Open Access Article
Open Access ArticleIonic liquids as solvents for PPTA oligomers†
Sven
Dewilde
,
Wim
Dehaen
and
Koen
Binnemans
*
KU Leuven, Department of Chemistry, Celestijnenlaan 200F, bus 2404, B-3001 Leuven, Belgium. E-mail: koen.binnemans@chem.kuleuven.be; Fax: +32 16 32 79 92
First published on 2nd November 2015
Abstract
Poly-p-phenyleneterephthalamide (PPTA) is an aramid polymer with high tensile strength which is currently industrially synthesized in a solvent mixture of N-methylpyrrolidone (NMP) and CaCl2. Due to the toxicity of NMP and the need for a salt to increase the solubility, ionic liquids are suggested as suitable, alternative solvents. A whole series of ionic liquids (ILs) were investigated for their solubilization strength towards PPTA. For this study, small PPTA oligomers were synthesized and used as model compounds in solubility tests with ionic liquids. This study gave insights in the types of cations and anions required for optimal dissolving behavior. Ionic liquids with coordinating anions are a requirement to solubilize PPTA by disrupting the intermolecular hydrogen bond network, just as is the case for cellulose dissolution. Infrared and NMR-spectroscopic studies revealed the interaction of the anions with the hydrogen atoms of the secondary amides of the aramid chains. However, there is no one-to-one relationship between ionic liquids suitable for PPTA and cellulose dissolution. Cations with hydrogen atoms capable of hydrogen bond formation, like imidazolium cations, are poor solvents for PPTA. These cations hamper the anions in using their full potential for coordination with the oligomers. Ammonium and phosphonium ionic liquids which contain only sp3-bonded hydrogen atoms on the cation, do not show a tendency to form hydrogen bonds and dissolve PPTA oligomers much better than their imidazolium analogues. This hypothesis was further confirmed by the fact that substitution of hydrogen atoms by methyl groups on imidazolium and pyridinium cations improves the solvent power of the ionic liquid significantly. This screening test has identified several types of ionic liquids that are able to dissolve larger amounts of the PPTA oligomers on a molar basis than the currently used industrial solvent NMP/CaCl2.
Introduction
Poly-p-phenyleneterephthalamide (PPTA) (commonly known under the brand names Kevlar® and Twaron®) is one of the most known and used aramids (aromatic polyamides) worldwide. PPTA fibers are used to strengthen materials owing to their high tensile strength-to-weight ratio and high thermal stability.1,2 These fibers are five times stronger than steel on an equal weight basis. The strength and high thermal stability of this para-aramid can be attributed to the intermolecular hydrogen bonds between the carbonyl groups and the NH-centers of the adjacent chain. Additional strength of great influence is derived from aromatic stacking interactions between different chains.3–5While these strong interactions between the polymer chains are beneficial for their applications, it makes PPTA very difficult to process. Preparing the polymer via melt polymerization is impossible since the polymer does not melt and only decomposes at temperatures above 500 °C.6 Therefore, a solution polymerization is needed during which the growing polymer chain has to be kept in a dissolved state as long as possible during the reaction. Initially, when the industrial production of PPTA started in the 1970s, a mixture of hexamethylphosphoramide (HMPA) and N-methylpyrrolidone (NMP) was used as polymerization medium, where terephthaloyl chloride (TDC) and p-phenylenediamine (PPD) reacted with each other, possibly in the presence of a tertiary amine.7 HMPA is a highly polar, aprotic solvent but it is a carcinogenic compound and its use in industrial applications has been discontinued. Nowadays, a mixture of N-methylpyrrolidone (NMP) and CaCl2 is used as an alternative solvent for the synthesis of PPTA.8,9 The strength of this solvent mixture relies on the interaction of the amide solvent with the salt. When dissolved, the Ca2+ ions interact with the amide bond of the solvent while the chloride ions show an interaction of intermediate strength with this complex.10 Therefore, when labile protons are present such as the protons of the secondary amide bonds of PPTA, the chloride anions form hydrogen bonds with them.11,12 This disrupts the aggregation of the growing polymer chain due to charge effects and interaction of the amide bonds with the binary solvent mixture. This results in the polymerization reaction continuing in a quasi-gel state.13 The NMP/CaCl2 solvent mixture does not act as a true solvent for PPTA, as it is not able to molecularly dissolve PPTA. However, the solvent–salt–PPTA-interactions enable satisfactory molecular masses suitable for spinning fibers for commercial use.
Although the binary mixture of NMP and CaCl2 is an useful replacement for HMPA, there are still some issues with this solvent. First of all, the use of two components as a solvent requires an extra recycling step since the washing water, NMP and CaCl2 all need to be recovered for reuse.14 Secondly, NMP also has toxicity issues. It is known to be teratogenic and has been placed on a list of substances of very high concern (SVHC-list) by the European Commission (EC).15 Being on the SVHC list means that the use of a substance within the EU will be subject to authorization under the REACH regulation. This implies that companies will have legal obligations concerning that compound and its use may be restricted.
Ionic liquids (ILs) could serve as yet another alternative solvent for the synthesis of PPTA since much of the dissolution power of the NMP/CaCl2 solvent mixture comes from the interaction of chloride anions with the intermolecular hydrogen bonds of the polymer chains. Ionic liquids are promising solvents with low melting points that consist entirely of ions.16,17 Typically, an ionic liquid is built up by large and/or asymmetric organic cations with an organic or inorganic anion. Since ionic liquids are known to have a very low vapor pressure, conventional molecular solvents could be replaced by ionic liquids to reduce the release of volatile organic compounds and greatly improve recyclability.18 Furthermore, ionic liquids have a low flammability, a high thermal stability and a wide liquidus range19,20 Ionic liquids have been used for the processing of polymers21–24 but so far, very little research has been done on the dissolution and synthesis of PPTA in ionic liquids. A few research groups have investigated the direct synthesis of new aramids in ionic liquids, but the synthesis of PPTA has not been reported yet.25–27 To the best of our knowledge, only Vygodskii et al. used ionic liquids to synthesize PPTA via a low-temperature polycondensation reaction.28 They used the ionic liquids 1-butyl-3-butylimidazolium bromide and tetrafluoroborate as solvents, but they obtained only low molecular weight oligomers.
A large number of ionic liquids are already known since a wide variety of different cations and anions exists. It is also possible to build in new functionalities in the ionic liquid by using easy synthetic steps.29 Therefore, ionic liquids are often called designer solvents since an ionic liquid can be selected or synthesized depending on the requirements of the process. To have a good starting point for the selection of suitable ionic liquids as polymerization medium for PPTA, inspiration was found in studies on the dissolution of cellulose in ionic liquids.30–37 Cellulose is a biopolymer that, just as PPTA, is difficult to dissolve in conventional organic solvents due to its extended hydrogen bond network built up by the large number of hydroxyl functional groups. It is now known that coordinating anions such as chlorides, carboxylates and phosphates are needed to break up the hydrogen bonds between the different chains to bring cellulose in dissolution.38 However, the choice of cation is important as well. Imidazolium and pyridinium ionic liquids dissolve cellulose better than other types of ionic liquids with the same anion. Acidic protons on heterocyclic rings of the cation are essential in the dissolution process of cellulose.40,41
In this paper, we describe the use of ionic liquids as solvents for PPTA oligomers. An extended screening test was done to discover which ionic liquids have the best solvent properties for PPTA oligomers. Both commercially available and newly synthesized ionic liquids were tested to get more insight in which cations and anions are needed to achieve the highest dissolution of PPTA oligomers. Since the current industrial solvent, NMP with 10.5 wt% CaCl2, does not act as a true solvent for high molecular weight PPTA, it is expected that this will also not be the case for ionic liquids. Therefore, low molecular weight oligomers were synthesized to use them as model compounds. After the initial screening tests, NMP/10.5 wt% CaCl2 and four selected ionic liquids were used as solvents for a more detailed investigation using all the available oligomers with the aim to get a better understanding of the solvent behavior of structurally different ionic liquids towards PPTA. To support the hypothesis that PPTA oligomers are soluble due to the breaking of hydrogen bonds by the coordinating anions of the ionic liquids, an infrared and NMR study was carried out on some solutions of an oligomer in an ionic liquid.
Experimental
Detailed synthetic procedures and analysis data of all the oligomers and ionic liquids are given in the ESI.†Chemicals and materials
All the commercial ionic liquids tested were purchased from IoLiTec (Heilbronn, Germany), Cytec (New Jersey, USA) or Sigma-Aldrich (Diegem, Belgium). For the synthesis of the functionalized ionic liquids, chloroacetyl chloride (99%), 1,6-dichlorohexane (98%), 2-ethylpyridine (98%), 1-methylimidazole (99%), tributylamine (99%), nicotinic acid (99.5%) were purchased from Acros Organics (Geel, Belgium). Tributylphosphine (97%), propylamine (≥99%), diethylamine (≥99.5% were purchased from Sigma-Aldrich (Diegem, Belgium). 1-ethyl-3-methylimidazolium dimethylcarbonate (30% in methanol) was purchased from IoLiTec (Heilbronn, Germany) and benzyl chloride (99%+) from Merck (Overijse, Belgium). For the synthesis of the oligomers, terephthaloyl chloride (99%+), benzoyl chloride (99%), aniline (99%), thionyl chloride (99.7%), pyridine extra dry (99.5%) and N-methylpyrrolidone extra dry (99.5%) were purchased from Acros Organics (Geel, Belgium). Mono-methylterephthalate (97%), p-phenylenediamine (≥99%), 4-aminobenzanilide (95%), triethylamine (99%) were purchased from Sigma-Aldrich (Diegem, Belgium). Triphenyl phosphite (>97%) was purchased from TCI Chemicals (Zwijndrecht, Belgium). All the ionic liquids and compounds were used without any further purification.Instrumentation and analysis methods
The 1H (300 MHz) and 13C NMR spectra (75 MHz) have been recorded on a Bruker Avance 300 MHz NMR spectrometer. 31P NMR spectra (242.92 MHz) with H3PO4 as an external reference, 13C NMR spectra (100 MHz) with DMSO-d6 as external reference for the dissolution studies were recorded with a Bruker Avance 400 MHz spectrometer at 70 °C. 15N NMR spectra (40.5 MHz) with were recorded with DMSO-d6 as external reference with a Bruker Avance 600 MHz spectrometer at 70 °C over a whole weekend. As deuterated solvents D2O, DMSO-d6 and D2SO4 96% in D2O were used. Analysis of the NMR spectra was done with the Bruker Topspin 2.1 software package. CHN analysis was performed on a CE Instruments EA-1110 element analyzer. Ionic liquids are hygroscopic; they are able to absorb quite some amount of water during sample preparation. Therefore, the presence of water has also being taken into account for the calculation of the composition. The higher oligomer compounds did not fully decompose in volatile degradation products, so it was not possible to determine their purity via CHN analysis. Mass spectra for the oligomers up to four aromatic rings were recorded with ESI-MS performed on a Thermo Electron LCQ Advantage ion trap mass spectrometer connected to an Agilent 1100 HPLC system, on its turn coupled to Xcalibur data system. Fourier transform infrared spectra were recorded on a Bruker Vertex 70 spectrometer with the attenuated total reflectance module (Platinum ATR) for direct sample examination and analyzed with OPUS software. Melting points were determined on a Mettler-Toledo 822 DSC instrument at a heating rate of 10 °C min−1 in a helium flow.Dissolution of oligomers in ionic liquids
All compounds were thoroughly dried prior to each experiment to minimize the water content of all the components as much as possible. Even the presence of low amounts of water can already disturb the solubility of the aramids. The oligomers (Fig. 1 and 2) were dried overnight in a vacuum oven at 60 °C and the ionic liquids were dried overnight on a Schlenk line at 80 °C or higher if a particular ionic liquid has a higher melting point. The scale of the experiment was typically 3 to 4 g of ionic liquid. To this ionic liquid was then added oligomer in increments of 1 wt% (or 0.5 wt%). Stirring and heating was performed on a Schlenk line since the volatility of both the ionic liquid and oligomers are negligible at these conditions. Solubility was checked visually. As long as the compound is soluble in the ionic liquid, the mixture stays completely transparent. When the mixture showed the first signs of turbidity, it was judged as being saturated. Since the solubility rises with increasing temperature, it was tried each time to determine the maximum solubility at 100 °C whenever possible. The maximum solubility values are simply calculated from the amount of oligomer added divided by the amount of ionic liquid used. The values are reported in both weight percentage (wt%) and mole percentage (mol%) since there exists a wide variety in molecular mass between different ionic liquids, which could skew the results.For the benchmark tests with NMP/10 wt% CaCl2, calcium chloride was dried in vacuo at 160 °C and dissolved in N-methylpyrrolidone dried on molecular sieve. These dissolution tests were performed in an argon atmosphere since NMP can evaporate after prolonged times in vacuum conditions, even at moderate temperatures.
Infrared spectroscopy study of trimer dissolved in room-temperature ionic liquids
According to the procedure of dissolution of oligomers in ionic liquids, 20 wt% of the trimer was dissolved in 1-ethyl-3-methylimidazolium acetate. The flask was transferred and kept under vacuum as long as possible to avoid the absorption of moisture from the atmosphere. A droplet was taken and an infrared spectrum was recorded. The same droplet was kept in contact with the atmosphere for a couple of minutes which allows for the uptake of water due to the hygroscopic nature of the ionic liquid. The addition of water caused the oligomer to precipitate and a new spectrum was recorded for a comparative study.NMR spectroscopy study of trimer in room-temperature ionic liquids
According to the procedure of dissolution of oligomers in ionic liquids, a 20 wt% and 40 wt% concentration of the trimer model compound in 1-ethyl-3-methylimidazolium acetate was made. Then pure 1-ethyl-3-methylimidazolium acetate and both solutions were transferred to a NMR tube and an internal standard of DMSO-d6 was added. 13C and 15N spectra were recorded and the changes in chemical shift of the compounds were analyzed.Results and discussion
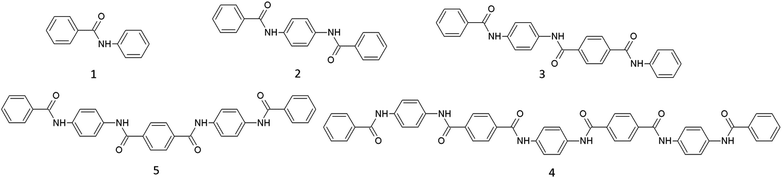
In order to make valuable comparisons between solubility data in different ionic liquids and those in NMP/10.5 wt% CaCl2, a set of short oligomers was synthesized. By using several accessible synthetic steps, oligomers were synthesized containing up to seven aromatic rings (Fig. 1). The names of these structures are simplified for clarity's sake. The oligomer with two aromatic rings will be referred as the dimer, three aromatic rings as the trimer, etc.For the initial screening tests, only the pentamer was used as a model compound since this compound is easily accessible via a one-step synthesis and because it already efficiently mimicks PPTA in solubility behavior. For example, the PPTA pentamer is barely soluble in polar, aprotic solvents such as N-methylpyrrolidone and dimethylsulfoxide. After the initial screening tests, a thorough study was done using all the synthesized oligomers with four selected commercially available ionic liquids and the industrial solvent, NMP/10.5 wt% CaCl2.
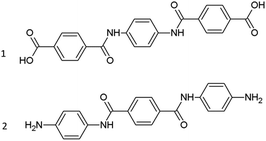
It has to be noticed that during the polycondensation reaction between p-phenylenediamine and terephthaloyl chloride intermediates are formed that will always contain functional groups on both ends of each structure. These structures are different than the non-end-functionalized oligomers discussed before and are expected to show a different dissolution behavior in ionic liquids. Therefore, a short dissolution study was performed with two different functionalized oligomers (Fig. 2) in the same set of selected ionic liquids.
Initial screening test
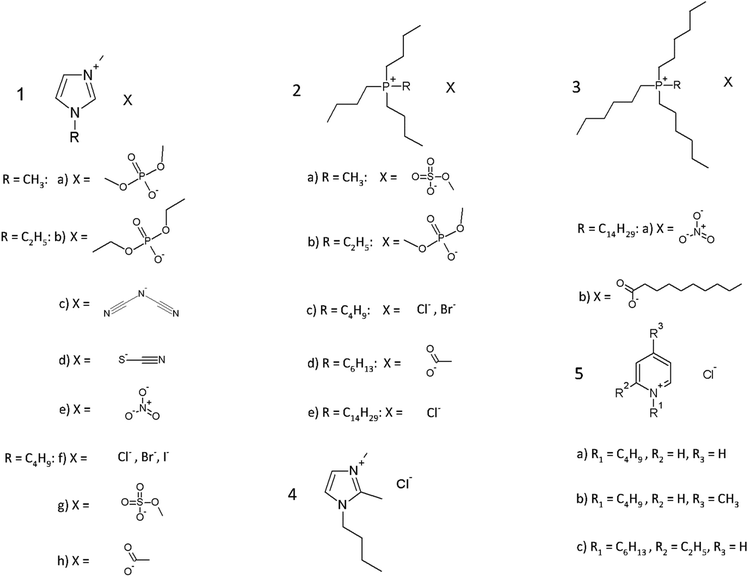
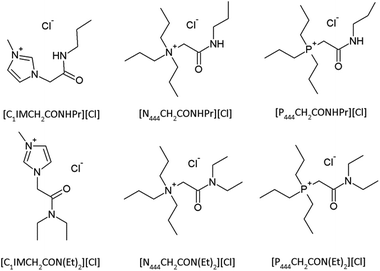
Fig. 3 shows all the commercially available ionic liquids that were used in the initial screening test. These ionic liquids were selected in such a manner that a wide variety of cations and anions were used and also tested in different combinations. This way, a better understanding can be found in which type of cations and anions are needed to become an ionic liquid that is strong in dissolving PPTA.To further investigate which properties an ionic liquid must possess in order to efficiently dissolve PPTA, some new functionalized ionic liquids were synthesized. First of all, in the search to replace NMP/CaCl2 with a suitable ionic liquid, inspiration was taken from the current industrial solvent and a new series of amide functionalized ionic liquids was synthesized with a chloride as anion (Fig. 4). As cation precursors, 1-methylimidazole, tributylphosphine and tributylamine were used. To these precursors a secondary and a tertiary amide functionality was attached, respectively. In the first case, it was tried to mimic the hydrogen-bonding interactions between the amide groups of PPTA. This was done in order to investigate if there is a preferential hydrogen bond interaction between solvent and solute over the solvent–solvent and solute–solute interactions. This, together with the hydrogen bond breaking character of the chloride anion, would give a driving force to keep the PPTA chains in dissolution. In the second case, a tertiary amide chloride ionic liquid was prepared, inspired by the solvent mixture NMP/CaCl2. In this way, it was tried to have similar solvent properties as the current industrial solvent while at the same time making use of the advantages ionic liquids possess.
Secondly, some new ionic liquids were synthesized which contain an aromatic moiety. Since part of the strength of PPTA is derived from aromatic stacking interactions, solubility could be increased by having such interactions with ionic liquids that contain an aromatic ring. For example, dendrimers end-capped with aromatic functionalities have proven to increase solubility of carbon nanotubes by aromatic stacking interactions.41 Two ionic liquids with an aromatic ring were tested to check if these functionalities are indeed an improvement: 1-ethyl-3-methylimidazolium nicotinate and 2-ethyl-1-benzylpyridinium chloride (Fig. 5). The former ionic liquid contains both a heteroaromatic ring on the anion and the cation, while the latter one contains both a heteroaromatic and aromatic ring on the cation.
 | ||
| Fig. 5 Ionic liquids containing an aromatic ring. Left: 1-Ethyl-3-methylimidazolium nicotinate [C2MIM][Nicot]. Right: 2-Ethyl-1-benzylpyridinium chloride [BenzylC2Pyr][Cl]. | ||
Finally, in the literature it has been reported that dicationic ionic liquids can perform better than their monocationic analogues for certain applications.42 Therefore, a dicationic phosphonium ionic liquid was synthesized to compare its solubility performance against a monocationic phosphonium ionic liquid (Fig. 6).
 | ||
| Fig. 6 1,6-Hexanediylbis(tributyl)phosphonium dichloride ([Cl][P444-C6H12-P444][Cl]), a dicationic phosphonium ionic liquid. | ||
Table 1 shows the solubility values of the pentamer model compound in all the commercially available and newly synthesized ionic liquids. Also, solubility was checked in N-methylpyrrolidone with 10 wt% CaCl2 which makes it possible to check how ionic liquids perform relative to the current industrial solvent and to point out candidates for possible replacements in the future.
| Entry | Ionic liquid | Solubility (wt%) | Solubility (mol%) |
|---|---|---|---|
| a Benchmark tests were performed on NMP/10 wt% CaCl2. The maximum solubility of the model compound in this solvent was 4 wt% (0.8 mol%). b Entry 3 is a dicationic ionic liquid, its solubility is calculated as amount oligomer soluble per anion. c n.s: not soluble at any temperature investigated. | |||
| 1 | [P4442][Et2PO4] | 10 | 7.6 |
| 2 | [P4446][CH3COO] | 12 | 6.9 |
| 3 | [Cl][P444-C6H12-P444][Cl] | 9 | 4.5b |
| 4 | [P4444][Cl] | 6 | 3.2 |
| 5 | [P444CH2CON(Et)2][Cl] | 5 | 3.2 |
| 6 | [P44414][Cl] | 4 | 3.1 |
| 7 | [N444CH2CON(Et)2][Cl] | 5 | 3.0 |
| 8 | [N8881][Cl] | 4 | 2.9 |
| 9 | [BenzylC2Pyr][Cl] | 5 | 2.1 |
| 10 | [C2C6Pyr][Cl] | 5 | 2.0 |
| 11 | [C4MIM][CH3COO] | 5 | 1.6 |
| 12 | [C4C1pyr][Cl] | 3 | 1.0 |
| 13 | [C2MIM][Nicot] | 3 | 1.3 |
| 14 | [C2MIM][Et2PO4] | 2 | 1.0 |
| 15 | [C4C1MIM][Cl] | 2 | 0.7 |
| 16 | [C1MIM][Me2PO4] | 1 | 0.4 |
| 17 | [C4MIM][Cl] | 0.5 | 0.16 |
| 18 | [C4MIM][MeSO4] | n.sc | n.s |
| 19 | [C4MIM][Br] | n.s | n.s |
| 20 | [C4MIM][I] | n.s | n.s |
| 21 | [C2MIM][S(CN)2] | n.s | n.s |
| 22 | [C2MIM][N(CN)2] | n.s | n.s |
| 23 | [C2MIM][NO3] | n.s | n.s |
| 24 | [C4Pyr][Cl] | n.s | n.s |
| 25 | [P4441][MeSO4] | n.s | n.s |
| 26 | [P4444][Br] | n.s | n.s |
| 27 | [P66614][NO3] | n.s | n.s |
| 28 | [P66614][C9H19COO] | n.s | n.s |
| 29 | [C1IMCH2CONHPr][Cl] | n.s | n.s |
| 30 | [C1IMCH2CON(Et)2][Cl] | n.s | n.s |
| 31 | [P444CH2CONHPr][Cl] | n.s | n.s |
| 32 | [N444CH2CONHPr][Cl] | n.s | n.s |
Since solubility of a solute increases with increasing temperature, each time it was tried to determine the maximum solubility at 100 °C except for ionic liquids that melt above 100 °C. For the latter, the maximum solubility was determined just above the melting temperature.
Also, the water content was continuously kept as low as possible by constantly working under high vacuum conditions since the presence of water can lower the maximum solubility significantly. The data are both reported in weight percentage (wt%) and mole percentage (mol%). While weight percentage is interesting to know how much mass of a solvent is needed to dissolve a certain amount of the oligomer, the mole percentage gives a better view on the inherent solubility power of the solvent i.e. how many particles of PPTA oligomer are able to be dissolved in 100 molecules of solvent.
Eleven structurally different ionic liquids were found capable of dissolving the pentamer in equal or higher amounts than the current industrial solvent on weight basis (4 wt%) with tributylhexylphosphonium acetate (entry 2) being able to dissolve the highest amount, a satisfactory three times more than NMP/10 wt% CaCl2. However, tributylethylphosphonium diethylphosphate (entry 1) has shown to be the most powerful solvent since it can dissolve the highest amount of PPTA pentamer per mole of ionic liquid, almost ten times higher than NMP/10 wt% CaCl2.
Influence of the anion
As predicted, the PPTA pentamer can only be dissolved in ionic liquids containing strongly coordinating anions just as is the case with cellulose dissolution. Only ionic liquids with chloride, carboxylate and phosphate anions are able to dissolve the aramid chains (depending on the cation, which will be discussed later), probably by coordination of the interchain hydrogen bonds of the amide groups.In the case of cellulose dissolution, where imidazolium ionic liquids are mostly investigated because of their strong dissolving properties, the chloride anion gives the strongest performance followed by the acetate and phosphate anion.33 Interestingly, the imidazolium ionic liquids with coordinating anions (entries 11, 13, 14 and 17) have here a reversed order when considering their performance: the imidazolium chloride ionic liquid shows poor pentamer solubility, while imidazolium acetate and phosphates are capable of dissolving significant amounts of this model compound. The same trend is observed for phosphonium ionic liquids: phosphonium phosphate and acetate are stronger solvents than their chloride analogue.
It is not fully understood yet why the preference of anion for disruption of hydrogen bonds shows an opposite trend in PPTA compared to cellulose. It has been suggested that the larger oxyanions are too bulky to efficiently interact with the tight intermolecular and intramolecular hydrogen bond network caused by the hydroxyl functional groups of cellulose.33 For instance, 3-butyl-1-methylimidazolium dibutylphosphate, which consists of an even larger anion, hinders efficient interaction with the hydrogen bond network of cellulose. Cellulose has three hydroxyl groups per glucose unit capable of hydrogen bonding, resulting in a tight hydrogen bond network. PPTA has only one hydrogen bond donor and acceptor site per amide bond while every amide bond is separated by an aromatic ring. The larger space in between the intermolecular hydrogen bonds of the amide groups of PPTA makes it less affected by the size of the anion. However, also here limitations in anion size were found: the very bulky anion of tributyl(tetradecyl)phosphonium decanoate (entry 28) was not able to dissolve the model compound while tributylhexylphosphonium acetate (entry 2) has proven to be one of the best solvents.
It seems that the basicity rather than the size of the anion is a crucial factor in the solvation strength of the ionic liquid. Independent of the cation, the order in strength is: chloride < diethylphosphate ≈ acetate, with pKa −6, 1.39 and 4.76, respectively. Theoretically, the most powerful ionic liquids would be the ones with a small and highly basic anion. For example, the powerful base sodium hydride (pKa = 35) dissolved in dimethylsulfoxide is able to dissolve high molecular weight PPTA.43,44 In this case, the dissolution mechanism is different: the strong base abstracts the hydrogen atoms from the amide bond, leaving the polymer unable to form hydrogen bonds and full of negative charges which repels the different polymer chains. Such a solvent with a strong base is obviously unsuitable as a polymerization medium since it can interact with the monomers. Care must be taken as this also could apply for ionic liquids with oxyanions such as acetates and phosphates since they could react with acid chlorides. In the current industrial synthesis process, terephthaloyl chloride is used as very reactive monomer to have a fast conversion into high molecular weight PPTA. Using too strongly basic anions could also mean that the anion gets protonated by the hydrochloric acid liberated during the reaction. This way the original anion can be lost and replaced by a chloride.
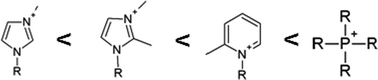
Influence of the cation
Some interesting results were obtained when investigations were done comparing different cations. In this case, no correlation was found between cations suitable for cellulose dissolution and those suitable for dissolution of PPTA oligomers. Moreover, it seems that the suitability of the cations are opposite to each other: ionic liquids that are good solvents for cellulose are poor solvents for PPTA and vice versa. To explain this difference, a closer look in cellulose dissolution must be taken, where it is already known that the cation actively participates in the dissolution process. The highest solubilities are obtained when a hydrogen atom on a sp2-bonded carbon atom is present next to a heteroatom which can be involved in forming hydrogen bonds with the hydroxyl groups of cellulose. This means that heteroaromatic rings such as imidazolium and pyridinium are excellent cations for dissolving cellulose. Tetrahedral cations such as ammonium and phosphonium only contain hydrogen atoms on sp3-bonded carbon atoms which are much less active in hydrogen bonding interactions. Moreover, these types of cations are usually bulky and are unable to interact efficiently with the hydrogen bond network. This makes tetrahedral cations unable to dissolve cellulose in large amounts.When analyzing the dissolution performance of ionic liquids with the same anion, the phosphonium and ammonium ionic liquids are the solvents of choice for dissolving the PPTA model compounds, rather than their imidazolium analogues. For example, 1-butyl-3-methylimidazolium chloride (entry 17) can barely dissolve any pentamer while tetrabutyl-phosphonium chloride (entry 4) is able to dissolve 6 wt% of the compound. It seems that the presence of a hydrogen bond donor on the cation disturbs the dissolution process instead of being an improvement to the overall coordination with PPTA. It seems that such cations prefer interaction with the anion, hindering its ability to break up hydrogen bonds. This hypothesis could be easily tested by removing the most acidic hydrogen atom on the 2′ position of the imidazolium ring and replacing it by a methyl group i.e. 1-butyl-2,3-dimethylimidazolium chloride (entry 15) (Fig. S1†). The methyl group on the 2′ position on the ring replaces the strongest interaction site, giving more freedom to the anion to complex with hydrogen atoms of the amide bonds of PPTA. However, the maximum solubility of this ionic liquid is still not on the same level as the quaternary phosphonium and ammonium ionic liquids. Further improvements could be made when the other hydrogen atoms on the imidazolium ring were to be replaced by methyl groups. Unfortunately, removal of hydrogen atoms on the imidazolium ring by methyl groups does increase the melting point and the viscosity of the ionic liquid substantially;45e.g. the melting point of 1-butyl-2,3-dimethylimidazolium chloride is 99 °C, while that of 1,2,4,5-tetramethylimidazolium chloride is 210 °C.46 The same trend can be observed for pyridinium chloride ionic liquids. When the hydrogen atom on the 2′ position of the ring is replaced by an alkyl group, a fairly good solvent for dissolving PPTA is obtained (entries 10 and 24). Unfortunately, pyridinium ionic liquids with chloride anions have high melting points, e.g. the melting point of 2-ethyl-1-benzylpyridinium chloride is 112 °C.
The same observations were made for the imidazolium acetate and imidazolium phosphate ionic liquids, but to a lesser extent. These ionic liquids are able to dissolve an acceptable amount of the model compound (entries 11, 14 and 16). It could be that these oxyanions are less susceptible to interaction with the imidazolium ring or that they are so strongly coordinating that coordination with the imidazolium cation only partially disturbs their ability to interact with PPTA. Nevertheless, the phosphonium analogues are able to dissolve much more of the model compound (entries 1 and 2). The tetrahedral conformation of the phosphonium and ammonium cations shields the positive charge from the anion. Also the lack of any sp2-bonded carbon atoms or electron-poor hydrogen atoms makes that these cations do not interrupt the anion in coordination with other compounds via hydrogen bonding. In our opinion, phosphonium ionic liquids with coordinating anions are the best candidates as solvents for PPTA. Less attention has been given in the literature to phosphonium ionic liquids compared to imidazolium or ammonium ionic liquids. However, they can have superior properties compared to the nitrogen-based analogues. For instance, they have a lower viscosity and higher thermal stability than their ammonium analogues.16,47–49 With these observations, an order of suitability of the cation for PPTA dissolution could be set up (Fig. 7).
Based on all these facts, a dissolution mechanism for PPTA in phosphonium ionic liquids can be proposed (Fig. S2†). Charge acts as a key role in dissolving PPTA. The presence of coordinating anions breaking up the hydrogen bonds give the PPTA chains a net negative charge and implements repulsive forces between different chains. The cations are in close proximity to the anion and form a shell around the PPTA/anions complex, further shielding different chains from each other. The phosphonium cations leave the complex unperturbed, giving the anions the full potential to interact with the hydrogen bond network.
Functionalized ionic liquids
None of the amide-functionalized imidazolium ionic liquids (entries 29 and 30) were able to dissolve the model compound. The reason is the too strong interaction of the imidazolium ring with the anion as discussed before. However, neither of the secondary amide-functionalized phosphonium and ammonium ionic liquids (entries 31 and 32) dissolved the pentamer. It seems that the hypothesis of extra beneficial interactions between solvent and solute does not hold up. In fact, it is a further confirmation that hydrogen bond donors on the cation disturb the ability of the anion to efficiently coordinate with the hydrogen bond network of PPTA.The tertiary amide-functionalized phosphonium and ammonium ionic liquids (entries 5 and 7) lack a hydrogen bond donor and did dissolve the model compound in an acceptable amount, 5 and 4 wt%, respectively. However, the goal was to improve solvent power by mimicking the NMP/CaCl2 solvent system. The solvent power in terms of mole units of N,N-diethyltributylacetamidephosphonium chloride (entry 5) and tetrabutylphosphonium chloride (entry 4) is almost exactly the same. This means that the implementation of a tertiary amide-functionality on the cation does not positively nor negatively influence the total solubility of the model compound. The viscosity of amide functionalized ionic liquids is significantly higher than that of their analogues with regular alkyl chains. This implies that amide-functionalized ionic liquids give rise to solvents which are more difficult in use while they do not give any significant improvement.
In the case of ionic liquids bearing an aromatic moiety, they were able to dissolve the model compound in a decent amount, 3 wt% and 5 wt% for entries 9 and 13 respectively. Since 1-ethyl-3-methylimidazolium nicotinate (entry 13) dissolves a comparable amount on a basis of mol% compared to 1-butyl-3-methylimidazolium acetate (entry 11) it can be concluded that the nicotinate anion did not give any significant improvement to the solubility. The same observation was made for 2-ethyl-1-benzylpyridinium chloride (entry 9), it dissolves a comparable amount of the pentamer as an analogous ionic liquid without an aromatic ring (entry 10).
Interestingly, the only dicationic ionic liquid that was investigated (entry 3) did indeed show a higher performance in dissolving the model compound than the monocationic analogue, tetrabutylphosphonium chloride (entry 4). The solubility in mole percentage was calculated as the amount of moles of the chloride anion, the active species in dissolving PPTA, present in the solvent to make a fair comparison. Here, it can be seen that the dicationic ionic liquid is able to dissolve 1.5 times more than the monocationic analogue. A clear explanation for this phenomenon has not been found but it is an indication that negative charges are of great importance in the solvent strength towards PPTA. Unfortunately, this ionic liquid has a higher melting point (105 °C) and a much higher viscosity than its monocationic analogue. Nevertheless, it would still be interesting to form mixtures of this ionic liquid with other ionic liquids to circumvent problems regarding the high melting point and viscosity.
Detailed dissolution tests
To gain further insight in the solubilizing properties of ionic liquids for PPTA oligomers and their suitability as possible polymerization medium, the dissolution of a series of low-molecular weight PPTA oligomers (Fig. 1) was investigated in four commercially available ionic liquids. The ionic liquids were selected in such a manner that different cations and anions are investigated: 1-ethyl-3-methylimidazolium acetate, 1-butyl-3-methylimidazolium chloride, tetrabutylphosphonium chloride and tributylethylphosphonium diethylphosphate. The solubility data of all the oligomers are compared with NMP/CaCl2 and are presented in Table 2.| Solubility (mol%) | ||||||
|---|---|---|---|---|---|---|
| # Aromatic rings | ||||||
| Solvent | 2 | 3 | 4 | 5 | 7 | b |
| n.s.: insoluble at any temperature. | ||||||
| [P4442][Et2PO4] | 209 | 44.7 | 13.2 | 7.62 | 4.12 | −3.21 |
| [C4MIM][CH3COO] | 203 | 31.3 | 5.46 | 1.79 | 0.13 | −5.80 |
| [P4444][Cl] | 194 | 32.6 | 7.45 | 3.20 | 1.67 | −4.01 |
| [C4MIM][Cl] | 137 | 9.40 | 2.00 | 0.16 | n.s. | −7.08 |
| NMP/10 wt% CaCl2 | 69 | 8.01 | 1.52 | 0.80 | 0.07 | −5.37 |
The data points of each ionic liquid were fitted according to the function Ax−b to investigate the behavior of solvent strength towards oligomers with increasing chain lengths. A selection of the fitted data is graphically depicted in Fig. 8. All the other graphical data are available in the electronic ESI.†
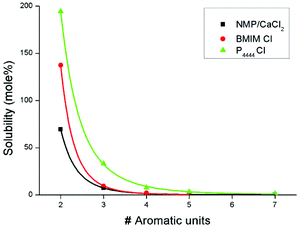 | ||
| Fig. 8 Graphical representation of the fitted solubility data of all the different oligomers in NMP/10 wt% CaCl2 (■), 1-butyl-3-methylimidazolium chloride (●) and tetrabutylphosphonium chloride (▲). | ||
As intuitively would be expected, the solubility of the oligomers show an exponential decrease when the chain length of the oligomer increases. The presence of extra secondary amide bonds on one molecule makes it increasingly harder for the solvent to break up all the intermolecular hydrogen bonds and separate different oligomer chains from each other. Some interesting additional conclusions can be taken when all the solubilities are compared between the different solvents. For instance, 1-butyl-3-methylimidazolium chloride which was described as a weak solvent during the screening tests, is actually able to dissolve the shorter oligomers in larger amounts than the benchmark, NMP/10 wt% CaCl2. However, this exponential decrease in solubility with increasing chain length is much faster than that of all other solvents. This ionic liquid is not able to dissolve the heptamer, the longest oligomer tested. The same can be observed for 1-ethyl-3-methylimidazolium acetate which shows a similar solubility for the shorter oligomers such as tetrabutylphosphonium chloride but falls significantly behind in performance when the chain length of the oligomer increases. As previously discussed, imidazolium ionic liquids are poorer solvents because the hydrogen atoms on the imidazolium ring prevent the anion to efficiently coordinate with the hydrogen bond network of the oligomers. It seems that these weakened anions start to struggle in effectively separating different oligomer chains when five or more amide bonds are present on each molecule.
The exponential decrease in solubility for the two tested phosphonium ionic liquids is much lower compared to all other solvents. Here, the coordinating anions suffer less in dissolving longer oligomer chains which is advantageous for using them as a polymerization medium since it is necessary to keep the growing polymer chain as long as possible in dissolution to have satisfactory molecular masses. Again, phosphonium ionic liquids with coordinating anions have shown to be promising possible candidates in replacing the current industrial solvent for the synthesis of PPTA. The next step in the search of the best possible polymerization medium would be the optimization of reaction conditions in order to implement these ionic liquids as solvents and maximize the full potential they possess.
Dissolution of functionalized oligomers
From the solubility data depicted in Table 3 it can be observed that the presence of functional groups strongly reduces the solubility of the oligomer when compared to the solubilities of the PPTA trimer without end-functionalities. The solubility of the trimer containing amines is always lower than the trimer containing carboxylic acid functionalities. The labile protons of the amine might efficiently interact with the coordinating anions, making them unavailable for interchain breaking interactions. In the case of tributylethylphosphonium diethyl-phosphate, tetrabutylphosphonium chloride and N-methyl-pyrrolidone with 10 wt% CaCl2, the solubilities of the functionalized compounds are comparable to the solubilities of the pentamer model compound. Interestingly, imidazolium ionic liquids perform better in dissolving the functionalized trimer compounds than the pentamer model compound. In some cases, their solvent properties are even on the same level or better as certain phosphonium ionic liquids. It could be that the hydrogen bond donor sites on the heteroaromatic ring are a beneficial interaction site for interaction with these functional groups. Nevertheless, since at the start of a polycondensation reaction mainly short oligomers with functional groups are present in the reaction mixture, it is advisable to not completely discard imidazolium ionic liquids as reaction solvents since they show an acceptable performance in dissolving functionalized oligomers. Possibly, a mixture of both a phosphonium and imidazolium ionic liquid could give rise to a solvent which is a strong solvent at the beginning of the reaction when many monomers and short functionalized oligomers are present but also at the end of the reaction where only long oligomers and polymers are present where end-functionalities are less of a factor.| Solubility (mol%) | Trimer-NH2 | |
|---|---|---|
| Solvent | Trimer-COOH | |
| [P4442][Et2PO4] | 11.40 | 6.10 |
| [C4MIM][CH3COO] | 8.83 | 4.58 |
| [P4444][Cl] | 2.46 | 1.80 |
| [C4MIM][Cl] | 3.02 | 2.01 |
| NMP/10 wt% CaCl2 | 1.36 | 0.80 |
Correlation of observed data with solvatochromic parameters
From all the solubility experiments with the pentamer model compound, a general trend was observed: strongly coordinating, hydrogen bond accepting, anions are needed for efficient dissolution while cations with no hydrogen bond donors are also necessary. The hydrogen-bond donating and accepting properties of a solvent can be quantified using solvatochromic parameters. These parameters are measured by adding to the solvent specific dyes which will exhibit a shift in the wavelength at maximal absorbance (λmax) dependent on their interactions with that particular solvent. In the literature, the three Kamlet–Taft parameters α, β and π* are widely used to investigate the hydrogen-bond accepting and donating properties of an ionic liquid.50–53 The parameter α describes the hydrogen-bond donating capability of a solvent. In the case of an ionic liquid this parameter is mainly dependent on the cation, if the anion does not contain any functional groups e.g. amines. The α-scale is normalized in such a manner that α = 1 for methanol. The parameter β describes the hydrogen-bond accepting capability of an ionic liquid. This parameter is mainly dependent on the anion, if the cation does not contain functional groups e.g. hydroxyls. The β-scale is normalized in such a manner that β = 1 for hexamethylphosporamide (HMPA), coincidentally the solvent that has been used in the past for the synthesis of PPTA. The parameter π* describes the polarity/polarizability of as solvent and its value does not vary much in the case of ionic liquids. It is of prime importance that the same set of dyes is used to determine the parameters of different solvents in order to make consistent comparisons. In the case of ionic liquids often Reichardt's dye, 4-nitroaniline and N,N-diethyl-4-nitroaniline are used as solvatochromic dyes.51Ab Rani et al.51 and Jessop et al.54 did an effort to collect all available Kamlet–Taft parameters of ionic liquids known at that time. This enables us to correlate the current observations to existing data of the used ionic liquids. Fig. 9 shows the β values of imidazolium ionic liquids with different anions compared to the amount of the pentamer model compound these ionic liquids were able to dissolve. Ionic liquids with a low β value, i.e. ionic liquids containing an anion with a low tendency to form hydrogen bonds, do not dissolve the pentamer model compound at all. There seems to be a cut-off point where the β value and thus the hydrogen-bond binding capacity is high enough to start dissolving the pentamer. After the cut-off point, increases the maximum solubility of the model compound with an increasing β value. This trend also corresponds to the pKa values of the anions as discussed before.
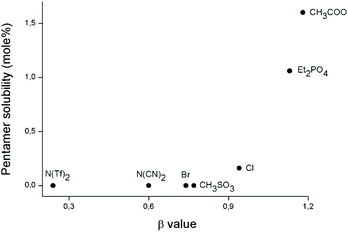 | ||
| Fig. 9 Correlation between the Kamlet–Taft β parameter of imidazolium ionic liquids with different anions and the amount of pentamer model compound they are able to dissolve. Ionic liquids analyzed are [C6MIM][N(Tf)2],55,56 [C4MIM][N(CN)2],56,57 [C6MIM][Br],58 [C4MIM][CH3SO3],51 [C6MIM][Cl],58 [C4MIM][Et2PO4],51 [C4MIM][CH3COO].59 | ||
Unfortunately, data of the α parameter of ionic liquids comparing different cations with the same anion are not widely available. Therefore, all the data of the ionic liquids with the same cations as used in this study are collected and a range of α values is set up or in the case of scarce data, an ionic liquid was selected which anion is not of great influence to the α value (Table 4).
When comparing this data to the order of cations capable of dissolving PPTA presented in Fig. 7, it is evident that more or less the same order is established when we order the cations from the higher to the lower α values. This means that the lower the α value and thus lower the tendency to form hydrogen bonds, the better it is able to dissolve the pentamer model compound, independently of the coordinating anion present.
From the point of view from the Kamlet–Taft parameters, it can be concluded that the best ionic liquids for dissolving PPTA oligomers are the ones with the highest possible β values, which means the ionic liquids need to have a strongly basic and strongly coordinating anion. Additionally, an as low as possible α value is needed to avoid interruption of the cation via hydrogen bonding with the anion.
Spectroscopic analysis of PPTA dissolution
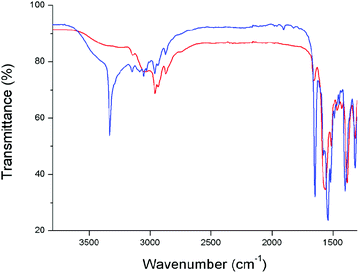
Throughout this work, the importance of breaking of the hydrogen bonds between the different PPTA chains in order to bring the polymer in solution has been stressed. The shifts in energy of the NH-vibrational stretching mode when a PPTA model compound is brought into dissolution was studied by infrared spectroscopy. A trimer was chosen as model compound for this study since this oligomer can be dissolved in much greater amounts than the pentamer and therefore gives much stronger signals in the infrared spectrum. Fig. 10 shows both the infrared spectra of pure 3-butyl-1-methylimidazolium acetate (black) and of the same ionic liquid with 20 wt% trimer dissolved in it (red).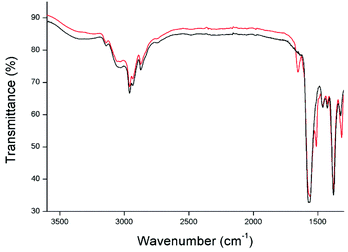 | ||
| Fig. 10 Comparison between the infrared spectrum of pure 1-butyl-3-methylimidazolium acetate (black) and the same ionic liquid containing 20 wt% trimer (red). | ||
Both spectra show a broad O–H stretch band of water with low intensity at 3500 cm−1 due to small uptake of water from the atmosphere when a droplet was transported from the flask to the FTIR-spectrometer. Also the typical vibrations from the alkyl chain and the carboxylate anion are present at 3000, 1570 and 1390 cm−1 respectively. The red spectrum represents the vibrations of the same ionic liquid but with 20 wt% trimer dissolved in it. A few peaks representing the vibrational signals of the trimer are superimposed on the original spectrum e.g amide I, amide II and the C–N of the amide bonds of the trimer. However, the vibrational N–H stretch at ca. 3350 nm which is typical for secondary amides is completely absent. The disappearance of this peak can be explained by the formation of new hydrogen bonds between the anion of the ionic liquid and the hydrogen of the secondary amide of the oligomer. A change in vibrational energy is expected due to the interaction with a heavier anion. Also the interaction with a liquid and changeability in chemical environment would explain the disappearance of a sharp N–H stretching peak.
The droplet containing 3-butyl-1-methylimidazolium acetate and 20 wt% trimer that was used to record the spectrum was left in contact with the atmosphere for a couple of minutes. The very hygroscopic nature of the ionic liquid caused the oligomer to precipitate out of solution by absorbing a significant amount of water. A new spectrum was recorded and compared with the original spectrum of the same droplet (Fig. 11).
This time the sharp N–H stretching vibration at 3350 cm−1 is clearly visible along with a stronger broad O–H stretch band (blue spectrum). In the presence of water, a hydration shell formed around the anions, causing the anions to lose their ability to form hydrogen bonds with the amides of the oligomer. This gives the oligomers the chance to re-aggregate and precipitate as a solid. The precipitation also caused the other vibration signals of the oligomer to be present in a stronger intensity. This shows that working in completely water-free conditions is crucial to use the full solvent potential of ionic liquids.
Inspired by 13C NMR measurements of cellulose in an ionic liquid,3913C and 15N NMR spectra were recorded to locate the interaction sites by investigating the important changes in chemical shift for both ionic liquid and trimer model compound when the latter is dissolved in increasingly larger amounts (S5–S9). Table 5 shows the chemical shifts of the carbon atoms of the model compound when it is dissolved in a 20 and 40 wt% concentration in 1-butyl-3-methylimidazolium acetate while Table 6 shows the chemical shifts of the carbon atoms of pure 1-butyl-3-methylimidazolium acetate and of the same ionic liquid when 40 wt% of the trimer model compound is dissolved in it. For easy understanding, the structure with the numbering of carbon atoms of the model compound and ionic liquid are given in Fig. 12 and 13.
| wt% trimer | δ/ppm | |||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C6 | C2 | C3 | C4 | C5 | C7 | N1 | |
| 20 | 121.00 | 127.68 | 128.02 | 130.71 | 135.31 | 135.87 | 165.71 | 130.07 |
| 40 | 120.99 | 127.63 | 127.97 | 130.71 | 135.24 | 135.81 | 165.70 | 129.80 |
| Δδ | 0.01 | 0.05 | 0.05 | 0.0 | 0.07 | 0.06 | 0.01 | +0.27 |
| wt% trimer | δ/ppm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C8 | C7 | C6 | C4 | C5 | C9 | C3 | C2 | C1 | C10 | N1 | N2 | |
| 0 | 12.70 | 18.82 | 25.43 | 31.78 | 35.17 | 48.34 | 122.99 | 124.00 | 138.84 | 174.14 | 171.33 | 183.51 |
| 40 | 12.66 | 18.76 | 25.43 | 31.54 | 35.03 | 48.35 | 122.64 | 123.70 | 138.46 | 174.52 | 170.87 | 183.25 |
| Δδ | +0.04 | +0.06 | 0.00 | +0.24 | +0.14 | −0.01 | +0.35 | +0.30 | +0.38 | −0.38 | +0.46 | +0.26 |
Interestingly, neither the carbon atoms of the aromatic rings nor the carbon atoms of the amide functional group showed any significant changes in chemical shift upon increasing the concentration of the model compound from 20 to 40 wt% in the ionic liquid. This could be a further indication that the most important interaction sites during dissolution of polyamides are located on the nitrogen atom of the secondary amide. The fact that the carbon atom of the carbonyl functional group (C7) does not show any changes in chemical shift could mean that the carbonyl group does not interact with the hydrogen bond donors on the imidazolium ring of the ionic liquid. This is an additional piece of evidence that coordinating cations, such as pyridinium and imidazolium cations, are not needed to improve solubility and that they should be avoided since they show the tendency to interrupt the coordination of the anion with the hydrogen bond network of the solute. When the recorded 15N NMR spectra are analyzed, the nitrogen atoms from the trimer model compound show a decrease in chemical shift (increase in electron density) upon increasing the concentration of this compound from 20 to 40 wt% in the ionic liquid. This could be attributed to the fact that intermolecular hydrogen bond between the secondary amides of the oligomer compound are broken up and that new hydrogen bonds are formed with the electron rich and well coordinating acetate group. With this observation, a confirmation was found that breaking up of hydrogen bonds between the amide groups is a key step in dissolving the oligomers.
Several atoms on both the anion and cation of the ionic liquid showed significant changes in chemical shift upon dissolving a high amount of the model compound. All the carbon and nitrogen atoms on the imidazolium ring and the neighboring carbon atom of the alkyl chains (C1 and C5) showed a change in chemical shift upfield, meaning that the electron density on these carbon atoms increased. Only the carbon of the carbonyl functional group on the acetate anion (C10) showed a change in chemical shift downfield, meaning that the electron density on this carbon atom decreased. The fact that the acetate anion shows a decrease in electron density, could be simply attributed to the formation of new and stronger hydrogen bonds with the labile protons of amide functionalities of the oligomer. The increase in electron density on the imidazolium cation is not that straightforwardly explained since the cation does not show the tendency to form hydrogen bonds with the amide group of the oligomer. It could be that the imidazolium cation partly loses its interaction with the strongly coordinating acetate anion because this anion has a preferable interaction with the secondary amides of the model compound. This would imply that the electron density taken away by the electron-withdrawing acetate group is reallocated to the imidazolium ring. The increase in electron density is then distributed around the imidazolium ring.
Conclusions
Ionic liquids were proposed as alternative and greener solvents for the synthesis of poly-p-phenyleneterephthalamide to replace the current industrial solvent mixture of N-methylpyrrolidone/calcium chloride (NMP/CaCl2). Since a wide variety of ionic liquids exist, a large screening test was set up to gain insights about which anions and cations are preferred to achieve a strong solvent for PPTA. For these tests a set of short PPTA oligomers without functional end-groups were synthesized and used as model compounds to investigate and compare the solvent strength of each ionic liquid. It was found that just as for dissolution of cellulose, coordinating anions such as chloride, carboxylates and phosphates are needed as active species to break up the hydrogen bond network formed by the secondary amide bonds between different oligomer molecules. An infrared and NMR spectroscopic study confirmed the breaking up of intermolecular hydrogen bonds as an important step in the dissolution process and showed the importance of working in absolute dry conditions to use the full capacity of the solvation strength of ionic liquids. Interestingly, imidazolium cations have proven to be unsuitable for dissolution of the model compounds, in contrast to what is observed for cellulose dissolution. The presence of hydrogen bond donors on the cation disturbs the anion in using its full potential to interact with the aramid compounds. Extended dissolution tests confirmed that imidazolium ionic liquids have difficulties to keep longer oligomer chains in dissolution. Elimination of hydrogen bond donor sites on the cation by replacing them by methyl groups such as with imidazolium rings with a methyl group on the 2′ position or certain methylpyridinium cations improves the solvent capabilities of the ionic liquid significantly. It has been found that tetrahedral cations such as phosphonium and ammonium which lack hydrogen bond donors in the structure give rise to the best performing ionic liquids when combined with a coordinating anion. Also new functionalized ionic liquids were synthesized that have proven to be good solvents, especially a dicationic phosphonium chloride ionic liquid. Tributylethylphosphonium diethylphosphate, tributylhexyl-phosphonium acetate and tetrabutylphosphonium chloride were found to be the best solvents for the PPTA model compounds and performed much better than the presently used industrial solvent NMP/CaCl2. It can thus be concluded that ionic liquids have potential to serve as a greener and better alternative for the synthesis of PPTA.Acknowledgements
The author thanks the Flemish Government Agency for Innovation by Science and Technology (IWT Flanders) for a PhD fellowship to SD. Support by Teijin Aramid (Arnhem, The Netherlands) and Iolitec (Heilbronn, Germany) is gratefully acknowledged. Karel Duerinckx and Dirk Henot are also thanked for their help with NMR and CHN measurements respectively.References
- J. M. Garcia, F. C. Garcia, F. Serna and J. L. de la Pena, Prog. Polym. Sci., 2010, 35, 623–696 CrossRef CAS.
- B. Crist, Annu. Rev. Mater. Sci., 1995, 25, 295–323 CrossRef CAS.
- M. Arpin and C. Strazielle, Polymer, 1977, 18, 591–598 Search PubMed.
- M. Zhou, V. Frydman and L. Frydman, J. Phys. Chem., 1996, 100, 19280–19288 CrossRef CAS.
- Y. Rao, A. J. Waddon and R. J. Farris, Polymer, 2001, 42, 5937–5946 CrossRef CAS.
- X. Li and M. Huang, J. Appl. Polym. Sci., 1999, 71, 565–571 CrossRef CAS.
- G. I. Kudryavtsev and T. I. Shein, Fibre Chem., 1978, 10, 101–115 CrossRef.
- L. Vollbracht and T. J. Veerman, PatentUS4308374, 1981 Search PubMed.
- A. E. M. Bannenberg-Wiggers, J. A. Van Omme and J. M. Surquin, PatentUS5726275, 1998 Search PubMed.
- A. K. Dibrova, V. B. Glazunov and S. P. Papkov, Fibre Chem., 1987, 18, 180–183 CrossRef.
- Zhou Xiang and Liu Shan, Polym. Sci., 1987, 5, 95–100 Search PubMed.
- A. A. Fedorov, V. M. Savinov and L. B. Sokolov, Polym. Sci. U. S. S. R., 1970, 12, 2475–2491 CrossRef.
- A. Mariani, O. Monticelli, S. Fiori and S. Russo, e-polym., 2003, 59, 1–7 Search PubMed.
- C. Bin, H. Ping, L. Yue and H. Juan, PatentCN103172902, 2013 Search PubMed.
- Scientific Committee on Consumer Safety (SCCS), Opinion on N-Methyl-2-pyrrolidone (NMP), European Union, 2011 Search PubMed.
- S. A. Forsyth, J. M. Pringle and D. R. MacFarlane, Aust. J. Chem., 2004, 57, 113–119 CrossRef CAS.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- G. Laus, G. Bentivoglio, H. Schottenberger, V. Kahlenberg, H. Kopacka, T. Röder and H. Sixta, Lenzinger Ber., 2005, 84, 71–85 CAS.
- P. Wasserscheid and T. Welton, Ionic liquids in synthesis, Wiley-VCH, Weinheim, 1st edn, 2008 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- D. Glas, J. Hulsbosch, P. Dubois, K. Binnemans and D. E. De Vos, ChemSusChem, 2014, 7, 610–617 CrossRef CAS PubMed.
- T. Zhao, Q. Zhou, X. L. He, S. D. Wei, L. Wang, J. M. N. van Kasteren and Y. Z. Wang, Green Chem., 2010, 12, 1062–1065 RSC.
- N. Winterton, J. Mater. Chem., 2006, 16, 4281–4293 RSC.
- M. Rahman and C. S. Brazel, Polym. Degrad. Stabil., 2006, 91, 3371–3382 CrossRef CAS.
- E.I. Lozinskaya, A.S. Shaplov and Y. Vygodskii, Eur. Polym. J., 2004, 40, 2065–2075 Search PubMed.
- I. Yavari, P. Yavari and E. Kowsari, Synth. Commun., 2009, 39, 2540–2548 CrossRef CAS.
- S. Mallakpour and M. Dinari, Iran. Polym. J., 2010, 19, 983–1004 CAS.
- Y. S. Vygodskii, E. I. Lozinskaya and A. S. Shaplov, Macromol. Rapid Commun., 2002, 23, 676–680 CrossRef CAS.
- S. G. Lee, Chem. Commun., 2006, 1049–1063 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- S. Fischer, K. Thümmler, K. Pfeiffer, T. Liebert and T. Heinze, Cellulose, 2002, 9, 293–300 CrossRef CAS.
- J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 1557–1559 Search PubMed.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- B. Zhao, L. Greiner and W. Leitner, RSC Adv., 2012, 2, 2476–2479 RSC.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- B. Lu, A. Xu and J. Wang, Green Chem., 2014, 16, 1326–1335 RSC.
- A. Pinkert, K.N. Marsh and S. Pang, Ind. Eng. Chem. Res., 2010, 49, 11121–11130 CrossRef CAS.
- G. J. Bahun and A. Adronov, J. Polym. Sci., Part A-1: Polym. Chem., 2010, 48, 1016–1028 CrossRef CAS.
- S. D. Hojniak, A. L. Khan, O. Holloczki, B. Kirchner, I. F. Vankelecom, W. Dehaen and K. Binnemans, J. Phys. Chem. B, 2013, 117, 15131–15140 CAS.
- M. Takayanagi and T. Katayose, J. Polym. Sci., Polym. Chem. Ed., 1981, 19, 1133–1145 CrossRef CAS.
- N. Ogata, K. Sanui and S. Kitayama, J. Polym. Sci., Polym. Chem. Ed., 1984, 22, 865–867 CrossRef CAS.
- P. A. Hunt, J. Phys. Chem. B, 2007, 111, 4844–4853 CrossRef CAS PubMed.
- T. Peppel, C. Roth, K. Fumino, D. Paschek, M. Köckerling and R. Ludwig, Angew. Chem., Int. Ed., 2011, 50, 6661–6665 CrossRef CAS PubMed.
- G. Adamová, R.L. Gardas, L.P.N. Rebelo, A.J. Robertson and K.R. Seddon, Dalton Trans., 2011, 40, 12750–12764 RSC.
- K. J. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 309–321 CrossRef CAS.
- C. J. Bradaric, A. Downard, C. Kennedy, A. J. Robertson and Y. Zhou, Green Chem., 2003, 5, 143–152 RSC.
- R.W. Taft and M.J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef CAS.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P.A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC.
- L. Crowhurst, R. Falcone, N. L. Lancaster, V. Llopis-Mestre and T. Welton, J. Org. Chem., 2006, 71, 8847–8853 CrossRef CAS PubMed.
- P. Jessop, D. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC.
- S. Coleman, R. Byrne, S. Minkovska and D. Diamond, Phys. Chem. Chem. Phys., 2009, 11, 5608–5614 RSC.
- C. Chiappe and D. Pieraccini, J. Phys. Chem. A, 2006, 110, 4937–4941 CrossRef CAS PubMed.
- C. Chiappe, C. S. Pomelli and S. Rajamani, J. Phys. Chem. B, 2011, 115, 9653–9661 CrossRef CAS PubMed.
- A. Jelicic, N. García, H.-G. Löhmannsröben and S. Beuermann, Macromolecules, 2009, 42, 8801–8808 CrossRef CAS.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967 RSC.
- K. A. Kurnia, F. Lima, A. Filipa, M. Claudio, J. A. P. Coutinhoa and M. G. Freire, Phys. Chem. Chem. Phys., 2015, 17, 18980–18990 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures for the ILs, characterization data (NMR, CHN, DSC, viscosity, infrared) and additional figures from results and discussion. See DOI: 10.1039/c5gc02185e |
| This journal is © The Royal Society of Chemistry 2016 |