Open Access Article
Open Access ArticleItaconic acid – a versatile building block for renewable polyesters with enhanced functionality
Tobias
Robert
* and
Stefan
Friebel
Fraunhofer Institute for Wood Research – Wilhelm-Klauditz-Institut WKI, Bienroder Weg 54E, 38108 Braunschweig, Germany. E-mail: tobias.robert@wki.fraunhofer.de
First published on 14th April 2016
Abstract
The increased implementation of renewable resources in the chemical industry is mainly driven by environmental reasons. However, as a rather underestimated side-effect of this development, new chemical building blocks have become available, which are not economically accessible from petrochemical sources. In this respect, itaconic acid has gained considerable attention, as it is biotechnologically produced from carbohydrates on an industrial scale. Its trifunctional structure allows for the synthesis of new polymers, like polyitaconates or related co-polymers which have been extensively studied in the past. Polyesters derived from this unsaturated dicarboxylic acid on the other hand have only recently started to gain interest, despite myriad potential applications, such as UV-curing resins for coatings, inks or adhesives or thermally curing compositions to name but a few. This work aims to review the most relevant work in the field of polyesters derived from itaconic acid and to show the unique properties of these materials and identify the potential for further research in this area.
1. Introduction
Over the last few years, chemical building blocks derived from renewable resources have gained more and more importance and started to be an alternative to chemicals from petrochemical feedstock.1–3 This is mostly driven by the imminent scarcity of fossil resources, which will be one of the major challenges in the decades to come. In addition, the increased public awareness and as a result the higher demand for bio-based and renewable products as well as the possibility of reducing dependency on imports of fossil fuels are of considerable importance. However, as another advantage of these bio-based building blocks, compounds with new structural features become available, that are not economically accessible from petrochemical feedstock. This in turn leads to new applications and materials with unprecedented properties.In this context, itaconic acid (IA) or methylene succinic acid (1, Fig. 1) has drawn considerable interest over the last few years.4 It is composed of two carboxylic acid functionalities and an α,β-unsaturated double bond, which makes it a promising precursor for a myriad chemical transformations.5,6 Itaconic acid was first synthesized in 1837 by the thermal decarboxylation of citric acid.7 Further synthetic approaches were reported, but none of them proved to be economically compatible.8,9 Biotechnological pathways based on carbohydrates have been known since the mid-forties of the last century.10 Since then considerable research efforts have been dedicated in this field to improve the yields and feasibility of this process.11–17 Nowadays, itaconic acid is produced on an industrial scale via fermentation with Aspergillus terreus with a production intensity of 80 g L−1.18 The worldwide production is estimated to be around 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year with a price of around 2 US$ per kg.19 Due to the high potential of this compound, the production capacity is expected to grow by 5.5% every year between 2016 and 2023.20
000 tons per year with a price of around 2 US$ per kg.19 Due to the high potential of this compound, the production capacity is expected to grow by 5.5% every year between 2016 and 2023.20
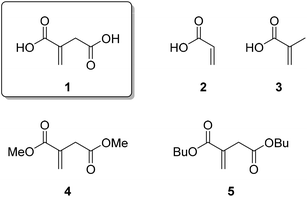 | ||
| Fig. 1 Chemical structure of acrylic (2) and methacrylic acid (3), itaconic acid (1) and their corresponding esters. | ||
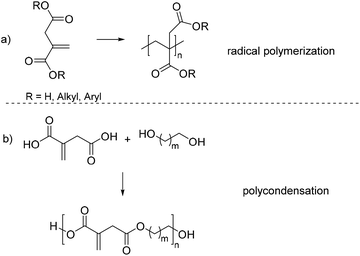
Due to the structural similarity to acrylic acid (2) and methacrylic acid (3), itaconic acid and the corresponding esters, such as dimethylitaconate (4) and dibutylitaconate (5), have been intensively studied as an alternative monomer or co-monomer to poly(meth)acrylates over the last few decades (Scheme 1a).3,21–31 Applications for this kind of polymer are widespread and range from corrosion inhibition32 to dental materials,33,34 elastomers,35 and drug-delivery.36–41
However, so far there have only been few studies in the field of polyesters based on itaconic acid (Scheme 1b). This is somewhat surprising as itaconic acid is a dicarboxylic acid with an α,β-unsaturated functionality. These structural features allow for a variety of new applications, such as an alternative for acrylated polyesters or modular building blocks for polyesters with tunable properties. Therefore, polyester-based polymers derived from itaconic acid could be useful in different fields of application, such as UV- and thermal curing or crosslinking, shape memory polymers (SMP). Also the possibility of modifying the unsaturated double bond after polycondensation exhibits a high potential to develop new materials with unprecedented properties. To the best of our knowledge, no comprehensive overview has been made on polyesters derived from itaconic acid. Therefore, this review aims to summarize the most important work done in this field and highlight the potential of this renewable building block.
2. Polyester derived from itaconic acid
2.1 Medical applications
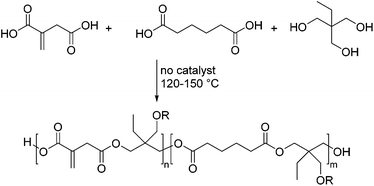
The first example of polyesters based on itaconic acid was reported as early as 1991 by Singh et al.42 The authors synthesized polyesters derived from itaconic acid and PEG-600 using p-toluenesulfonic acid as a catalyst. Also hydroquinone was added as an inhibitor to prevent radical crosslinking of the unsaturated double bonds. These polyesters were used as precursors for bio-erodible vaccine-loaded hydrogel microspheres. The crosslinking in this case was initiated through an aqueous solution of ammonium persulfate. Subsequently the rates of vaccine release were studied as a function of the degree of cross-linking.A milestone in the synthesis and application of bio-based polyesters derived from itaconic acid was made by Yousaf and co-workers.43 They synthesized four different polyesters by thermal polycondensation of itaconic acid, a second dicarboxylic acid and trimethylolpropane (TMP) without any catalysts and inhibitors (Scheme 2). This is quite remarkable, as the unsaturated double bond present in the itaconic acid usually leads to undesired crosslinking, if no inhibitors such as hydroquinones or methoxyphenols are used to quench the radicals arising at elevated temperatures. This results eventually in gel formation during the polycondensation process. In addition, despite the lack of a catalyst, the molecular weights were reasonably high (up to 2200 g mol−1) and polydispersity indices (Đ) quite narrow.
They further synthesized three polyesters by an enzymatically catalyzed reaction. This resulted in even higher molecular weights up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. Through the absence of any catalysts or co-reagents on the one hand or the use of enzymes on the other, this methodology is especially attractive for the synthesis of biomaterials. The materials synthesized were subjected to UV-light induced crosslinking in the presence of diethoxyacetophenone (DEAP), a photoinitiator commonly used in biomaterials.44 The properties of the resulting cross-linked polyesters were examined. As expected, the properties of the materials changed substantially with the ratio of itaconic acid to adipic acid (AA). By increasing the amount of longer chain aliphatic adipic acid the materials became more flexible, resulting in a drop in the Young's modulus and the ultimate tensile strength, while the rupture strain increased. As these polyesters were designed for drug delivery, tissue engineering, and other biomedical applications, the cytotoxicity as well as polymer molding was examined in the course of this study. Both properties are important for the design of drug releasing particles, stents, and sutures.45 The results suggested that polyesters of this type have a high potential in biomedical and biotechnological fields.
000 g mol−1. Through the absence of any catalysts or co-reagents on the one hand or the use of enzymes on the other, this methodology is especially attractive for the synthesis of biomaterials. The materials synthesized were subjected to UV-light induced crosslinking in the presence of diethoxyacetophenone (DEAP), a photoinitiator commonly used in biomaterials.44 The properties of the resulting cross-linked polyesters were examined. As expected, the properties of the materials changed substantially with the ratio of itaconic acid to adipic acid (AA). By increasing the amount of longer chain aliphatic adipic acid the materials became more flexible, resulting in a drop in the Young's modulus and the ultimate tensile strength, while the rupture strain increased. As these polyesters were designed for drug delivery, tissue engineering, and other biomedical applications, the cytotoxicity as well as polymer molding was examined in the course of this study. Both properties are important for the design of drug releasing particles, stents, and sutures.45 The results suggested that polyesters of this type have a high potential in biomedical and biotechnological fields.
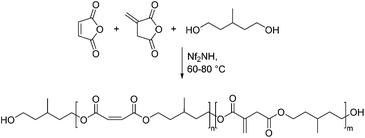
Recently Takasu and co-workers reported a low temperature approach for the polycondensation of unsaturated polyesters containing maleic anhydride and itaconic anhydride.46 This was done by reacting both anhydrides with 3-methyl-1,5-propandiol in the presence of bis(nonafluorobutanesulfonyl)imide (Nf2NH, Scheme 3).
The resulting polyesters had high molecular weights up to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. They studied the reactivity of both polyesters to undergo photo-crosslinking in the presence of a photoinitiator to obtain hydrogels that can be used as vehicles for drug delivery. One of the advantages of this polycondensation technique is that at these rather low temperatures no inhibitors are needed. However, one of their main arguments that thermal polycondensations lead to a high degree of double bond isomerization to the less reactive mesaconic acid, which would hamper the photo induced crosslinking is not very valid. NMR-measurements in several studies presented in this review showed only a negligible degree of isomerization during the thermal polycondensation processes.47,48 The scope of the methodology was examined by using other diacids and anhydrides, as well as alcohols. In most cases, the yields and molecular weights were high. By using maleic anhydride as a second dicarbonic acid moiety in the polyester, they were able to show that these two unsaturated diacids can be selectively reacted. The itaconic acid building blocks were able to undergo crosslinking through radiation curing, while the maleic acid moieties did not react under these conditions. Furthermore, they were able to show that the latter species could undergo a Z/E-isomerization to the corresponding fumaric acid functionality (Scheme 4). This proved to have an influence on the swelling behavior of the resulting hydrogels.
000 g mol−1. They studied the reactivity of both polyesters to undergo photo-crosslinking in the presence of a photoinitiator to obtain hydrogels that can be used as vehicles for drug delivery. One of the advantages of this polycondensation technique is that at these rather low temperatures no inhibitors are needed. However, one of their main arguments that thermal polycondensations lead to a high degree of double bond isomerization to the less reactive mesaconic acid, which would hamper the photo induced crosslinking is not very valid. NMR-measurements in several studies presented in this review showed only a negligible degree of isomerization during the thermal polycondensation processes.47,48 The scope of the methodology was examined by using other diacids and anhydrides, as well as alcohols. In most cases, the yields and molecular weights were high. By using maleic anhydride as a second dicarbonic acid moiety in the polyester, they were able to show that these two unsaturated diacids can be selectively reacted. The itaconic acid building blocks were able to undergo crosslinking through radiation curing, while the maleic acid moieties did not react under these conditions. Furthermore, they were able to show that the latter species could undergo a Z/E-isomerization to the corresponding fumaric acid functionality (Scheme 4). This proved to have an influence on the swelling behavior of the resulting hydrogels.
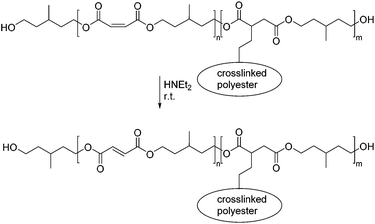
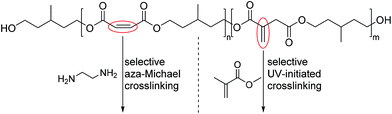
In a subsequent study the authors were able to show that mixed polyesters of this type can be selectively modified by means of aza-Michael additions.49 When the maleic acid/itaconic acid co-polyester was reacted with primary amines, such as benzylamine, n-butylamine or i-butylamine the aza-Michael addition exclusively occurred on the maleic acid moiety, which in the course of the addition rearranged to the corresponding Z-configurated fumaric acid. This methodology allows therefore for the synthesis of maleic acid itaconic acid co-polyesters, that can first be modified with primary amines and in a second step radically crosslinked through the external α,β-unsaturated C–C double bond of the itaconic acid building blocks. By using diamines, such as 1,2-ethanediamine this methodology allows in addition for two different crosslinking mechanisms: the first one being a selective aza-Michael addition crosslinking and the second a UV-initiated radical crosslinking (Scheme 5).
Inspired by the good biocompatibility of poly(butylene succinate) (PBS), Liu and co-workers reported the synthesis of a co-polyester of this type, by partial replacement of succinic acid by itaconic acid.50 By using a combination of titanium tetraisopropoxide (TTP) and diphenylphosphonic acid (DPPA), they were able to obtain high molecular weights up to 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 at a low percentage of itaconic acid incorporated into the co-polymer (5 mol% of the total diacid content). However, with increasing amount of itaconic acid, the molecular weights decrease to 14
000 g mol−1 at a low percentage of itaconic acid incorporated into the co-polymer (5 mol% of the total diacid content). However, with increasing amount of itaconic acid, the molecular weights decrease to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 with 40 mol% itaconic acid. This is most probably due to the lower reactivity of the itaconic acid compared to succinic acid. In addition, as no inhibitors were used during the polycondensation process, crosslinking of the polymer was observed, when 50% of itaconic acid was used. This resulted in an insoluble polymer, which could not be examined by means of size exclusion chromatography (SEC). In addition to the somewhat higher reactivity, the catalytic system also seems to suppress the THF formation by an intramolecular etherification of 1,4-butandiol. The authors examined this effect by comparing the feed ratio to the actual co-polymer ratio determined by means of NMR. Unlike the other examples in this review, the co-polyesters were not further radically crosslinked. However, biodegradability studies showed that the materials do degrade under in vitro conditions. This reveals their potential for biomedical applications, even though the degradation rate decreases with increasing amount of itaconic acid incorporated into the polymer.
600 g mol−1 with 40 mol% itaconic acid. This is most probably due to the lower reactivity of the itaconic acid compared to succinic acid. In addition, as no inhibitors were used during the polycondensation process, crosslinking of the polymer was observed, when 50% of itaconic acid was used. This resulted in an insoluble polymer, which could not be examined by means of size exclusion chromatography (SEC). In addition to the somewhat higher reactivity, the catalytic system also seems to suppress the THF formation by an intramolecular etherification of 1,4-butandiol. The authors examined this effect by comparing the feed ratio to the actual co-polymer ratio determined by means of NMR. Unlike the other examples in this review, the co-polyesters were not further radically crosslinked. However, biodegradability studies showed that the materials do degrade under in vitro conditions. This reveals their potential for biomedical applications, even though the degradation rate decreases with increasing amount of itaconic acid incorporated into the polymer.
2.2 Shape memory polymers (SMP)
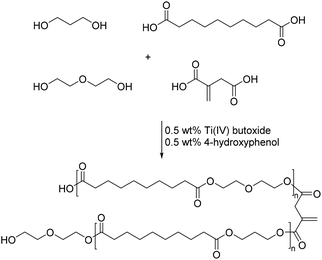
A very impressive example of the use of itaconic acid derived polyesters as SMP was described by Guo and co-workers.51 The polyesters were almost exclusively composed of bio-based monomers with itaconic acid, sebacic acid and 1,3-propandiol. Only diethylene glycol was used in order to influence the crystallinity of the polymers. The polycondensation was conducted in the presence of 0.5 wt% 4-methoxyphenol as the inhibitor and 0.5 wt% Ti(IV)-butoxide as the condensation catalyst (Scheme 6). Heating the mixture to rather high temperatures of 220 °C under vacuum allowed for the synthesis of polyesters with high molecular weights of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.
000 g mol−1.
These polyesters were then subjected to compression molding with dicumylperoxide as the radical initiator resulting in a set of SMP with interesting properties and potential fields of application. However, the polyesters were synthesized with a rather low ratio of itaconic acid and it would be of interest to study the influence of higher ratios on the properties of the resulting SMP.
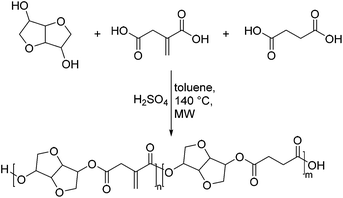
Later, Ritter and co-workers followed a similar approach by synthesizing polyesters from renewable resources as precursors for SMP.52 In this case they used polyesters based on isosorbide as diol and different ratios of succinic and itaconic acids (Scheme 7).
In detail, they reacted an equimolar mixture of dicarboxylic acids and diols in toluene in the presence of 0.5 mol% sulfuric acid as the catalyst and 0.25 mol% phenothiazine as the inhibitor. However, a standard polycondensation procedure with a Dean–Stark apparatus at 140 °C for 48 h only resulted in very low yields below 10% and low molecular weights of 500–800 g mol−1. These results were improved by using microwave irradiation instead of thermal heating. By this method, both yields and molecular weights were slightly increased to 17% and 1200 g mol−1 after 4 h. However, neither increased catalyst loading nor longer reaction times resulted in further improvement. This is quite surprising, especially as an equimolar ratio of monomers was used. Besides the somewhat low reactivity of the isosorbide, the most plausible explanation might be the low solubility of the polyesters in toluene, which results in the precipitation of the oligomers during the reaction. Subsequently, these unsaturated polyesters were also subjected to a crosslinking step by a thermally induced radical reaction initiated by the diazo-based initiator VA-65. However, in this case they used dimethyl itaconate as a co-monomer. The crosslinked polyester showed a shape memory effect with a deformation at temperatures below the Tg, which could be reversed after reheating above the Tg. However, in comparison with the SMP obtained by Guo and co-workers the materials proved to be very brittle. In a subsequent study the authors used bio-derived dinitrones based on isosorbide to crosslink the unsaturated polyesters by an 1,3-dipolar cycloaddition.53
2.3 Elastomers and composites
Also in the field of elastomers, itaconic acid derived polyesters were used as crosslinkable components. Wei et al. reported the synthesis of a fully bio-derived polyester based on succinic acid, sebacic acid, 1,3-propanediol and 1,4-butanediol with 5–15 mol% of itaconic acid in relation to the total acid content.54 MeHQ and phosphoric acid were used as inhibitors. In this case the monomers were reacted for 2 h at 180 °C before Ti(IV) butoxide was added as a catalyst and the reaction was continued at 220 °C at reduced pressure. Following this protocol, a polyester with a number-average molecular weight (Mn) of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 was obtained. This polyester was crosslinked with different amounts of dicumylperoxide as the thermal initiator and the properties like tensile strength and stress and Tg of the resulting elastomers were examined. In the last step, the material was reinforced with nanosilica, which resulted in a bio-based, transparent rubber-like material with a low Tg of −54 °C and mechanical properties that could compete or even outperform commercially available elastomers.
000 g mol−1 was obtained. This polyester was crosslinked with different amounts of dicumylperoxide as the thermal initiator and the properties like tensile strength and stress and Tg of the resulting elastomers were examined. In the last step, the material was reinforced with nanosilica, which resulted in a bio-based, transparent rubber-like material with a low Tg of −54 °C and mechanical properties that could compete or even outperform commercially available elastomers.
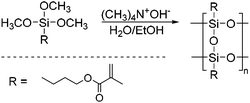
Sakuma et al. were able to show that itaconic acid-based polyesters can also be used as organic components in organic–inorganic hybrid composites.55 Methacryl-substituted polysilsesquioxanes (Me-PSQ) were used as inorganic compounds, which could be prepared from 3-(trimethoxysilyl)propyl methacrylate (TMSPM, Scheme 8). Three different composites were synthesized by reacting the unsaturated polyester with the Me-PSQ in different ratios in the presence of benzoylperoxide. Subsequently the properties of the hybrid composites were compared to the uncured and cured polyesters. The hybrids proved to be more temperature stable, with a Td (5 wt% weight loss temperature) up to 100 °C higher than the uncured polyester. In addition, the hybrids exhibited a higher storage modulus in the rubbery state (T > Tg).
2.4 Coatings
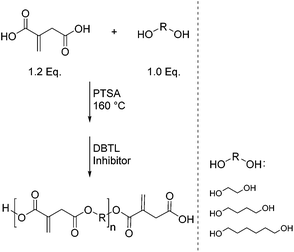
Due to the structural similarity to acrylic and methacrylic acid, which are often used as reactive groups in radiation curing binders for coating and printing ink applications, itaconic acid has a high potential to serve as a renewable alternative in this area. An example for coating applications with itaconic acid as the crosslinking group was reported by Dai et al.56 In this case three different primary diols were used as monomers for the synthesis (Scheme 9). In addition, the syntheses were conducted in the presence of two different catalysts. First prepolymers were formed in the presence of 0.5 wt% p-toluenesulfonic acid (PTSA). After 2 h, as a second catalyst, 1 wt% dibutyltin dilaurate (DBTL) was added and water was removed under vacuum. To prevent crosslinking of the unsaturated double bond, 0.5 wt% of 4-methoxyphenol was used as the inhibitor.With this procedure COOH-terminated polyesters were obtained, but despite the use of two catalysts, the molecular weights were still quite low (750–1250 g mol−1). However, the values were not measured by means of SEC, but calculated based on AV and OHV determined after the reaction.57 These polyesters were then converted into a water-based emulsion with a 40% solid content by mixing the COOH-terminated resin with an aqueous solution of NaHCO3. After the addition of a waterborne photoinitiator and a curing promoter, the emulsions were applied to a metal surface and were crosslinked after a thermal drying step by means of UV-curing. The resulting coatings exhibited a high hardness and good water and solvent resistance. However, in terms of adhesion and flexibility these systems proved to be rather insufficient.
To solve these problems, the researchers developed a similar system with improved performance.58 By incorporation of different ratios of glycerol into the polyester, they were able to improve the adhesion of the system. In addition, the use of acrylated epoxidized soybean oil as a second bio-based radically crosslinking component led to more flexibility of the coatings obtained after UV-curing.
In addition to UV-curing systems, Dai et al. further developed a thermally curing composition based on these polyesters and AESO.59 They were able to show that in the presence of tert-butyl peroxybenzoate as a radical initiator this mixture of unsaturated compounds can be used as resins for heat cured coatings. In most cases, the properties such as Tg and tensile strength of the coating were improved by using this combination of AESO and itaconic acid-based polyesters. However, also in this case the polyesters used in this study had a very low molecular weight despite the use of a tin catalyst. No effort was made to obtain polyesters with a higher molecular weight, which should have a considerable impact on the coating performance and the molecular weight was not determined by means of SEC. In addition, the polyesters were again acid terminated. As no water-based emulsions were made in this case, it raises the question, why no OH-terminated polyesters were examined. This is particularly important as the beneficial effect of the OH-functionality of glycerol-derived polyesters has been mentioned several times throughout the study.
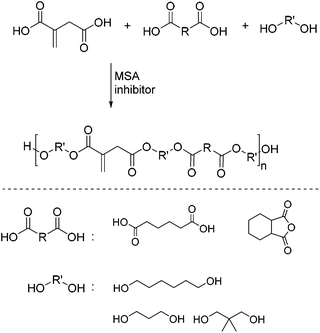
Over the past few years, our research group has placed a focus on the synthesis of bio-based polymers for coating applications.60–62 Due to its renewable nature and its high potential as an alternative crosslinking moiety, itaconic acid is an interesting building block for bio-based coatings. Therefore, within the scope of a governmentally-funded project itaconic acid-based dispersions for wood coating applications were successfully developed.63 In detail, this involved the development of a reliable method to synthesize polyester resins derived from itaconic acid and other (bio-based) building blocks, such as adipic acid (AA), hexahydrophthalic anhydride (HPSA), 1,6-hexanediol (HDO), 1,3-propandiol (PDO), and neopentylglycol (NPG, Scheme 10).
The polycondensation reactions were conducted in the presence of 0.4 wt% methanesulfonic acid (MSA) as the catalyst and a mixture of MeHQ and BHT as inhibitors. Using n-heptane as the entrainer, the reaction proceeded smoothly under Dean–Stark conditions between 120 and 180 °C within 8 hours depending on the choice of monomers. The course of the reaction was monitored by analysis of the acid value (AV). In a first set of experiments, the OHV at the end of the reaction was lower than theoretically expected. This can be explained by loss of the more volatile diols during the azeotropic distillation and etherification side-reactions. To compensate this loss up to 5% excess of diols were added at the start of the reactions. A variety of polyesters synthesized including AV and the hydroxyl value (OHV) at the completion of the reaction, Mw and viscosity are shown in Table 1. The products were obtained in molecular weights ranging from 3100 to 5600 g mol−1 with no sign of crosslinking detectable by means of SEC. Fig. 2 shows the SEC traces of the itaconic-acid based polyesters I–V.
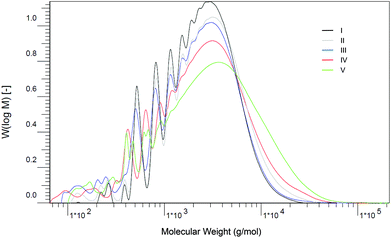 | ||
| Fig. 2 Overview of the molecular weight distribution of the polyesters I–V analyzed by means of SEC. | ||
| # | Diacids (eq.) | Diols (eq.) | AV (mg g−1) | OHV (mg g−1) | M w (g mol−1) | Đ | Viscosity (Pa s) |
|---|---|---|---|---|---|---|---|
| I | 0.60 IA | 0.8 HDO | 3 | 120 | 3200 | 1.9 | 27 |
| 0.25 AA | 0.45 NPG | ||||||
| 0.15 HPSA | |||||||
| II | 0.70 IA | 0.75 HDO | 3 | 118 | 3700 | 2.4 | 15 |
| 0.30 AA | 0.50 NPG | ||||||
| III | 0.58 IA | 0.65 HDO | 4 | 117 | 3100 | 2.8 | 8 |
| 0.42 AA | 0.45 NPG | ||||||
| 0.15 PDO | |||||||
| IV | 0.80 IA | 0.70 HDO | 4 | 101 | 4100 | 3.4 | 31 |
| 0.20 AA | 0.48 NPG | ||||||
| V | 0.95 IA | 0.20 HDO | 7 | 103 | 5600 | 4.0 | 45 |
| 0.05 AA | 1.05 PDO |
The IR spectra of the polyesters clearly show signals at ∼1670 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-stretch) and 810 cm−1 (C–H-deformation), which are derived from the α,β-unsaturated C–C-double bond of the itaconic acid. These two signals can also be used to track the progression or the degree of a subsequent radical crosslinking reaction. In addition, NMR spectra of the polyesters clearly show the typical signals of the terminal double bond of the itaconic acid moiety with signals at around 6.3 and 5.6 ppm and a small geminal coupling constant of around 3 Hz. In addition, no sign of the rearrangement of the terminal C
C-stretch) and 810 cm−1 (C–H-deformation), which are derived from the α,β-unsaturated C–C-double bond of the itaconic acid. These two signals can also be used to track the progression or the degree of a subsequent radical crosslinking reaction. In addition, NMR spectra of the polyesters clearly show the typical signals of the terminal double bond of the itaconic acid moiety with signals at around 6.3 and 5.6 ppm and a small geminal coupling constant of around 3 Hz. In addition, no sign of the rearrangement of the terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-double bond to the C4-chain to form mesaconic acid can be detected in the NMR spectra.
C-double bond to the C4-chain to form mesaconic acid can be detected in the NMR spectra.
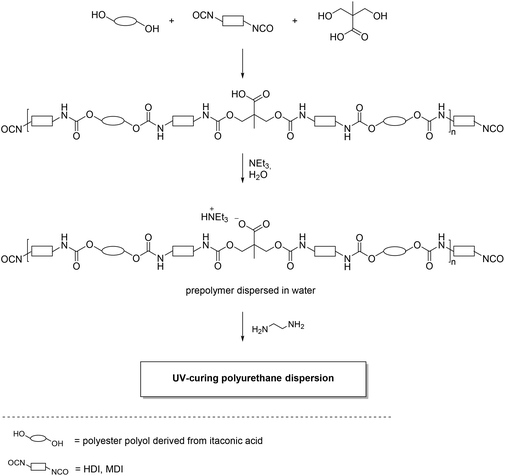
The polyesters obtained were used subsequently to synthesize water-based polyurethane dispersions for wood coating applications. This was done by reacting the polyesters with different diisocyanates, such as MDI or HDI in the presence of dimethylolpropionic acid (DMPA), which acts as an internal emulsifier after activation by a subsequent reaction with triethylamine and water (Scheme 11). With this procedure an isocyanate-terminated prepolymer dispersion was obtained, which was subjected in a last step to a chain elongation with ethylenediamine to obtain the final UV-curing polyurethane dispersion based on itaconic acid. The resulting dispersion was used as a binder component for wood coating applications with excellent properties, which proved to be compatible with commercially available products of this type.
A somewhat different example in the field of coatings was recently reported by Jain and co-workers.64 In this case a polyester with only 2 wt% itaconic acid was used as the backbone for an acrylic resin. In detail, the unsaturated double bonds of the polyester were reacted with methyl methacrylate, butyl acrylate and styrene by means of radical polymerization. These acrylic modified polyesters were mixed with 20–30% of a melamine-formaldehyde resin and subsequently applied to a steel surface and cured for 30 min at 140 °C. The resulting baking finishes exhibited very promising properties and might be an addition to existing materials in this field of coatings.
2.5 Further studies on the polycondensation of itaconic acid
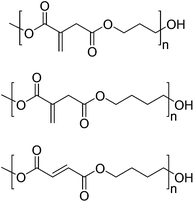
Another study on the polycondensation of itaconic acid was conducted by Retuert and co-workers in 1993.65 In this rather early example they reacted the unsaturated acid with ethylene glycol in the presence of a catalytic amount of p-toluenesulfonic acid and hydroquinone as the inhibitor at 120 °C under partial vacuum. However, they had to keep the conversion under 85% to prevent a high amount of crosslinking. To circumvent this problem, the authors synthesized this kind of polyester through the reaction of dimethyl itaconate or itaconoyl dichloride with ethylene glycol, which could be conducted at lower temperatures of 100 °C and 60 °C, respectively. But in all the cases the molecular weights were low (up to 1200 g mol−1). Only preliminary crosslinking experiments were reported, in which they were able to show that the polyesters obtained in this study readily co-polymerize with a range of vinyl polymers such as styrene, acrylates, etc.Recently Clark and co-workers studied the synthesis of itaconic acid-containing polyesters starting from the corresponding methylesters of itaconic and fumaric acid.48 In a first step three polyesters were synthesized by a reaction with 1,3-propanediol and 1,4-butanediol (Fig. 3).
By a detailed NMR study of these polyesters they were able to analyze the degree of mesomerization of the itaconic acid moiety to the corresponding mesaconic acid species and the multiplet complexity of the protons in the α-position to the ester functionality. In addition, they used NMR to elucidate the possible crosslinking mechanism of the polyester chains, which according to their results proceeds through a nucleophilic addition of a hydroxyl functionality at the α,β-unsaturated double bond of the itaconic acid, the so called Ordelt-reaction or condensation.66,67 By further NMR experiments they examined the percentage of itaconic acid incorporated in a co-polyester of succinic and itaconic acid, which proved to be as high as 96%. Further analyses, like TGA, DSC and solubility of the polyesters, were also conducted in the course of this study, however, no crosslinking experiments of these materials were undertaken.
Teramoto et al. studied the influence of the crosslink density on the solubility and biodegradability of poly(butylene succinate) prepolymers that contained different amounts of either maleic or itaconic acid units in the main chain.68 For this they incorporated 5, 10, and 15% of the unsaturated acids into the polyesters which were subsequently crosslinked using benzoyl peroxide as the thermal initiator. As expected, a higher amount of itaconic or maleic acid in the polyester leads to a higher crosslinking density, which can be analyzed by the amount of polymer insoluble in chloroform. The highest amount of 90.9% insoluble fraction was obtained when 15% itaconic acid was present in the polyester. During the crosslinking process the differences in the curing speed became apparent, with a complete conversion of the itaconic acid double bond within 20 min, whereas curing times of 40 min were needed for the polymers containing maleic acid. In addition, it was shown that the biodegradability decreased with increasing crosslink density. However, the biodegradation of these polyesters did not shut down completely, even when crosslinked with 15% of the itaconic acid present in the main chain.
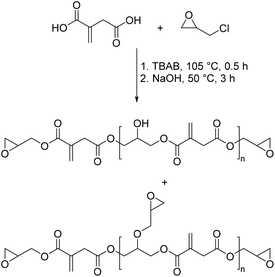
Another possibility for obtaining polyesters derived from itaconic acid was described by Ma et al.69 They reacted itaconic acid with an excess of epichlorohydrin (Scheme 12) to obtain a polyester epoxy resin as a renewable alternative to the most commonly used epoxy resin bisphenol A diglycidyl ether (BADGE). The polyesters synthesized exhibited rather low molecular weights and were cured with methyl hexahydrophthalic anhydride with different additives such as divinyl benzene or acrylated epoxidized soybean oil. The authors were able to show that the cured resins can compete with commercial ones derived from BADGE. Especially the fact that these bio-based epoxy resins have the possibility of an additional thermally induced radical curing mechanism through the α,β-unsaturated double bond of the itaconic acid is a valuable asset of these compounds. In a subsequent study the authors developed further epoxy resins derived from itaconic acid70 and also gave an overview on bio-based thermosetting resins with some examples based on itaconic acid.71
3. Enzymatic polycondensations of itaconic acid
Besides “classical” synthetic routes, polyesters derived from itaconic acid can also be synthesized through enzymatic catalysis. As described earlier Yousaf and co-workers performed some of their polycondensations via enzymatic catalysis.43 By using lipase B from Candida antarctica (CaLB) they were able to obtain linear polyesters derived from different diols with molecular weights of 200–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1. However, long reaction times (94 h) and high reaction temperatures (90 °C) were needed, which might result in a degradation of the enzymes. Under somewhat similar conditions Loos and co-workers were able to obtain co-polyesters derived from 1,4-butanediol, itaconic acid and a second saturated dicarbonic acid with very high molecular weights up to 58
900 g mol−1. However, long reaction times (94 h) and high reaction temperatures (90 °C) were needed, which might result in a degradation of the enzymes. Under somewhat similar conditions Loos and co-workers were able to obtain co-polyesters derived from 1,4-butanediol, itaconic acid and a second saturated dicarbonic acid with very high molecular weights up to 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.72 In this very extensive study they were able to show that the molecular weights of the resulting co-polyesters are very dependent on the chain-length of the other dicarbonic acid and usually decrease with increasing amounts of itaconic acid. In addition to this screening the resulting unsaturated polyesters were subjected to UV-induced crosslinking and the mechanical properties of the resulting materials were determined. Gardossi and co-workers developed a new type of immobilized enzyme catalyst by covalently bonding CaLB to an epoxy functionalized methacrylic resin.73 The authors were able to show that this catalyst can be used in the polycondensation of dimethyl itaconate with butanediol. In addition, it can indeed be recycled with only a little loss in catalytic reactivity. However, only low molecular weights of the corresponding polyesters were obtained even after 96 h reaction time. In a subsequent study, the authors analyzed the enzymatic polycondensation reaction in more detail to improve the low reactivity of the system.74 A key element for high reactivity is the optimized mass transfer and the homogeneous dispersion of the enzyme. This is hampered by the need to immobilize the enzyme to avoid contamination of the product and to ensure recyclability of the costly catalyst. However, the authors were able to show that the reactivity can also be influenced by the concentration and the structure of the diols used. Both theoretical and experimental studies show that the more rigid cyclic diol, 1,4-cyclohexanedimethanol is a more promising substrate to achieve higher molecular weight polyesters.
000 g mol−1.72 In this very extensive study they were able to show that the molecular weights of the resulting co-polyesters are very dependent on the chain-length of the other dicarbonic acid and usually decrease with increasing amounts of itaconic acid. In addition to this screening the resulting unsaturated polyesters were subjected to UV-induced crosslinking and the mechanical properties of the resulting materials were determined. Gardossi and co-workers developed a new type of immobilized enzyme catalyst by covalently bonding CaLB to an epoxy functionalized methacrylic resin.73 The authors were able to show that this catalyst can be used in the polycondensation of dimethyl itaconate with butanediol. In addition, it can indeed be recycled with only a little loss in catalytic reactivity. However, only low molecular weights of the corresponding polyesters were obtained even after 96 h reaction time. In a subsequent study, the authors analyzed the enzymatic polycondensation reaction in more detail to improve the low reactivity of the system.74 A key element for high reactivity is the optimized mass transfer and the homogeneous dispersion of the enzyme. This is hampered by the need to immobilize the enzyme to avoid contamination of the product and to ensure recyclability of the costly catalyst. However, the authors were able to show that the reactivity can also be influenced by the concentration and the structure of the diols used. Both theoretical and experimental studies show that the more rigid cyclic diol, 1,4-cyclohexanedimethanol is a more promising substrate to achieve higher molecular weight polyesters.
In a somewhat different enzymatic approach, Yamaguchi et al. used a lipase-catalyzed ring-opening addition condensation polymerization (ROACP) at 25 °C for 2 hours to obtain polyesters with molecular weights ranging from 560–3690 g mol−1 (Scheme 13).75 Besides itaconic anhydride, succinic anhydride and glutaric anhydride were used in combination with different diols, such as 1,4-butanediol, 1,6-hexanediol, 1,8-octanediol, and 1,10-decanediol. In addition, it was shown that the α,β-unsaturated C–C-bond in the polyesters is not affected by the lipase-catalyzed polymerization and can be further modified after the polycondensation reaction. As a proof of principle, a sample polyester composed of itaconic and glutaric anhydride and 1,8-octanediol was heated without any initiator to 150 °C. Gelation occurred after 3 h and was monitored by means of FT-IR spectroscopy. The crosslinking reaction was complete after 27 h when the characteristic IR-signal of the vinylidine group at ∼770 cm−1 was no longer detectable. Unfortunately, the cross-linking was only examined with one polyester and no further experiments with thermal or photo initiators were conducted to investigate the reactivity of these polyesters in this kind of radical reaction.
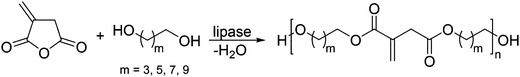 | ||
| Scheme 13 Synthesis of different polyesters by lipase-catalyzed ring-opening addition condensation polymerization (ROACP). | ||
4. Post-polymerization modification of itaconic acid-derived polyesters
Besides the major part of itaconic acid-based polyesters being used as radically crosslinking polymers as an alternative to (meth)acrylates, other fields of application have been reported. A very promising approach is the exploitation of the periodically located exo-chain double bond for post-polymerization modifications.In this context, Ramakrishnan and co-worker demonstrated that polyesters derived from itaconic acid are susceptible to Michael additions with a range of different nucleophiles.47 For this they synthesized in a first step polyesters derived from dibutylitaconate and four different diols. The polycondensation reaction was conducted under reduced pressure at 160 °C in the presence of quinol as the radical inhibitor and dibutyltin dilaurate as the catalyst. The resulting polyesters were characterized by NMR and SEC analysis and revealed very high molecular weights from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 up to 815
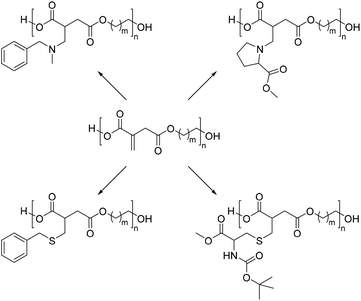
000 up to 815![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. However, the high molecular weights and the shape of the SEC chromatograms indicate a certain degree of crosslinking of the C–C-double bonds during the polycondensation reaction. In the next step, these polyesters were subjected to a series of Michael additions with several sulfur and nitrogen-based nucleophiles including protected amino acids such as proline and cysteine (Scheme 14). These reactions were nearly quantitative resulting in modified polyesters that might be interesting for a broad range of applications, including biological uses.
000 g mol−1. However, the high molecular weights and the shape of the SEC chromatograms indicate a certain degree of crosslinking of the C–C-double bonds during the polycondensation reaction. In the next step, these polyesters were subjected to a series of Michael additions with several sulfur and nitrogen-based nucleophiles including protected amino acids such as proline and cysteine (Scheme 14). These reactions were nearly quantitative resulting in modified polyesters that might be interesting for a broad range of applications, including biological uses.
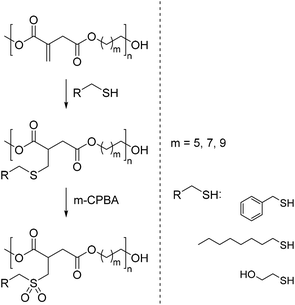
A similar approach was undertaken by Meier and co-workers.76 They reported the synthesis of three polyesters derived from dimethyl itaconate. In order to obtain polyesters with high molecular weights of around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 the use of tin ethylhexanoate (1 mol%) as the catalyst and reaction temperatures of 130 °C were necessary. In addition, 0.5 wt% of 4-methoxyphenol as the inhibitor was used to prevent crosslinking of the unsaturated double bonds. In the next step, the polyesters were subjected to an aza-Michael addition of three sulfur nucleophiles. The resulting polysulfides were subsequently oxidized to the corresponding polysulfones (Scheme 15). This reaction sequence reveals the potential of itaconic acid based polyesters to yield novel types of bio-based polymers.
000 g mol−1 the use of tin ethylhexanoate (1 mol%) as the catalyst and reaction temperatures of 130 °C were necessary. In addition, 0.5 wt% of 4-methoxyphenol as the inhibitor was used to prevent crosslinking of the unsaturated double bonds. In the next step, the polyesters were subjected to an aza-Michael addition of three sulfur nucleophiles. The resulting polysulfides were subsequently oxidized to the corresponding polysulfones (Scheme 15). This reaction sequence reveals the potential of itaconic acid based polyesters to yield novel types of bio-based polymers.
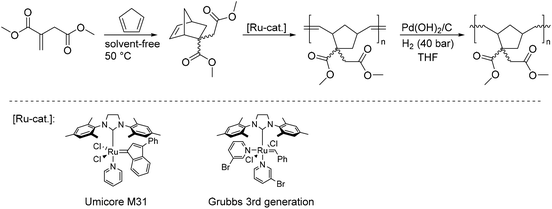
In addition to the polysulfones, renewable polynorbonenes derived from dimethyl itaconate were synthesized in the course of this study. For this, DMI was subjected to a solvent-free Diels–Alder reaction with cyclopentadiene, followed by a ring-opening metathesis polymerization (ROMP) catalyzed by Ru-catalysts (Scheme 16). The resulting polymers were obtained at room temperature within 30 min with good control over molecular weights (up to 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1) and low polydispersities. These polynorbonenes were also hydrogenated with a heterogeneous Pd-catalyst and the properties of the resulting saturated polymers were compared to the unsaturated analogs.
500 g mol−1) and low polydispersities. These polynorbonenes were also hydrogenated with a heterogeneous Pd-catalyst and the properties of the resulting saturated polymers were compared to the unsaturated analogs.
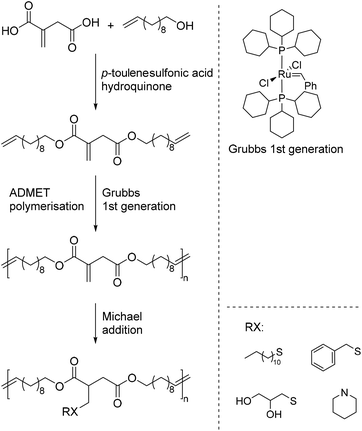
Lv et al. reported a different strategy to obtain itaconic acid-based polyesters.77 By a condensation reaction of itaconic acid with 10-undecenol a linear diene monomer was obtained. This diene was then polymerized by means of acyclic diene metathesis (ADMET) polymerization to yield an unsaturated linear polyester with a long alkyl spacer between the itaconic acid moieties. Two different polyesters were synthesized by this method with high molecular weights of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 44
000 and 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively. The polyesters were further modified by means of aza- and thio-Michael additions using different thiols and amines (Scheme 17).
000 g mol−1, respectively. The polyesters were further modified by means of aza- and thio-Michael additions using different thiols and amines (Scheme 17).
 | ||
| Scheme 17 Synthesis of itaconic acid-based polyesters through ADMET polymerization and post-polymerization modification via thio- and aza-Michael addition. | ||
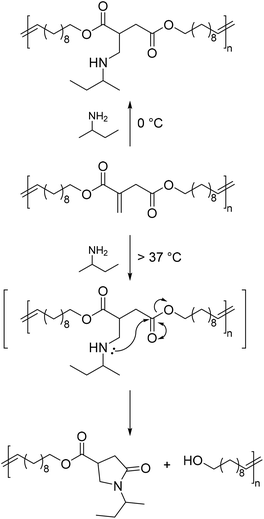
Like in the reports described earlier,47,76 the post-polymerization modifications proceeded with high yields of more than 90%. In addition, the authors were able to show that sterically demanding primary amines, such as sec-butylamine can also be added to the polyesters at low temperatures (Scheme 18). This is interesting as primary amines usually further react to form a lactam, which leads to the cleavage of the polyester.78–80 In fact, at higher temperatures of more than 37 °C, even these bulky amines undergo a lactam formation leading to a smart polyester with a temperature-controlled self-degradation. This thermally induced degradation of polyesters exhibits high potential for biomedical applications.
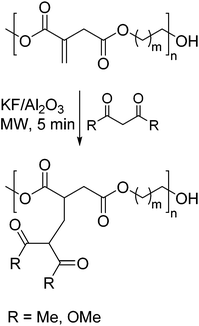
In a very recent example, Farmer et al. also exploited the α,β-unsaturated double bond to functionalize polyesters derived from itaconic acid.81 However, in this case they used the 1,3-dicarbonyl compounds acetylacetonate and dimethyl malonate as C-nucleophiles in a Michael-addition (Scheme 19). The reactions were performed in the presence of a heterogeneous catalyst under microwave radiation within 5 min without any additional solvent added. Most of the resulting modified polyesters showed an increase in Tg. In addition, they were able to show that the metal chelating abilities of the covalently bonded acetylacetonate can be retained.
 | ||
| Scheme 19 Michael-addition of acetylacetonate and dimethyl malonate to itaconic acid-based polyesters. | ||
5. Conclusion and outlook
The aim of this concise overview of polyesters derived from itaconic acid was to highlight the potential of this type of material. The interesting chemical structure of this bio-based dicarbonic acid with an α,β-unsaturated exo-double bond allows for new compositions of polyesters that are not accessible with standard building blocks derived from petrochemical sources, like methacrylic acid or acrylic acid. Admittedly, these two compounds possess a higher reactivity of the α,β-unsaturated double bond towards radical crosslinking compared to itaconic acid. However, this review tried to show that the reactivity of the latter is sufficient for a broad range of crosslinking applications, such as drug delivery, shape memory polymers, elastomers and coatings, with properties comparable or even superior to standard crosslinking compositions. In addition, the periodical distribution of the unsaturated double bond in the polyester backbone makes it a superb macromolecular scaffold for post-polymerization modifications, such as Michael-additions with different kinds of nucleophiles, Diels–Alder reactions or such like.Due to the promising crosslinking properties of itaconic acid-based polyesters, these polymers can be a renewable alternative to existing materials based on acrylic and methacrylic acid and might find more and more applications in the fields described above. But even more important, further modifications of this type of polymer can open access to a myriad of new materials with unprecedented properties and new fields of applications, making this bio-based building block a valuable renewable tool in the synthesis of innovative polymeric materials.
Acknowledgements
The authors would like to thank Dr Toine Biemans from Worlée Chemie GmbH for fruitful discussions and the Fachagentur Nachwachsende Rohstoffe e. V. and the Federal Ministry of Food and Agriculture for financial support.Notes and references
- R. Ulber, K. Muffler, N. Tippkötter, T. Hirth and D. Sell, in Renewable Raw Materials, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 1–5 Search PubMed.
- M. N. Belgacem and A. Gandini, Monomer, Polymers and Composites from Renewable Resources, Elsevier, 2008 Search PubMed.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Chem. Rev., 2016, 116, 1540–1599 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- F. M. A. Geilen, B. Engendahl, A. Harwardt, W. Marquardt, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2010, 49, 5510–5514 CrossRef CAS PubMed.
- A. M. Medway and J. Sperry, Green Chem., 2014, 16, 2084–2101 RSC.
- S. Baup, Ann. Chim. Phys., 1837, 19, 29–38 Search PubMed.
- R. G. Berg and D. S. Hetzel, Preparation of Citraconic Anhydride, US4100179A, 1978 Search PubMed.
- G. P. Chiusoli, Process for preparing itaconic acid, and 2,3-butandienoic acid, US3025320, 1962 Search PubMed.
- L. B. Lockwood and G. E. N. Nelson, Arch. Biochem., 1946, 10, 365–374 CAS.
- H. Hajian and W. M. W. Yusoff, Curr. Res. J. Biol. Sci., 2015, 7, 37–42 CAS.
- T. Klement and J. Buechs, Bioresour. Technol., 2013, 135, 422–431 CrossRef CAS PubMed.
- A. H. Mondala, J. Ind. Microbiol. Biotechnol., 2015, 42, 487–506 CrossRef CAS PubMed.
- K. S. Vuoristo, A. E. Mars, J. V. Sangra, J. Springer, G. Eggink, J. P. M. Sanders and R. A. Weusthuis, AMB Express, 2015, 5, 61–71 CrossRef PubMed.
- P. Songserm, S. Thitiprasert, V. Tolieng, J. Piluk, S. Tanasupawat, S. Assabumrungrat, S.-T. Yang, A. Karnchanatat and N. Thongchul, Appl. Biochem. Biotechnol., 2015, 177, 595–609 CrossRef CAS PubMed.
- S. Okamoto, T. Chin, K. Nagata, T. Takahashi, H. Ohara and Y. Aso, J. Biosci. Bioeng., 2015, 119, 548–553 CrossRef CAS PubMed.
- J. Becker and C. Wittmann, Angew. Chem., Int. Ed., 2015, 54, 3328–3350 CrossRef CAS PubMed.
- T. Willke and K. D. Vorlop, Appl. Microbiol. Biotechnol., 2001, 56, 289–295 CrossRef CAS PubMed.
- M. Okabe, D. Lies, S. Kanamasa and E. Park, Appl. Microbiol. Biotechnol., 2009, 84, 597–606 CrossRef CAS PubMed.
- Transparency Market Research, Market Report Itaconic Acid, 2015, 2015.
- F. Lopez-Carrasquero, A. M. de Ilarduya, M. Cardenas, M. Carrillo, M. L. Arnal, E. Laredo, C. Torres, B. Mendez and A. J. Muller, Polymer, 2003, 44, 4969–4979 CrossRef CAS.
- J. M. G. Cowie, M. Y. Pedram and R. Ferguson, Eur. Polym. J., 1985, 21, 227–232 CrossRef CAS.
- J. M. G. Cowie and N. M. A. Wadi, Br. Polym. J., 1985, 17, 27–31 CrossRef CAS.
- R. Diazcalleja, L. Gargallo and D. Radic, J. Appl. Polym. Sci., 1992, 46, 393–399 CrossRef CAS.
- A. Ribesgreus, R. Diazcalleja, L. Gargallo and D. Radic, Polymer, 1991, 32, 2755–2759 CrossRef CAS.
- J. M. G. Cowie and R. Ferguson, J. Polym. Sci., Polym. Phys. Ed., 1985, 23, 2181–2191 CrossRef CAS.
- V. Arrighi, A. Triolo, I. J. McEwen, P. Holmes, R. Triolo and H. Amenitsch, Macromolecules, 2000, 33, 4989–4991 CrossRef CAS.
- V. Arrighi, I. J. McEwen and P. F. Holmes, Macromolecules, 2004, 37, 6210–6218 CrossRef CAS.
- S. Bednarz, A. Blaszczyk, D. Blazejewska and D. Bogdal, Catal. Today, 2015, 257, 297–304 CrossRef CAS.
- K. Satoh, Polym. J., 2015, 47, 527–536 CrossRef CAS.
- S. Okada and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 822–827 CrossRef CAS.
- T. Sugama and M. Cook, Prog. Org. Coat., 2000, 38, 79–87 CrossRef CAS.
- X. Yang, G. Ma and J. Nie, J. Appl. Polym. Sci., 2012, 125, 1330–1338 CrossRef CAS.
- L. Howard, Y. Weng and D. Xie, Dent. Mater., 2014, 30, 644–653 CrossRef CAS PubMed.
- R. Wang, J. Ma, X. Zhou, Z. Wang, H. Kang, L. Zhang, K.-c. Hua and J. Kulig, Macromolecules, 2012, 45, 6830–6839 CrossRef CAS.
- T. Betancourt, J. Pardo, K. Soo and N. A. Peppas, J. Biomed. Mater. Res., Part A, 2010, 93, 175–188 Search PubMed.
- K.-S. Chen, Y.-A. Ku, H.-R. Lin, T.-R. Yan, D.-C. Sheu, T.-M. Chen and F.-H. Lin, Mater. Chem. Phys., 2005, 91, 484–489 CrossRef CAS.
- S. L. Tomić, S. I. Dimitrijević, A. D. Marinković, S. Najman and J. M. Filipović, Polym. Bull., 2009, 63, 837–851 CrossRef.
- M. Şen and O. Güven, Eur. Polym. J., 2002, 38, 751–757 CrossRef.
- C. Erbil, Y. Yildiz and N. Uyanik, Polym. Adv. Technol., 2009, 20, 926–933 CrossRef CAS.
- M. M. Babic, B. D. Bozic, B. D. Bozic, J. M. Filipovic, G. S. Uscumlic and S. L. Tomic, J. Mater. Sci., 2015, 50, 6208–6219 CrossRef CAS.
- M. Singh, R. Rathi, A. Singh, J. Heller, G. P. Talwar and J. Kopecek, Int. J. Pharm., 1991, 76, R5–R8 CrossRef CAS.
- D. G. Barrett, T. J. Merkel, J. C. Luft and M. N. Yousaf, Macromolecules, 2010, 43, 9660–9667 CrossRef CAS.
- S. E. A. Gratton, S. S. Williams, M. E. Napier, P. D. Pohlhaus, Z. Zhou, K. B. Wiles, B. W. Maynor, C. Shen, T. Olafsen, E. T. Samulski and J. M. DeSimone, Acc. Chem. Res., 2008, 41, 1685–1695 CrossRef CAS PubMed.
- D. G. Barrett, W. Luo and M. N. Yousaf, Polym. Chem., 2010, 1, 296–302 RSC.
- T. Tang, T. Moyori and A. Takasu, Macromolecules, 2013, 46, 5464–5472 CrossRef CAS.
- S. Chanda and S. Ramakrishnan, Polym. Chem., 2015, 6, 2108–2114 RSC.
- T. Farmer, R. Castle, J. Clark and D. Macquarrie, Int. J. Mol. Sci., 2015, 16, 14912 CrossRef CAS PubMed.
- T. Tang and A. Takasu, RSC Adv., 2015, 5, 819–829 RSC.
- Q. Liu and X.-M. Zhou, J. Macromol. Sci., Part A: Pure Appl. Chem., 2015, 52, 745–751 CrossRef CAS.
- B. Guo, Y. Chen, Y. Lei, L. Zhang, W. Y. Zhou, A. B. M. Rabie and J. Zhao, Biomacromolecules, 2011, 12, 1312–1321 CrossRef CAS PubMed.
- O. Goerz and H. Ritter, Polym. Int., 2013, 62, 709–712 CrossRef CAS.
- O. Goerz and H. Ritter, Beilstein J. Org. Chem., 2014, 10, 902–909 CrossRef PubMed.
- T. Wei, L. Lei, H. Kang, B. Qiao, Z. Wang, L. Zhang, P. Coates, K.-C. Hua and J. Kulig, Adv. Eng. Mater., 2012, 14, 112–118 CrossRef CAS.
- T. Sakuma, A. Kumagai, N. Teramoto and M. Shibata, J. Appl. Polym. Sci., 2008, 107, 2159–2164 CrossRef CAS.
- J. Dai, S. Ma, X. Liu, L. Han, Y. Wu, X. Dai and J. Zhu, Prog. Org. Coat., 2015, 78, 49–54 CrossRef CAS.
- B. S. Kim, H. Y. Jeong and B. K. Kim, Colloids Surf., A, 2005, 268, 60–67 CrossRef CAS.
- J. Dai, S. Ma, Y. Wu, J. Zhu and X. Liu, Prog. Org. Coat., 2015, 87, 197–203 CrossRef CAS.
- J. Dai, S. Ma, Y. Wu, L. Han, L. Zhang, J. Zhu and X. Liu, Green Chem., 2015, 17, 2383–2392 RSC.
- C. Philipp and S. Eschig, Prog. Org. Coat., 2012, 74, 705–711 CrossRef CAS.
- J. Bullermann, S. Friebel, T. Salthammer and R. Spohnholz, Prog. Org. Coat., 2013, 76, 609–615 CrossRef CAS.
- J. Bullermann, R. Spohnholz, S. Friebel and T. Salthammer, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 680–690 CrossRef CAS.
- S. Friebel and T. Biemans, Synthesis of wood coatings with binder resins derived from itaconic acid, funded by the German Federal Ministry of Food and Agriculture through the Fachagentur für nachwachsende Rohstoffe e.V., FKZ: 22020408, http://www.fnr.de, 2013.
- A. B. Shivarkar, D. V. Gaykar and R. K. Jain, Prog. Org. Coat., 2015, 89, 75–81 CrossRef CAS.
- J. Retuert, M. Yazdanipedram, F. Martinez and M. Jeria, Bull. Chem. Soc. Jpn., 1993, 66, 1707–1708 CrossRef CAS.
- J. Lehtonen, T. Salmi, K. Immonen, E. Paatero and P. Nyholm, Ind. Eng. Chem. Res., 1996, 35, 3951–3963 CrossRef CAS.
- Z. Ordelt and B. Krátký, Farbe und Lack, 1969, 6, 523–531 Search PubMed.
- N. Teramoto, M. Ozeki, I. Fujiwara and M. Shibata, J. Appl. Polym. Sci., 2005, 95, 1473–1480 CrossRef CAS.
- S. Ma, X. Liu, Y. Jiang, Z. Tang, C. Zhang and J. Zhu, Green Chem., 2013, 15, 245–254 RSC.
- S. Ma, X. Liu, L. Fan, Y. Jiang, L. Cao, Z. Tang and J. Zhu, ChemSusChem, 2014, 7, 555–562 CrossRef CAS PubMed.
- S. Ma, T. Li, X. Liu and J. Zhu, Polym. Int., 2016, 65, 164–173 CrossRef CAS.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda van Ekenstein and K. Loos, Polym. Chem., 2015, 6, 5451–5463 RSC.
- A. Pellis, L. Corici, L. Sinigoi, N. D'Amelio, D. Fattor, V. Ferrario, C. Ebert and L. Gardossi, Green Chem., 2015, 17, 1756–1766 RSC.
- L. Corici, A. Pellis, V. Ferrario, C. Ebert, S. Cantone and L. Gardossi, Adv. Synth. Catal., 2015, 357, 1763–1774 CrossRef CAS.
- S. Yamaguchi, M. Tanha, A. Hult, T. Okuda, H. Ohara and S. Kobayashi, Polym. J., 2013, 46, 2–13 CrossRef.
- M. Winkler, T. M. Lacerda, F. Mack and M. A. R. Meier, Macromolecules, 2015, 48, 1398–1403 CrossRef CAS.
- A. Lv, Z.-L. Li, F.-S. Du and Z.-C. Li, Macromolecules, 2014, 47, 7707–7716 CrossRef CAS.
- F. Felluga, G. Pitacco, M. Prodan, S. Pricl, M. Visintin and E. Valentin, Tetrahedron: Asymmetry, 2001, 12, 3241–3249 CrossRef CAS.
- Z. Wang, T. Wei, X. Xue, M. He, J. Xue, M. Song, S. Wu, H. Kang, L. Zhang and Q. Jia, Polymer, 2014, 55, 4846–4856 CrossRef CAS.
- P. L. Paytash, E. Sparrow and J. C. Gathe, J. Am. Chem. Soc., 1950, 72, 1415–1416 CrossRef CAS.
- T. J. Farmer, J. H. Clark, D. J. Macquarrie, J. K. Ogunjobi and R. L. Castle, Polym. Chem., 2016, 7, 1650–1658 RSC.
| This journal is © The Royal Society of Chemistry 2016 |