance polymers and fibers, which find applications in automobiles, carpets and apparel. Continuing in this dynamic, the company keeps developing new products from bio-based and non-toxic 1,3-propane diol.19
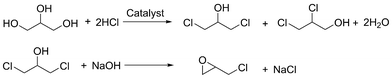
Another sustainable process to convert biomass into a key molecule for polymer synthesis was established by Solvay. The company produces bio-based epichlorohydrine (“Epicerol”) from non-toxic glycerol, a by-product of biodiesel production or oleochemistry (Scheme 3). Additionally to the use of renewable resources, the environmental impact of the entire process is reduced due to the avoidance of chlorine, less gas emissions and lower amounts of chlorinated by-products and water effluent. However, although bio-based, the molecule epichlorohydrin itself of course remains toxic, causes respiratory irritation, and is carcinogenic, mutagenic and reprotoxic. Thus, safer handling alternatives or replacements still need to be developed in the near future.20
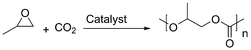
A different strategy towards more sustainable materials was adopted by Novomer and Bayer MaterialSciences (now restructured as Covestro). The greenhouse gas CO2 is employed as building block for the synthesis of polypropylene carbonate (Scheme 4). Although the technology still employs fossil-based propylene oxide as starting material, leading to an only partially bio-based material, the partial substitution of the fossil feedstock by CO2 along with the biodegradability of the final material is a step towards sustainability.21,22 A commercialization with an annual production of 5000 kt of polyol building blocks for polyurethane foams was realized this year. The product, now commercially available under the trade name Cardyon, finds applications in mattresses and upholstered furniture and shows similar performance to conventional products.23,24 Additionally, recently, with the aim of reducing its environmental impact, the automotive company Ford has started to work in collaboration with Novomer on the incorporation of CO2-based polyols into the production of vehicles.25 The use of these more sustainable and recyclable foams and plastics in vehicles should become feasible within the next five years. However, the obtained propylene carbonate polyols with different molecular weights are then used for the synthesis of polyurethanes employing toxic diisocyanates. In order to address this issue in the future, academic research intensively investigates the synthesis of non-isocyanate-based polyurethanes.26
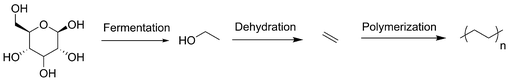
Finally, bioethylene and thereof derived “green” polyethylene is produced by Braskem from sugarcane under the name “I'm green” (Scheme 5). Instead of the traditional cracking of fossil-based naphtha, in the first step, sugarcane is fermented and distilled to generate bioethanol and electricity with the remaining sugarcane. At Braskem, further dehydration of ethanol leads to ethylene, which is directly polymerized, extruded and transformed into the final product used in our daily lives.27 Even if polyethylene can be recycled, it is not biodegradable and causes problems for the environment. Biodegradable PLA often constitutes a good alternative.
As shown by the previous successful examples, the industrial production of chemicals and polymers from renewable resources is feasible and several companies have reinforced their development in this direction. Selling products from renewables constitutes a marketing advantage for the companies, both by making customers less scared about the toxicity of the products and conscious of protecting the environment, as evidenced by the success of the Plantbottle of Coca-Cola, made of 30% plant-based material.28 However, renewability does not necessarily correlate with sustainability and the overall process has to be compared and evaluated to judge if a sustainable solution is found. Some benefit should be given to renewable routes, if compared to petroleum based ones, for the reasons discussed above. Ultimately, humankind has to return to a fully bio-based way of life – the only truly sustainable option for our (long term) future.
Notes and references
- Life Cycle Assessment Handbook: A Guide for Environmentally Sustainable Products, ed. M. A. Curran, John Wiley & Sons, 2012 Search PubMed.
- Green chemistry: Theory and practice, ed. P. T. Anastas and J. C. Warner, Oxford University Press, 1998 Search PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- S. Sorrell, J. Speirs, R. Bentley, R. Miller and E. Thompson, Energy, 2012, 37, 709–724 CrossRef.
- C. Okkerse and H. van Bekkum, Green Chem., 1999, 1, 107–114 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- A. Llevot, P.-K. Dannecker, M. von Czapiewski, L. C. Over, Z. Söyler and M. A. R. Meier, Chem. – Eur. J., 2016, 22, 11510–11521 CrossRef CAS PubMed.
- M. Eissen, J. O. Metzger, E. Schmidt and U. Schneidewind, Angew. Chem., Int. Ed., 2002, 41, 414–436 CrossRef CAS.
- PlasticsEurope, Plastics – the Facts 2015, from: http://www.plasticseurope.org/plastics-sustainability-14017.aspx.
- R. E. Chapin, J. Adams, K. Boekelheide, L. E. Gray, S. W. Hayward, P. S. J. Lees, B. S. McIntyre, K. M. Portier, T. M. Schnorr, S. G. Selevan, J. G. Vandenbergh and S. R. Woskie, Birth Defects Res., Part B, 2008, 83, 157–395 CrossRef CAS PubMed.
- C. Héliès-Toussaint, L. Peyre, C. Costanzo, M.-C. Chagnon and R. Rahmani, Toxicol. Appl. Pharmacol., 2014, 280, 224–235 CrossRef PubMed.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J.-P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- 3 M, Reduce your impact on the environments: 2013 sustainable office products, from: http://www.command.com/3MContentRetrievalAPI/BlobServlet?lmd=1371695663000&assetId=1258560969199&assetType=MMM_Image&blobAttribute=ImageFile.
- R. Auras, B. Harte and S. Selke, Macromol. Biosci., 2004, 4, 835–864 CrossRef CAS PubMed.
- E. T. H. Vink, D. A. Glassner, J. J. Kolstad, R. J. Wooley and R. P. O'Connor, Ind. Biotechnol., 2007, 58–81 CrossRef CAS.
- V. Nagarajan, A. K. Mohanty and M. Misra, ACS Sustainable Chem. Eng., 2016, 4, 2899–2916 CrossRef CAS.
- Dupont, The Manufacturing Process of Bio-PDO™ and Bio-Based Fibers, from: http://www.dupont.com/products-and-services/fabrics-fibers-nonwovens/fibers/brands/dupont-sorona/articles/how-dupont-sorona-is-made.html.
- Solvay, Epicerol™, from: http://www.solvay.com/en/markets-and-products/featured-products/epicerol.html.
- Novomer, CO2 business overview, from: http://www.novomer.com/co2-business-overview-0.
- Covestro, from: http://www.covestro.de/en/projects-and-cooperations/co2-project.
- Covestro, from: http://presse.covestro.de/news.nsf/id/aazc76-premiere-fuer-neuen-rohstoff.
- Covestro, from: http://press.covestro.com/news.nsf/id/covestro-starts-brand-launch-for-co2-products.
- Ford, from: https://media.ford.com/content/fordmedia/fna/us/en/news/2016/05/17/preserving-mother-earth-ford-first-automaker-captured-co2.html.
- L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau and H. Cramail, Chem. Rev., 2015, 115, 12407–12439 CrossRef CAS PubMed.
- Braksem, I'm green™ polyethylene, from: http://www.braskem.com/site.aspx/How-it-is-produced.
- Coca-Cola, PlantBottle, from: http://www.coca-colacompany.com/our-company/plantbottle.
| This journal is © The Royal Society of Chemistry 2016 |