Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical cascades in water for the synthesis of functionalized aromatics from furfurals†
Sally
Higson
,
Fabiana
Subrizi
,
Tom D.
Sheppard
* and
Helen C.
Hailes
*
Department of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK. E-mail: tom.sheppard@ucl.ac.uk; h.c.hailes@ucl.ac.uk
First published on 7th January 2016
Abstract
One-pot synthetic routes from furfurals to polysubstituted aromatic compounds have been developed in water, without the need for any organic solvents. The reaction proceeds via an uncatalysed, one-pot reaction cascade through formation of a hydrazone derivative, in situ cycloaddition with a dienophile, then aromatisation. A range of substituted phthalimides can be accessed with complete control over the substitution pattern. The reaction was also extended to other dienophiles and the diene 2-furylacrolein. The phthalimide products were further elaborated to produce a variety of polysubstituted benzenes including pharmaceutically relevant compounds.
Introduction
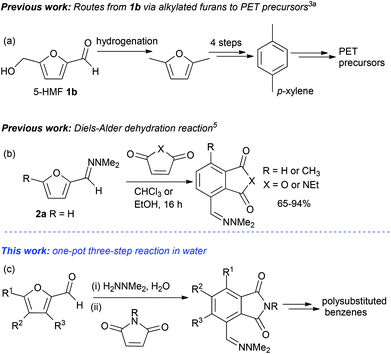
Furfural 1a and 5-(hydroxymethylfurfural) (5-HMF) 1b are renewable chemical feedstocks obtained from the hydrolysis and dehydration of cellulosic biomass, which is available from plant waste matter.1 The use of furans in Diels–Alder cycloaddition reactions has been well documented: in general good yields have been observed in reactions between electron rich furans such as 2,5-dialkylated furans or 3-alkoxyfurans and electron deficient dieneophiles.2 However, for many substrates Lewis acid catalysts, high temperatures/pressures or a large excess of the furan are required.3 Of particular recent interest is the use of biomass-derived furans such as 2,5-dimethylfuran for the preparation of p-xylene for applications in polyethylene terephthalate (PET) synthesis, and one of the first synthetic routes required a lengthy reaction sequence using multiple reagents/catalysts (Scheme 1a).3a A more recent strategy employed the direct reaction of 2,5-dimethylfuran and ethylene in the presence of Lewis acid or heterogeneous acid catalysts at high temperature and pressure to generate p-xylene.3b Since 2,5-dimethylfuran is generated by the reduction of 5-HMF 1b, a new strategy has been reported involving first the oxidation of 1b, then reaction with ethylene at high temperature to generate 4-(hydroxymethyl)benzoic acid for subsequent conversion into PET precursors.4 An alternative approach to the use of catalysts or forcing reaction conditions in furan Diels–Alder cycloadditions, is modification of the electron-withdrawing aldehyde moiety in biomass derived furans. For example, furfural dimethylhydrazone 2a, prepared from furfural 1a, was reacted with maleic anhydride or N-ethyl maleimide 3a in chloroform to give aromatic products via a Diels–Alder-dehydration cascade in 65%–94% yield (Scheme 1b).5 The approach utilising 2a and maleic anhydride was subsequently used to generate phthalimides for the treatment of cutaneous lupus, and thalidomide analogues developed for the treatment of hematological cancers.6,7We are interested in developing non-petrochemical routes to functionalized pharmaceutically relevant aromatics using renewable chemical feedstocks and environmentally benign solvents such as water,8 together with reaction cascades. It was envisaged that hydrazones such as 2a had significant potential for developing an efficient route to polysubstituted benzenes from sustainable furfural building blocks, if efficient reaction conditions could be developed which avoided the need to employ toxic organic solvents or catalysts. Furthermore, it should be noted that polysubstituted benzenes (>3 substituents) are still often extremely difficult to prepare regioselectively, despite the fact that they have numerous applications in medicinal chemistry. Herein the synthesis of polysubstituted phthalimides is described from furfurals via a one-pot reaction cascade, which does not require organic solvents for either the reaction or for product purification. We also demonstrate subsequent modifications of the phthalimide products to access a selection of polysubstituted aromatic compounds (Scheme 1c).
Results and discussion
Initial studies using furfural 1a, dimethylhydrazine 4 and N-ethylmaleimide 3a focused on establishing the synthesis of the hydrazone 2a and then the Diels–Alder-aromatisation two-step reaction in the same solvent – one with a good environmental profile for subsequent combination into a reaction cascade.9While hydrazones are traditionally prepared by heating at reflux in organic solvents under dehydrating conditions, they have also been prepared in refluxing aqueous–alcoholic solutions.10 Interestingly, the formation of hydrazone 2a was achieved in 76% yield at 50 °C in water, despite the fact that the reaction involves a dehydration; although the product required isolation via an organic extraction. Pleasingly, however, reaction of 2a with maleimide 3a in water11 (also at 50 °C, pH 6) gave phthalimide 5a in 94% yield, giving a combined 2-step yield of 71%. When performed as a one-pot sequential reaction under the same conditions (Scheme 2), 5a was formed in 95% yield and could be isolated directly as it precipitated out of the aqueous reaction mixture. Scaling the reaction up to 20 g (of 1a) gave 5a in 97% isolated yield. This suggests that the cycloaddition reaction can drive the initial hydrazone formation to completion by consuming 2a, as the two-step yield was considerably higher than that observed for the hydrazone formation alone in water. The simultaneous addition of all three reaction components (1a, 3a, and 4) gave 5a in approximately 10% lower yield due to side reactions; for this reason the reaction with other substrates was performed as a one-pot reaction by initially mixing 1 + 4, before adding 3 after allowing time for hydrazone formation to reach equilibrium. The general utility of the reaction sequence was exemplified using five maleimides (3a–3e) and 13 furfural derivatives (1a, 1b, 1f–1r) to give phthalimides 5a–5r. In most cases, the total reaction time was less than 5 h for the conversion of 1 to 5 (Scheme 2). In addition, products were isolated by filtration with no organic solvents being used, making the reactions very amenable for scale-up. A range of different maleimides could readily be utilized, including 3b (R = H) giving 5b and 5i in high yields (>85%). Phthalimides 5f–5m were obtained in good to excellent yields from furfurals 1 with alkyl or heterocyclic groups at R1, and from a dialkylated furfural (5n).
When R1 = Br (1o), the phenolic product 5o was generated due to the elimination of bromide during the aromatization step. With substituents at C-3 in the furfural (R3 = Br) or C-4 (R2 = Br, Ph), the corresponding phthalimides 5p–5r were also formed in good yield. No reaction was observed with an aryl substituent at R1.
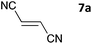
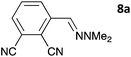
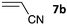
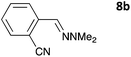
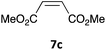
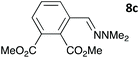
The one-pot three-step cascade was also extended to furfuryl acrolein 6 to give 5s in 64% isolated yield. In addition, a selection of non-maleimide dienophiles were examined (Table 1). Fumaronitrile 7a has previously been reacted with hydrazone 2a in refluxing benzene with SnCl4 catalyst, and 8a was formed in only 13% yield due to extensive polymerisation.5,12 With no catalyst, hydrazone 2a reacted with 7a in water to give 8a in 68% isolated yield (Table 1). Acrylonitrile 7b and dimethyl maleate 7c were also used in reactions with the hydrazone 2a or furfural 1a, and 8b/8c respectively were formed but in lower yield.
When the dimethylhydrazone 2a was reacted with methyl vinyl ketone 7d in water, a Michael-addition took place instead of a cycloaddition to give hydrazone 2t. Optimisation of the first 2 steps gave 2t in 39% yield (from 1a), and subsequent cycloaddition and aromatisation gave 5t in 58% yield that was readily isolated by filtration (Scheme 3). Hydrazone 2a has previously been reported to undergo Michael addition to 1,4-naphthoquinone in boiling benzene,5,12 however, it is notable here that conjugate addition to a less activated Michael-acceptor could be achieved in water without a catalyst.
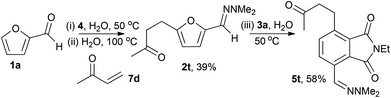 | ||
| Scheme 3 One-pot formation of hydrazone 2t in water and subsequent Diels–Alder cycloaddition and aromatisation in water. | ||
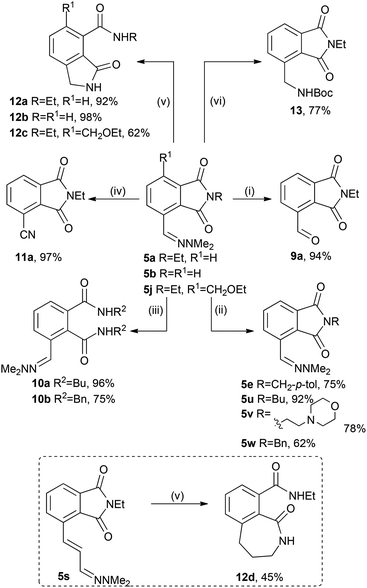
Modification of phthalimide–hydrazones 5a, 5b, 5j was investigated to demonstrate the versatility of the hydazones for the synthesis of polysubstituted benzenes. Hydrazone 5a could be hydrolysed in excellent yield to the aldehyde 9a (Scheme 4); 5b readily underwent transamidation to a range of other phthalimides (5e, 5u–5w) in 62%–92% yield using catalytic boric acid.13 Notably, this reaction could be performed using 5b isolated by filtration (but not dried) from the one-pot cascade. The phthalimide 5a could also be ring opened with excess amine to give the diamides 10a–10b in excellent yields. Oxidation of 5a to the nitrile 11 was readily achieved in 97% yield, as was hydrogenation of 5a, 5b, 5j to the amine, which was either converted to lactams 12a–c in 62–98% yield or directly isolated as the Boc-amine 13 in 77% yield. In a similar fashion hydrazone 5s was reduced to tetrahydrobenzoazepin-1one 12d in 45% isolated yield (Scheme 4).
Finally, synthesis of the poly(ADP-ribose) polymerase inhibitor and potential cancer chemotherapeutic 1414 was carried out using hydrazone 5b (Scheme 5). Hydrolysis to the aldehyde 9b was followed by imine formation with 15/B(OCH2CF3)3,15 reduction then acid mediated Boc-deprotection and lactam formation to give the target compound 14 as the hydrochloride salt in 72% yield over the four step sequence.
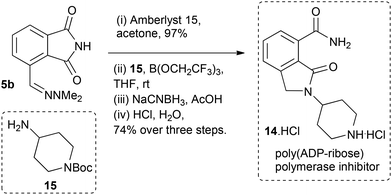 | ||
| Scheme 5 Synthesis of poly(ADP-ribose) polymerase inhibitor 14·HCl from furfural-derived phthalimide 5b. | ||
Conclusions
In conclusion, one-pot cascade reaction sequences in water which provide access to polysubstituted phthalimides have been developed, without the need for organic solvents for either the reaction or product purification. The products generated are useful precursors to a range of polysubstituted benzenes including medicinally relevant compounds.Acknowledgements
We gratefully acknowledge the Department of Chemistry at University College London for funding S. H. and the Engineering and Physical Sciences Research Council (EPSRC, EP/K014897/1) for funding F. S. as part of their Sustainable Chemical Feedstocks programme. Input and advice from the project Industrial Advisory Board is also acknowledged. We would also like to thank the EPSRC national mass spectrometry facility in Swansea for analysing some compound samples.Notes and references
- (a) G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed; (b) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 4211 CrossRef PubMed; (c) J. N. Chheda, Y. Roman-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342 RSC; (d) O. O. James, S. Maity, L. A. Usman, K. O. Ajanaku, O. O. Ajani, T. O. Siyanbola, S. Sahu and R. Chaubey, Energy Environ. Sci., 2010, 3, 1833 RSC; (e) S. A. Sanchez-Vazquez, H. C. Hailes and J. R. G. Evans, Polym. Rev., 2013, 53, 627 CrossRef CAS.
- Examples of reactions between furan or electron rich furans and electron deficient dienophiles: (a) O. Diels and K. Alder, Chem. Ber., 1929, 62, 557 Search PubMed; (b) G. H. Grogan and L. M. Rice, J. Med. Chem., 1963, 6, 802–805 CrossRef PubMed; (c) J. M. Fraile, J. I. Garcia, M. A. Gómez, A. de la Hoz, J. A. Mayoral, A. Moreno, P. Prieto, L. Salvatella and E. Vázquez, Eur. J. Org. Chem., 2001, 2891 CrossRef CAS; (d) G. Caillot, S. Hegde and E. Gras, New J. Chem., 2013, 37, 1195 RSC; (e) R. W. Foster, M. J. Porter, K. Bucar, L. Benhamou, H. C. Hailes, C. J. Tame and T. D. Sheppard, Chem. – Eur. J., 2015, 21, 6107 CrossRef CAS PubMed.
- For example: (a) M. Shiramizu and F. D. Toste, Chem. – Eur. J., 2011, 17, 12452 CrossRef CAS PubMed; (b) C. L. Williams, C.-C. Chang, P. Do, N. Nikbin, S. Caratzoulas, D. G. Vlachos, R. F. Lobo, W. Fan and P. J. Dauenhauer, ACS Catal., 2012, 2, 935 CrossRef CAS; (c) Y.-T. Cheng and G. W. Huber, Green Chem., 2012, 14, 3114 RSC; (d) C.-C. Chang, S. K. Green, C. L. Williams, P. J. Dauenhauer and W. Fan, Green Chem., 2014, 16, 585 RSC.
- J. P. Pacheco and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 8363 CrossRef CAS PubMed.
- K. T. Potts and E. B. Walsh, J. Org. Chem., 1984, 49, 4099 CrossRef CAS.
- G. W. Muller, M. Saindane, C. Ge, M. A. Kothare, L. M. Cameron and M. E. Rogers, WO2007/136640 A2, 2007.
- V. Jacques, A. W. Czarnik, T. M. Judge, L. H. T. Van der Ploeg and S. H. DeWitt, Proc. Natl. Acad. Sci. U. S. A., 2015, E1471–E1479 CAS.
- (a) C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC; (b) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114 CrossRef CAS; (c) M. B. Gawande, V. D. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC.
- R. K. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854 RSC.
- D. Todd, J. Am. Chem. Soc., 1949, 71, 1353 CrossRef CAS.
- M. V. Gill, V. Luque-Agudo, E. Román and J. A. Serrano, Synlett, 2014, 2179 Search PubMed.
- K. T. Potts and E. B. Walsh, J. Org. Chem., 1988, 53, 1199 CrossRef.
- T. B. Nguyen, J. Sorres, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 3202 CrossRef CAS PubMed.
- V. B. Gandhi, Y. Luo, X. Liu, Y. Shi, V. Klinghofer, E. F. Johnson, C. Park, V. L. Giranda, T. D. Penning and G. D. Zhu, Bioorg. Med. Chem. Lett., 2010, 20, 1023 CrossRef CAS PubMed.
- (a) R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512 CrossRef CAS PubMed; (b) J. T. Reeves, M. D. Visco, M. A. Marsini, N. Grinberg, C. A. Busacca, A. E. Mattson and C. H. Senenayake, Org. Lett., 2015, 17, 2442 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H NMR and 13C NMR spectra, and compound characterisation data. See DOI: 10.1039/c5gc02935j |
| This journal is © The Royal Society of Chemistry 2016 |

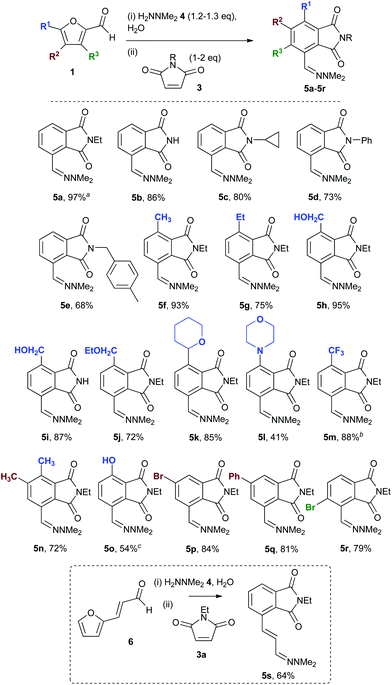
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 g scale;
20 g scale;