Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recovery of an antidepressant from pharmaceutical wastes using ionic liquid-based aqueous biphasic systems†
Maciej
Zawadzki
a,
Francisca A.
e Silva
b,
Urszula
Domańska
a,
João A. P.
Coutinho
 b and
Sónia P. M.
Ventura
*a
b and
Sónia P. M.
Ventura
*a
aDepartment of Physical Chemistry, Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
bCICECO - Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: spventura@ua.pt
First published on 15th March 2016
Abstract
This study is aimed at developing a sustainable process for the recovery of valuable drugs from pharmaceutical wastes using ionic liquid (IL)-based aqueous biphasic systems (ABS). Because in pharmaceutical wastes, excipients represent the major contaminants, the search for selective routes for their elimination is of primordial relevance and for that purpose IL-based ABS were evaluated. The effects of different process parameters, namely the IL nature, pH and mixture composition used in the extraction system, were studied and the process was optimized to maximize the extraction of the antidepressant from pharmaceutical wastes. Moreover, the maximum amount of amitriptyline able to be processed using such systems was assessed. The set of ABS investigated herein revealed a high extraction performance, as indicated by the outstanding logarithmic functions of the amitriptilyne partition coefficients ranging from 2.41 ± 0.05 to >2.5 and extraction efficiencies between 66% ± 1% and 100%. The best ABS and conditions were considered in the development of an integrated multi-step purification process. The process here proposed comprises three main stages as follows: the solid–liquid extraction of the antidepressant from ADT 25 pills, its purification using the optimal IL-based ABS and the antidepressant isolation by precipitation with anti-solvent. After the removal of most water insoluble excipients in the first step, with the selected IL-based ABS, it was possible to further eliminate water soluble contaminants. A high capability of extraction and purification, leading to the selective separation of amitriptyline hydrochloride from the main contaminants contained in solid pharmaceutical wastes was achieved. Finally, the isolation of the amitriptilyne in a pure state was successfully accomplished through precipitation with the anti-solvent.
Introduction
The growing production and consumption of pharmaceuticals worldwide is responsible for large levels of waste generated both in their production and final disposal and their occurrence in natural ecosystems.1 The household disposal of drugs, the expiration date and the excessive package size are the main reasons behind such waste production.2 Currently, wastes are mainly incinerated, providing the complete disintegration of the active compounds and preventing their entry in the environment.2 Beyond its inherent hazardous nature and high cost, there are valuable active pharmaceutical ingredients being completely destroyed, because circa 90% of the active ingredients is still in its active form past the expiration date.3Antidepressants are one of the most intensively prescribed pharmaceutical classes throughout the globe.4 The prescription of antidepressants was around 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 packages in Portugal in 2001 and this number is growing.5 This group of drugs has been detected in surface and treated drinking water, wastewater treatment plants and aquatic organisms’ tissues, showing a huge environmental persistency and signs of possible bioaccumulation.6 In addition, this class of pharmaceuticals is one of the hottest considering their market price. The free access data available are representative of their global market, revealing a total revenue of 8.7 billion U.S. dollars, considering the top antidepressant drugs sold in the United States between July of 2011 and June of 2012.7 The large amount of pharmaceutical waste that this creates, together with the idea behind the Horizon 2020 societal challenge, “Waste is a resource to recycle, reuse and recover raw materials”,8 drives the challenge to develop processes to use pharmaceutical wastes as a source of active ingredients, and particularly antidepressant chemicals, which can be applied either as starting materials for other chemicals or as industrial or commercial standards. Thus, the development of strategic technologies to recover and purify these antidepressant ingredients of high value from waste-based matrices is of high relevance.
000 packages in Portugal in 2001 and this number is growing.5 This group of drugs has been detected in surface and treated drinking water, wastewater treatment plants and aquatic organisms’ tissues, showing a huge environmental persistency and signs of possible bioaccumulation.6 In addition, this class of pharmaceuticals is one of the hottest considering their market price. The free access data available are representative of their global market, revealing a total revenue of 8.7 billion U.S. dollars, considering the top antidepressant drugs sold in the United States between July of 2011 and June of 2012.7 The large amount of pharmaceutical waste that this creates, together with the idea behind the Horizon 2020 societal challenge, “Waste is a resource to recycle, reuse and recover raw materials”,8 drives the challenge to develop processes to use pharmaceutical wastes as a source of active ingredients, and particularly antidepressant chemicals, which can be applied either as starting materials for other chemicals or as industrial or commercial standards. Thus, the development of strategic technologies to recover and purify these antidepressant ingredients of high value from waste-based matrices is of high relevance.
Liquid–liquid extraction (LLE) is an important separation and purification process in several industrial domains due to its benefits regarding energetic and operational costs and easy scale-up.9 As substitutes with outstanding potential for the traditional LLE processes, aqueous biphasic systems (ABS) gained favor across academia and industry.10 They can replace LLE processes constituted by volatile organic solvents, because ABS are formed by two aqueous-rich phases of incompatible and structurally different polymers,11 salts12 or a polymer and a salt,13 therefore creating milder conditions for biomolecules. In this study, ABS composed of two salts, one of those belonging to the ionic liquids (ILs) class, are applied.
ILs have emerged as promising solvents to be used in LLE processes,14 and particularly in ABS,15 due to their unique properties16 (e.g. negligible vapor pressure, high chemical and thermal stabilities and good solvation ability) and tunable characteristics17 (meaning that different combinations of cations, anions and alkyl chains allow the preparation of a specific IL with controlled properties for a desired final application). A wide variety of IL-based ABS have been reported, based on an IL and inorganic or organic salts, polymers, carbohydrates and amino-acids, or even being used as additives, e.g. adjuvants, electrolytes and co-surfactants.15,18,19 Actually, this versatility plays an important role in the creation of ABS for specific applications, allowing the manipulation of the phases’ polarities and affinities for different molecules/compounds.15,20 The wide range of results reported in literature suggests that these systems present high effectiveness and selectivity if properly designed and optimized.15
Recently, the successful use of ABS to recover paracetamol from pharmaceutical wastes was reported.21 Following the same line of study, the main objective of this work was to recover and purify an antidepressant from its solid wastes through the application of IL-based ABS for its purification, thus enlarging the spectrum of active pharmaceutical ingredients recovered by this approach. ABS composed of phosphonium- and quaternary ammonium-based ILs together with distinct phosphate-based salts and buffers were selected to ascertain the partitioning behavior of amitriptyline hydrochloride (herein used as an antidepressant model compound). Finally, the best systems were integrated in a multi-stage process for the extraction, purification and isolation (or polishing) of amitriptyline hydrochloride directly from the pharmaceutical waste.
Experimental
Materials
Amitriptyline hydrochloride (Ami, 1-propanamine,3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-hydrochloride, CAS number 549-18-8, purity ≥ 98 wt%) was purchased from Sigma-Aldrich (see Fig. 1A). The ILs used in this study belong to two distinct families, quaternary ammonium such as tetrabutylammonium bromide, [N4444]Br (purity ≥ 98 wt%) and tetrabutylammonium chloride, [N4444]Cl (purity ≥ 97 wt%) both purchased from Sigma-Aldrich and phosphonium salts, such as tetrabutylphosphonium bromide, [P4444]Br (purity = 95.2 wt%), tributylmethylphosphonium methylsulfate, [P4441][MeSO4] (purity = 98.6 wt%) and triisobutyl(methyl)phosphonium tosylate, [Pi(444)1][Tos] (purity = 99 wt%), all kindly supplied by Cytec. Their chemical structures and abbreviation names are shown in Fig. 1B. The purity of each IL was further checked through 1H and 13C NMR spectroscopy and found to match the purity levels given by the suppliers. The salts used were potassium phosphate tribasic, K3PO4 (purity ≥ 98 wt%) and potassium phosphate monobasic, KH2PO4 (purity ≥ 99.5 wt%), both from Sigma-Aldrich and potassium phosphate dibasic, K2HPO4 (purity ≥ 98 wt%), purchased from JMVP, Portugal. For the HPLC-UV-Vis mobile phase, the ammonium acetate (purity ≥ 99.99%) and acetic acid (purity ≥ 99.99%) were acquired from Sigma-Aldrich, the triethylamine (HPLC grade) was acquired from Fischer Chemical and the acetonitrile (HPLC grade) was purchased from HiPerSolv CHROMANORM. Potassium hydroxide (KOH, pure) was supplied by Pronalab. The water used was double distilled, passed through a reverse osmosis system and further treated with a Milli-Q plus 185 water purification apparatus.The pharmaceutical drug ADT 25 mg was produced in Portugal by Wynn Industrial Pharma, S. A. and obtained from a local pharmacy (Aveiro, Portugal).
Phase diagrams and tie-lines
The ternary phase diagrams for the systems [Pi(444)1][Tos] + K2HPO4/KH2PO4 + H2O, [P4441][MeSO4] + K2HPO4/KH2PO4 + H2O, [N4444]Br + K2HPO4/KH2PO4 + H2O, [P4444]Br + K2HPO4/KH2PO4 + H2O, [P4444]Cl + K2HPO4/KH2PO4 + H2O and [N4444]Cl + K3PO4 + H2O were already established in previous studies.22,23 Aiming at completing the array of IL-based ABS evaluated in this study, additional experimental ternary phase diagrams for the systems composed of [N4444]Br + K2HPO4 + H2O, [N4444]Br + K3PO4 + H2O and [N4444]Cl + K2HPO4 + H2O were determined using the cloud point titration method,24 at 298 (±1) K and atmospheric pressure. The experimental binodal curves were correlated using the Merchuk equation25 (eqn (S1) provided in ESI†).The tie-lines (TLs) were measured through a well-established gravimetric method first reported by Asenjo and collaborators25 and widely used and validated by us for IL-based ABS.21–24 A ternary mixture of IL + salt + H2O at the biphasic region was prepared, stirred vigorously and allowed to reach thermodynamic equilibrium by the separation of phases for at least 18 hours at 298 (±1) K. After separation of the coexisting phases, they were separated carefully and weighed with a precision of ±10−4 g. The TLs were determined by the application of the lever-arm rule through the relationship between the weight of the top (IL-rich) phase and that of the overall system. For the calculation of each TL, a system of four equations and four unknown variables was solved (eqn (S2)–(S5) described in more detail in the ESI†). The tie-line length (TLL) was determined as the Euclidean distance between the IL-rich (top) and salt-rich (bottom) phases compositions (eqn (S6) of the ESI†).
Quantification of amitriptyline hydrochloride
The liquid chromatograph HPLC Gilson was equipped with a UV-Vis detector 156 and a pump 321. The analytical column (100 × 4.6 mm) and precolumn were composed of a LiChrosphere 100 RP–C18 (5 μm) sorbent and were acquired from Merck. The mobile phase consisted of an aqueous phase (A) containing 21.5 mM acetic acid, 5 mM ammonium acetate buffer, 0.1 wt% pf trimethylamine (pH 4.8) and 5 wt% acetonitrile (to prevent bacteria growth), and an organic phase (B) composed of pure acetonitrile. Separation was carried out under isocratic conditions with a mobile phase ratio of 50% of phase B, using a flow rate of 1 mL·min−1. Quantification was based on the internal calibration using the respective peak areas, being two calibration curves prepared for higher and lower concentration regimes. The injection volume was 20 μL and the UV-Vis detector was set to measure at 240 nm. The validation parameters of the analytical method prepared are given in ESI Table S1.† The retention time of amitriptyline depends on the type of IL employed, being those with the bromide anion responsible for lower values (5.3 min for chloride-based vs. 3.4 min for bromide-based).Optimization study of amitriptyline hydrochloride partition
Optimization of the extraction of amitriptyline hydrochloride from aqueous solution was carried using three variables as follows: the ionic liquid, the pH (by varying the salt) and the mixture point compositions (along the same tie line). For each optimization assay, 5 g total mixtures of IL + salt + water + amitriptyline hydrochloride (≈10−3 g) were prepared by weighing the appropriate amounts of each component (within an uncertainty of 10−4 g). The mixtures were stirred vigorously and the systems were placed at 298 (±1) K for at least 18 hours to ensure complete separation of the two aqueous phases. Those were separated and collected for the measurement of weight (with an uncertainty of 10−4 g) and volume (with an uncertainty of 0.1 mL) and for amitriptyline quantification. Under these conditions, the top and bottom phases correspond to an IL-rich and a salt-rich layer, respectively. Each system was carried out in triplicate (the standard deviations are reported along with the extraction parameters determined) and at least three injections per sample were performed. Moreover, the maximum amount of antidepressant to be processed by this technology was assessed using the same procedure described above with few modifications. Mixtures composed of 10 wt% [N4444]Br + 25 wt% K3PO4 and varying amounts of amitriptyline hydrochloride, from 2.53 up to 100.6 mg (which correspond to 0.51 up to 20.9 mg per gram of ABS), were added.The extraction efficiency (EEAmi, %) was calculated using eqn (1) as follows:
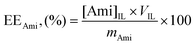 | (1) |
The logarithmic functions of the partition coefficients, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi, were calculated as the ratio between the concentration of amitriptyline hydrochloride found in the IL-rich (top), [Ami]IL, and in the salt-rich (bottom) phases, [Ami]Salt, represented by eqn (2) as follows:
KAmi, were calculated as the ratio between the concentration of amitriptyline hydrochloride found in the IL-rich (top), [Ami]IL, and in the salt-rich (bottom) phases, [Ami]Salt, represented by eqn (2) as follows:
 | (2) |
Recovery and isolation of amitriptyline hydrochloride from ADT 25 mg
The extraction of amitriptyline hydrochloride from ADT 25 mg was carried out in a multi-stage process designed specifically for that purpose. In the first step, a solid–liquid extraction with water was performed, wherein amitriptyline hydrochloride was recovered from powdered ADT 25 mg tablets (under constant stirring, for 24 hours). The liquid solution (ADT pill solution) was then centrifuged and filtered (diameter of the pore 0.45 μm), to remove any insoluble excipients from the drug. The amitriptyline hydrochloride solution previously filtered was subsequently used in the preparation of the IL-based ABS selected previously from the optimization data as the best extraction systems. The systems elected were composed of 10.0 wt% of [N4444]Br + 27.1 wt% K2HPO4/KH2PO4 at pH 6.6, 10.0 wt% of [N4444]Br + 25.1 wt% K3PO4 at pH 13.2, 10.0 wt% of [P4444]Br + 23.5 wt% K2HPO4/KH2PO4 at pH 6.6 and 10.0 wt% [N4444]Cl + 27.0 wt% K3PO4, wherein the filtered extract was added in the appropriate amount to achieve 57 mg of amitriptyline hydrochloride in the 10 g total extraction system. The last step, i.e., the polishing or isolation of amitriptyline hydrochloride from the IL-rich phase, was performed by applying two distinct approaches are as follows: (i) for the phases from the system [N4444]Br + K3PO4 and [N4444]Cl + K3PO4 both at pH 13.2, phases were diluted 6 times with pure water; and (ii) for the system [N4444]Br + K2HPO4/KH2PO4 at pH 6.6 and [P4444]Br + K2HPO4/KH2PO4 pH 6.6, the phases were diluted 6 times with an aqueous solution containing 5 wt% KOH. After proper dilution, the IL-rich phases became cloudy and after 24 hours at 277 (±1) K, a precipitate was formed. After centrifugation of each sample, the concentration of amitriptyline hydrochloride in the solution obtained was measured. The isolation efficiency (IEAmi, %) was calculated based on the concentrations of amitriptyline hydrochloride in the IL-rich (top) phase before ([Ami]IL) and after the precipitation step ([Ami]A.PIL), as shown in eqn (3). | (3) |
pH assessment
The pH of the salt solutions was monitored at 298 (±1) K using a Mettler Toledo S47 SevenMulti™ dual meter pH equipment with an uncertainty of ±0.02.Results and discussion
The main objective of this study was the development of a new process based on IL-based ABS for the recovery of amitriptyline hydrochloride from pharmaceutical residues (ADT 25 mg). In this context, this study starts with an initial optimization of ABS (carried out with a commercial standard of the antidepressant), aiming at the analysis and interpretation of the extraction results and driving forces behind the partition of the antidepressant drug. From the insights gathered in the optimization task, the best systems will be further studied in the definition of an integrated process of purification, which will contemplate the extraction of the active drug from the solid pharmaceutical wastes, its purification using the most efficient ABS in terms of the extraction efficiency and purification performance and then the isolation of the antidepressant compound from the solvents used in the ABS preparation.Partition of amitriptyline hydrochloride with ABS
The applicability of the IL-based ABS to the recovery of amitriptyline hydrochloride was investigated by the optimization of several parameters, namely, the IL structure, pH and mixture composition. An overview of the results obtained for the extraction parameters along with the conditions tested is reported in Table 1. The results indicate the complete partition of amitriptyline hydrochloride for the IL-rich phase, as confirmed by the large partition coefficients logarithmic functions obtained, generally higher than 2.5. This excellent capability to extract amitriptyline hydrochloride is corroborated by the remarkable extraction efficiency data obtained, which varied between 93% ± 3% and 100%. Its preferential distribution towards the IL-top phase, the most hydrophobic layer in these systems, is related to the lipophilic nature of this tricyclic antidepressant, in accordance with its high octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ko/w of 4.85
Ko/w of 4.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26). To facilitate the analysis of each experiment, the results are represented in Fig. 2–4, which are organized according to the effect of ILs’ structural features (Fig. 2), pH (Fig. 3) and mixture composition (Fig. 4).
26). To facilitate the analysis of each experiment, the results are represented in Fig. 2–4, which are organized according to the effect of ILs’ structural features (Fig. 2), pH (Fig. 3) and mixture composition (Fig. 4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi) of amitriptyline hydrochloride and the corresponding standard deviations (σ) for the IL-based ABS used during the optimization studies
KAmi) of amitriptyline hydrochloride and the corresponding standard deviations (σ) for the IL-based ABS used during the optimization studies
| IL | Salt | pH | [IL]M/(wt%) | [Salt]M/(wt%) | [Water]M/(wt%) | EEAmi ± σ (%) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi± σ KAmi± σ |
|---|---|---|---|---|---|---|---|
| [Pi(444)1][Tos] | K2HPO4/KH2PO4 | 6.6 | 30.03 | 15.07 | 54.90 | 95 ± 1 | >2.5 |
| [P4441][MeSO4] | K2HPO4/KH2PO4 | 6.6 | 29.86 | 14.98 | 55.16 | 98 ± 4 | 2.41 ± 0.05 |
| [N4444]Br | K2HPO4/KH2PO4 | 6.6 | 37.50 | 10.55 | 51.95 | 100 | >2.5 |
| 30.01 | 15.01 | 54.98 | 93 ± 3 | >2.5 | |||
| 20.10 | 21.05 | 58.85 | 99 ± 6 | >2.5 | |||
| 10.06 | 26.98 | 62.96 | 98 ± 5 | >2.5 | |||
| K2HPO4 | 9.6 | 30.00 | 15.06 | 54.94 | 97 ± 3 | >2.5 | |
| K3PO4 | 13.2 | 30.05 | 15.11 | 54.84 | 97.5 ± 0.6 | >2.5 | |
| [P4444]Br | K2HPO4/KH2PO4 | 6.6 | 29.80 | 14.99 | 55.21 | 96 ± 2 | >2.5 |
| 19.99 | 19.60 | 60.41 | 97 ± 3 | >2.5 | |||
| 9.99 | 23.30 | 66.71 | 94 ± 4 | >2.5 | |||
| [N4444]Cl | K2HPO4/KH2PO4 | 6.6 | 27.34 | 13.79 | 58.87 | 100 | >2.5 |
| K2HPO4 | 9.6 | 29.67 | 15.03 | 55.30 | 100 | >2.5 | |
| K3PO4 | 13.2 | 29.88 | 15.05 | 55.07 | 100 | >2.5 | |
| 20.14 | 20.91 | 58.92 | 100 | >2.5 | |||
| 10.06 | 27.09 | 62.85 | 100 | >2.5 |
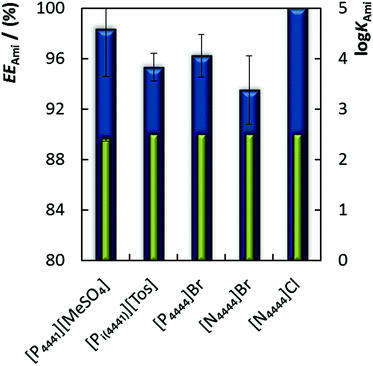
The study of the IL structure effect on the extractive performance of amitriptyline hydrochloride was carried using ABS composed of circa 30 wt% of IL + 15 wt% of K2HPO4/KH2PO4 (pH 6.6). The results presented in Fig. 2 indicate that [N4444]Cl is the best choice to efficiently extract this drug (EEAmi of 100% and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi > 2.5). When comparing the influence of the cation structure, based on two ILs sharing the Br− anion, [P4444]Br and [N4444]Br, it is possible to observe a slightly higher ability of the [P4444]+ (which is more hydrophobic than [N4444]+) to extract the antidepressant (again, the partition phenomenon seems to be controlled by the relative lipophilic/hydrophilic nature of the phases). The other phosphonium-based ILs ([P4441][MeSO4] and [Pi(444)1][Tos]) display extraction efficiencies similar to those of [P4444]Br. These results indicate that although the phosphonium-based compounds appear to be better candidates, careful optimization of the cation/anion combination is a key issue in the successful preparation of an adequate extraction system as gauged from the enhanced results obtained by applying the [N4444]Cl. Moreover, it is not only the extraction and partition parameters obtained that should be taken into account, but also the cost, environmental impact and chemical characteristics of the ILs involved in these systems. Although slightly more corrosive, the halide-based compounds ([N4444]Br, [N4444]Cl and [P4444]Br) are cheaper27 and less toxic28 (especially when compared with the [Pi(444)1][Tos]). An additional benefit of ammonium-based cations utilization compared to their phosphonium-based congeners is both their lower cost27 and toxicity.29 ILs based on ammonium cations and halide anions were thus selected.
KAmi > 2.5). When comparing the influence of the cation structure, based on two ILs sharing the Br− anion, [P4444]Br and [N4444]Br, it is possible to observe a slightly higher ability of the [P4444]+ (which is more hydrophobic than [N4444]+) to extract the antidepressant (again, the partition phenomenon seems to be controlled by the relative lipophilic/hydrophilic nature of the phases). The other phosphonium-based ILs ([P4441][MeSO4] and [Pi(444)1][Tos]) display extraction efficiencies similar to those of [P4444]Br. These results indicate that although the phosphonium-based compounds appear to be better candidates, careful optimization of the cation/anion combination is a key issue in the successful preparation of an adequate extraction system as gauged from the enhanced results obtained by applying the [N4444]Cl. Moreover, it is not only the extraction and partition parameters obtained that should be taken into account, but also the cost, environmental impact and chemical characteristics of the ILs involved in these systems. Although slightly more corrosive, the halide-based compounds ([N4444]Br, [N4444]Cl and [P4444]Br) are cheaper27 and less toxic28 (especially when compared with the [Pi(444)1][Tos]). An additional benefit of ammonium-based cations utilization compared to their phosphonium-based congeners is both their lower cost27 and toxicity.29 ILs based on ammonium cations and halide anions were thus selected.
The effect of pH on the extraction process was conducted using systems composed of [N4444]Br or [N4444]Cl, by varying the salting-out species, K3PO4 at pH 13.2, K2HPO4 at pH 9.6 and K2HPO4/KH2PO4 at pH 6.6. For this purpose, additional binodal curves for the systems composed of [N4444]Br + K2HPO4, [N4444]Br + K3PO4 and [N4444]Cl + K2HPO4 were determined to fulfill the series of ABS at distinct pH values. The data in mass fraction units of the ternary phase diagrams (Tables S2–S4), Merchuk parameters (Table S5) and information on the TLs and TLLs (Table S6) are provided in ESI.† It was verified that the main effects induced by the changes at the level of the IL's structural features and “salting-out” agents were in agreement with those well-described in the literature (decreasing order of ABS formation ability: K3PO4 > K2HPO4 and [N4444]Br > [N4444]Cl).15 The influence of pH on the charge of amitriptyline and on its subsequent extraction partition was also analyzed. Speciation of this molecule as a function of pH is presented in Fig. S1 in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 Amitriptyline has an amine group in its structure that can become protonated and change its hydrophilicity (pKa = 9.41
30 Amitriptyline has an amine group in its structure that can become protonated and change its hydrophilicity (pKa = 9.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31). The three salts investigated herein provided a totally different pH to this heterocyclic drug: pH 6.6, where amitriptyline is in its ionized form, pH 9.6 in which only around of 60% of the species in solution are in their protonated form and pH 13.2, wherein amitriptyline is its neutral form.30 It is known that these charge modifications can affect the partitioning on the ABS, as the solubility in water of amitriptyline in its non-ionized form decreases drastically compared to the charged form.32 The results related to the pH effect on the extraction efficiencies of this antidepressant are presented in Fig. 3. The pH does not affect the extraction performance of [N4444]Cl-based ABS, being constant at values of 100% and has a small effect for the systems based on the [N4444]Br, in which this value increases slightly from 93% ± 3% (at pH 6.6) to 97% ± 3% (at pH 9.6) closer to 97.5% ± 0.6% (at pH 13.2). Moreover, from the partition coefficients results, wherein their logarithmic functions were larger than 2.5, it was observed that even changing the pH, the antidepressant always migrates extensively towards the top phase. This can only be explained by a process dominated by the salting-out from the salt-rich phase induced by the phosphate salts, coupled with the change in solvation resulting from the loss of electrostatic interactions as the drug with increasing pH becomes neutral, decreasing its solubility in water and increasing its lipophilicity. Moreover, the extraction performance achieved for the [N4444]Cl-based ABS (pH 6.6) is higher than that obtained for the [N4444]Br-based ABS (pH 6.6); however, this tendency is attenuated at higher pH. This may be a direct consequence of the higher lipophilic character of [N4444]Br compared to [N4444]Cl, conjugated with the poorer water content in the [N4444]Br-rich phase. Indeed, a longer TLL was achieved for [N4444]Br than for [N4444]Cl, taking into account the results for the same mixture point composition, i.e., larger amounts of IL and lower water contents in the IL-rich phase (for more details in the TLs see Table S6 in ESI†).
31). The three salts investigated herein provided a totally different pH to this heterocyclic drug: pH 6.6, where amitriptyline is in its ionized form, pH 9.6 in which only around of 60% of the species in solution are in their protonated form and pH 13.2, wherein amitriptyline is its neutral form.30 It is known that these charge modifications can affect the partitioning on the ABS, as the solubility in water of amitriptyline in its non-ionized form decreases drastically compared to the charged form.32 The results related to the pH effect on the extraction efficiencies of this antidepressant are presented in Fig. 3. The pH does not affect the extraction performance of [N4444]Cl-based ABS, being constant at values of 100% and has a small effect for the systems based on the [N4444]Br, in which this value increases slightly from 93% ± 3% (at pH 6.6) to 97% ± 3% (at pH 9.6) closer to 97.5% ± 0.6% (at pH 13.2). Moreover, from the partition coefficients results, wherein their logarithmic functions were larger than 2.5, it was observed that even changing the pH, the antidepressant always migrates extensively towards the top phase. This can only be explained by a process dominated by the salting-out from the salt-rich phase induced by the phosphate salts, coupled with the change in solvation resulting from the loss of electrostatic interactions as the drug with increasing pH becomes neutral, decreasing its solubility in water and increasing its lipophilicity. Moreover, the extraction performance achieved for the [N4444]Cl-based ABS (pH 6.6) is higher than that obtained for the [N4444]Br-based ABS (pH 6.6); however, this tendency is attenuated at higher pH. This may be a direct consequence of the higher lipophilic character of [N4444]Br compared to [N4444]Cl, conjugated with the poorer water content in the [N4444]Br-rich phase. Indeed, a longer TLL was achieved for [N4444]Br than for [N4444]Cl, taking into account the results for the same mixture point composition, i.e., larger amounts of IL and lower water contents in the IL-rich phase (for more details in the TLs see Table S6 in ESI†).
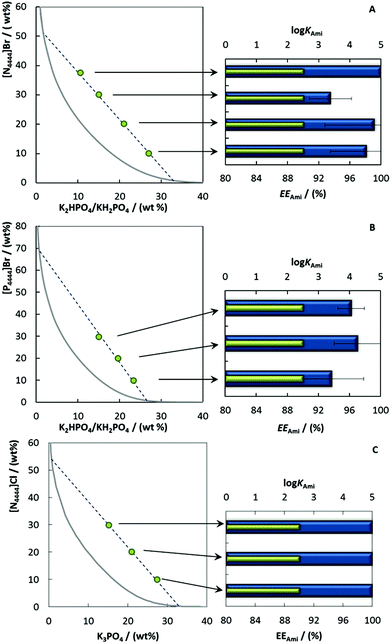
The influence of different mixture points, along the same TL, on the partitioning behavior of amitriptyline hydrochloride was also investigated. Herein, the main objective was to tune the volume ratio of the coexisting aqueous phases by reducing the IL-rich phase volume as much as possible to yield an as high as possible concentration of amitriptyline. For this purpose, mixture compositions laying on the same TL for [N4444]Br + K2HPO4/KH2PO4 + H2O, [P4444]Br + K2HPO4/KH2PO4 + H2O and [N4444]Cl + K3PO4 + H2O were prepared. The extraction parameters obtained are presented in Fig. 4 and show that both the extraction efficiencies and partition coefficients were persistently high, with no significant changes (EEAmi > 93% ± 3% for [N4444]Br + K2HPO4/KH2PO4 + H2O > 94% ± 4% for [P4444]Br + K2HPO4/KH2PO4 + H2O and ≈100% for [N4444]Cl + K3PO4 + H2O and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi > 2.5 for the entire set of systems). Systems composed of smaller top phases are better alternatives, not only from an operational point of view (improved extractive performances at the same time that facilitate further isolation strategies), but also from an economic perspective because the amounts of the IL used are minimized.
KAmi > 2.5 for the entire set of systems). Systems composed of smaller top phases are better alternatives, not only from an operational point of view (improved extractive performances at the same time that facilitate further isolation strategies), but also from an economic perspective because the amounts of the IL used are minimized.
Given the promising extraction results afforded by the use of alkaline pH environments and the lower IL-rich phase volumes, systems composed of circa 10 wt% [N4444]Br + 25 wt% K3PO4 + 65 wt% H2O + distinct concentrations of antidepressant, were chosen to evaluate the maximum capacity of the present technology. The results in Fig. 5 show that the extraction efficiency increases with the amitriptyline concentration in the ABS (the detailed conditions and data are provided in ESI Table S7†). A maximum EEAmi = 97% ± 3% is then reached at circa 50 mg of amitriptyline fed in the ABS, followed by a significant decrease (down to 66% ± 1%) – Fig. 5A. In contrast, the logarithmic function of the partition coefficients does not depend on the amitriptyline concentration, being >2.5 (strong tendency to partition towards the IL-rich phase). The increase in the amitriptyline hydrochloride concentration in the ABS leads to its accumulation in the IL-rich phase, until saturation (CAmi = 93.5 mg of amitriptyline hydrochloride per g of IL-rich phase, as shown in Fig. 5B). As the amitriptyline hydrochloride concentration is increased further in the system, the formation of a third layer between the IL-rich phase and salt-rich phase is observed, which is likely the reason for the decrease in the extraction efficiencies (due to drug precipitation/losses) and thus can be considered an indication of the maximum capacity of the current technology.
Recovery and isolation of amitriptyline hydrochloride from ADT 25 mg
After the development of the amitriptyline hydrochloride purification process by the refinement of several IL-based ABS, their application to a real pharmaceutical waste-based matrix, i.e. ADT 25 mg pills, was carried out. A multi-stage process was set up including a solid–liquid extraction step followed by the physical separation of insoluble excipients, a purification stage involving the use of the best IL-based ABS selected in the optimization step and finally the isolation of the target drug. The proposed process diagram is depicted in Fig. 6. The solid–liquid extraction was performed using water as the principal solvent, in which amitriptyline hydrochloride is highly soluble. The ground pills were added to water and kept stirring for 24 hours to extract the total amount of the antidepressant. Subsequently, the resulting extract was submitted to two physical separation methods (filtration and centrifugation) to remove any insoluble excipients present in the aqueous extract rich in the antidepressant. At the end of these steps, the expected final concentration of amitriptyline hydrochloride (circa 31 g·dm−3) was determined theoretically based on the total amount of active ingredient in each pill (information retrieved from medicine flyers) and the number of pills added to the solid–liquid extracting agent. The actual concentration attained was confirmed by HPLC-UV-Vis, which was then regularly considered during the calculations of the efficiencies in the following steps.The filtered aqueous extract obtained from the solid–liquid extraction is rich in amitriptyline and other compounds, namely, calcium hydrogenophosphate dehydrate and tartrazine, two of the excipients used in ADT 25 mg formulation with high solubility in water (information detailed by Infarmed for the medicine used in this study). Thus, the purification task was developed taking into account the most efficient IL-based ABS (considering the extraction efficiency results) according to optimization studies (10 wt% [N4444]Br + 25 wt% K3PO4 + 65 wt% H2O, 10 wt% [N4444]Br + 27 wt% K2HPO4/KH2PO4 + 63 wt% H2O, 10 wt% [P4444]Br + 23 wt% K2HPO4/KH2PO4 + 67 wt% H2O and 10 wt% [N4444]Cl + 27 wt% K3PO4 + 63 wt% H2O). These results in Table 2 show that the extraction efficiency and the logarithmic function of the partition coefficients always exceed 92% ± 1% and 2.5, respectively; the results also consistent with those assessed in the optimization step using the commercial standard.
| IL-based ABS | [IL]M/(wt%) | [Salt]M/(wt%) | [Water]M/(wt%) | EEAmi ± σ (%) | IEAmi ± σ (%) |
|---|---|---|---|---|---|
| [N4444]Br + K2HPO4/KH2PO4 | 10.07 | 27.14 | 62.79 | 98.5 ± 0.7 | 98.73 ± 0.07 |
| [N4444]Br + K3PO4 | 10.40 | 25.08 | 64.52 | 95 ± 6 | 96.9 ± 0.1 |
| [P4444]Br + K2HPO4/KH2PO4 | 10.08 | 23.47 | 66.45 | 92 ± 1 | 97 ± 1 |
| [N4444]Cl + K3PO4 | 10.09 | 26.89 | 63.01 | 100 | 95 ± 2 |
During the purification of the antidepressant from the medicine ADT 25 mg using the [N4444]Br and [N4444]Cl + K3PO4 + H2O systems, a white precipitate was formed in the interphase, which is in contrast to what was observed during the optimization studies with the pure standard (formation of two clear phases). In this context and to exclude any possibility of amitriptyline losses, the aqueous phases were separated and its concentration assessed by HPLC-UV-Vis. The extraction efficiency was 95% or 100%, respectively, meaning that this technology maintains its high performance and that the precipitate is not significantly composed by amitriptyline hydrochloride. This probably means that at this pH, some soluble excipients (simultaneously extracted during the solid–liquid extraction step) precipitate, allowing for a first step of purification considering the physical elimination of some of the contaminants from the amitriptyline rich-phase.
The final step consisted in the isolation of the target antidepressant from the top (IL-rich) phase, through the manipulation of the pH to cause an inherent decrease in the solubility of the antidepressant, when it is present in its neutral form. For that purpose, an aqueous solution of KOH was added to the IL-rich phase in the case of the K2HPO4/KH2PO4 (pH 6.6)-based systems or only water in the K3PO4-based ABS (as the inherent pH of these systems guarantees the presence of amitriptyline hydrochloride as a neutral species). This pH-driven isolation was conducted at a low temperature of 277 (±1) K, a suitable way to further decrease the solubility of the antidepressant, thus enhancing its crystallization. In general, the isolation step was developed successfully as the isolation efficiencies (IEAmi) obtained for the three systems were higher than 95% ± 2%.
At the end, the highest extraction and isolation efficiencies were observed for the ABS composed of [N4444]Br + K2HPO4/KH2PO4 and [N4444]Cl + K3PO4 and the lowest values were attained using [P4444]Br + K2HPO4/KH2PO4. The promising performance of the systems composed of [N4444]Br and [N4444]Cl + K3PO4 + H2O should be highlighted as it allows an “extra” purification step (precipitation of excipients extracted along with the target antidepressant during the solid–liquid extraction) and a simpler precipitation procedure (no need for additional species in solution, which is in contrast to the remaining systems that required the addition of KOH).
For the proposed process to be of industrial relevance, the recovery and reuse of the main phase components must be considered after the purification and polishing steps. It is proposed that the two phases are recycled by the application of H3PO4 for the neutralization of the phase (from which the amitriptyline hydrochloride was isolated) to neutralize the small amount of KOH added in the polishing step and then the phase can be re-introduced in the preparation of the ABS, as described in the process diagram of Fig. 6. The other phase, which is practically free of amitriptyline hydrochloride and rich in excipients, can be treated by ultrafiltration to remove the high molecular weight excipients and then reused directly in the preparation of ABS. It is also highlighted that the concentration of excipients/contaminants is residual at this stage; thus, the reuse of the phase components is facilitated.
Conclusions
A novel process for the extraction of amitriptyline hydrochloride able to selectively separate it from its main contaminants (i.e. ADT 25 pills’ excipients) present in the pharmaceutical wastes was successfully developed. It comprises a solid–liquid extraction step (wherein most of the water insoluble excipients were removed), purification using IL-based ABS (wherein the water soluble excipients were eliminated), and isolation of the target antidepressant (wherein the amitriptyline hydrochloride was recovered from the solvent matrix). The extraction step was optimized using various IL + phosphate salts-based ABS, wherein the IL nature, the type of phosphate salt (which induced distinct pH media) and the mixture composition were varied. During this task, it was concluded that the antidepressant partition occurs towards the top (IL-rich) phase (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAmi > 0) with very high extraction efficiencies, ranging from 66% ± 1% to 100%. The most appropriate conditions for the partition phenomenon were selected based not only on the extractive performances, but also on the predicted cost and environmental impact of the ABS formation agents. In this context, the halide-based ILs, two extreme pH environments (K2HPO4/KH2PO4 and K3PO4) and mixture compositions containing low quantities of at circa 10 wt% of IL corresponding to short volume top phases, were selected as the best solvents and conditions to be adopted in the development of the process of purification considering the use of a multi-step process comprising the extraction, purification and isolation of amitriptyline hydrochloride from the pharmaceutical residues of ADT 25. At the end, the process herein designed was shown to be efficient for both extraction (92% ± 1% < EEAMI < 100%) and isolation (95% ± 2% < IEAMI < 98.73% ± 0.07%) steps regarding the recovery of amitriptyline hydrochloride. The essential role of pH (above amitriptyline hydrochloride's pKa – 9.41) in both the purification and isolation stages is shown in this study. In the first stage, some water soluble excipients are precipitated, while in the second stage, a simple isolation procedure of amitriptyline hydrochloride from the top (IL-rich) phase is obtained. With this study, new perspectives for the recovery of valuable drugs from pharmaceutical wastes (very low cost raw materials) are being created, converting them from toxic liabilities into a source of valuable chemicals, thus minimizing the life cycle impact of these compounds.
KAmi > 0) with very high extraction efficiencies, ranging from 66% ± 1% to 100%. The most appropriate conditions for the partition phenomenon were selected based not only on the extractive performances, but also on the predicted cost and environmental impact of the ABS formation agents. In this context, the halide-based ILs, two extreme pH environments (K2HPO4/KH2PO4 and K3PO4) and mixture compositions containing low quantities of at circa 10 wt% of IL corresponding to short volume top phases, were selected as the best solvents and conditions to be adopted in the development of the process of purification considering the use of a multi-step process comprising the extraction, purification and isolation of amitriptyline hydrochloride from the pharmaceutical residues of ADT 25. At the end, the process herein designed was shown to be efficient for both extraction (92% ± 1% < EEAMI < 100%) and isolation (95% ± 2% < IEAMI < 98.73% ± 0.07%) steps regarding the recovery of amitriptyline hydrochloride. The essential role of pH (above amitriptyline hydrochloride's pKa – 9.41) in both the purification and isolation stages is shown in this study. In the first stage, some water soluble excipients are precipitated, while in the second stage, a simple isolation procedure of amitriptyline hydrochloride from the top (IL-rich) phase is obtained. With this study, new perspectives for the recovery of valuable drugs from pharmaceutical wastes (very low cost raw materials) are being created, converting them from toxic liabilities into a source of valuable chemicals, thus minimizing the life cycle impact of these compounds.
Acknowledgements
The authors acknowledge Warsaw University of Technology for the funding to this study. The authors would like to acknowledge COST for funding STSM within CM1206 action (Ref. COST-STSM-ECOST-STSM-CM1206-200114-040305). This study was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. The authors are thankful to Fundação para a Ciência e Tecnologia for the financial support on the frame of the doctoral grant SFRH/BD/94901/2013 of F. A. e Silva and the post-doctoral grant SFRH/BPD/79263/2011 of S. P. M. Ventura. The authors also acknowledge Cytec Industries Inc. for the phosphonium-based ionic liquids samples kindly supplied.References
- Pharmaceuticals in the environment - Results of an EEA workshop, European Environment Agency, http://www.eea.europa.eu/publications/pharmaceuticals-in-the-environment-result-of-an-eea-workshop, 2010.
- K. Kümmerer, J. Environ. Manage., 2009, 90, 2354–2366 CrossRef PubMed.
- J. Natarajan, S. Altan and D. Raghavarao, Drug Inf. J., 1997, 31, 589–595 Search PubMed.
- Antidepressants: global trends, http://www.theguardian.com/news/2013/nov/20/mental-health-antidepressants-global-trends, 2013.
- Evolução do consumo de antidepressivos em Portugal Continental de 1995 a 2001: Impacto das medidas reguladoras, Infarmed, http://www.infarmed.pt/portal/page/portal/INFARMED/MONITORIZACAO_DO_MERCADO/OBSERVATORIO/INTRODUCAO_DE_FICHEIROS/rel_antidepressivos.pdf, 2002.
- V. Calisto and V. I. Esteves, Chemosphere, 2009, 77, 1257–1274 CrossRef CAS PubMed.
- Top antidepressant drugs in the United States based on revenue in 2011-2012 (in million U.S. dollars), Statistica - The Statistics Portal, http://www.statista.com/statistics/242644/revenues-of-top-depression-drugs-in-the-us-2011-2012/, 2012.
- Horizon 2020 Work programme 2016-2017, European Commission, http://ec.europa.eu/research/participants/data/ref/h2020/wp/2016_2017/main/h2020-wp1617-climate_en.pdf, 2015.
- P. G. Mazzola, A. M. Lopes, F. A. Hasmann, A. F. Jozala, T. C. V. Penna, P. O. Magalhaes, C. O. Rangel-Yagui and A. Pessoa Jr., J. Chem. Technol. Biotechnol., 2008, 83, 143–157 CrossRef CAS.
- P. A. Albertsson, Partitioning of Cell Particles and Macromolecules, Wiley, New York, 3rd edn, 1986 Search PubMed.
- A. D. Diamond and J. T. Hsu, AIChE J., 1990, 36, 1017–1024 CrossRef CAS.
- N. J. Bridges, K. E. Gutowski and R. D. Rogers, Green Chem., 2007, 9, 177–183 RSC.
- G. Tubio, B. B. Nerli, G. A. Picó, A. Venâncio and J. Teixeira, Sep. Purif. Technol., 2009, 65, 3–8 CrossRef CAS.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765–1766 RSC.
- M. G. Freire, A. F. M. Cláudio, J. M. M. Araújo, J. A. P. Coutinho, I. M. Marrucho, J. N. C. Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 4966–4995 RSC.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297 CrossRef CAS.
- M. Freemantle, Chem. Eng. News Arch., 1998, 76, 32–37 CrossRef.
- J. H. P. M. Santos, F. A. e Silva, J. A. P. Coutinho, S. P. M. Ventura and A. Pessoa Jr., Proc. Biochem., 2015, 50, 661–668 CrossRef CAS.
- F. A. Vicente, L. P. Malpiedi, F. A. e Silva, A. Pessoa Jr., J. A. P. Coutinho and S. P. M. Ventura, Sep. Purif. Technol., 2014, 135, 259–267 CrossRef CAS.
- J. F. B. Pereira, L. P. N. Rebelo, R. D. Rogers, J. A. P. Coutinho and M. G. Freire, Phys. Chem. Chem. Phys., 2013, 15, 19580–19583 RSC.
- F. A. e Silva, T. Sintra, S. P. M. Ventura and J. A. P. Coutinho, Sep. Purif. Technol., 2014, 122, 315–322 CrossRef CAS.
- T. E. Sintra, R. Cruz, S. P. M. Ventura and J. A. P. Coutinho, J. Chem. Thermodyn., 2014, 77, 206–213 CrossRef CAS.
- H. Passos, A. C. A. Sousa, M. R. Pastorinho, A. J. A. Nogueira, L. P. N. Rebelo, J. A. P. Coutinho and M. G. Freire, Anal. Methods, 2012, 4, 2664–2667 RSC.
- C. M. S. S. Neves, S. P. M. Ventura, M. G. Freire, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 5194–5199 CrossRef CAS PubMed.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr., B Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS.
- F. Faassen, G. Vogel, H. Spanings and H. Vromans, Int. J. Pharm., 2003, 263, 113–122 CrossRef CAS PubMed.
- H. Passos, M. G. Freire and J. A. P. Coutinho, Green Chem., 2014, 16, 4786–4815 RSC.
- S. P. M. Ventura, C. S. Marques, A. A. Rosatella, C. A. M. Afonso, F. Gonçalves and J. A. P. Coutinho, Ecotoxicol. Environ. Saf., 2012, 76, 162–168 CrossRef CAS PubMed.
- P. J. Carvalho, S. P. M. Ventura, M. L. S. Batista, B. Schröder, F. Gonçalves, J. Esperança, F. Mutelet and J. A. P. Coutinho, J. Chem. Phys., 2014, 140, 064505 CrossRef PubMed.
- The free chemical database at, http://www.chemspider.com (accessed on 10th October 2014).
- A. S. Yazdi, N. Razavi and S. R. Yazdinejad, Talanta, 2008, 75, 1293–1299 CrossRef CAS PubMed.
- S. Hansen, S. Pedersen-Bjergaard and K. Rasmussen, Introduction to pharmaceutical chemical analysis, John Wiley & Sons, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Equations for tie-lines' determination, validation parameters for the HPLC method, weight fraction data of the phase diagrams, tie-lines and tie-line lengths, correlation parameters of the phase diagrams, weight fraction data and extraction parameters of the systems employed in assessing the maximum capacity, speciation curves of amitriptyline hydrochloride. See DOI: 10.1039/c5gc03052h |
| This journal is © The Royal Society of Chemistry 2016 |