CO2-switchable drying agents†
Kyle J.
Boniface
,
Ryan R.
Dykeman
,
Alex
Cormier
,
Hong-Bo
Wang
,
Sean M.
Mercer
,
Guojun
Liu
*,
Michael. F.
Cunningham
* and
Philip G.
Jessop
*
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, ON, Canada K7L 3N6. E-mail: jessop@queensu.ca; guojun.liu@chem.queensu.ca; michael.cunningham@queensu.ca; Fax: +1 613 533 6669, +1 613 533 6669, +1 613 533 6637; Tel: +1 613 533 3212, +1 613 533 6996, +1 613 533 2782
First published on 24th August 2015
Abstract
CO2-switchable desiccants have been prepared and evaluated for the drying of isobutanol. CO2 addition triggered the binding of water to the drying agent, while CO2 displacement triggered the water's facile release. The switchable desiccants were capable of absorbing more water and were able to regenerate at much milder conditions than traditional desiccants like molecular sieves.
Introduction
Drying organic liquids (i.e. removing water from them) is a particularly important task in many fields of chemistry as well as several industrial processes such as solvent recycling and the production of ethanol and biodiesel. Many industries use large volumes of organic solvents; solvent recycling helps to save money and the environment. Used solvent is usually contaminated with water, rendering it useless for the industrial process for which it was intended. Ethanol, when it is manufactured by fermentation, is also contaminated with water.1 The presence of water in fuel grade ethanol reduces the heat of fuel combustion and causes phase separation in ethanol–petroleum blends.2 The removal of water from ethanol, as well as many other solvents, is complicated by the formation of azeotropes, which necessitates the use of additional means beyond distillation to reduce the water content; desiccants being a commonly employed agent to address this issue. Desiccants can be broken down into two categories: single use desiccants like sodium sulfate; and reusable desiccants such as molecular sieves. The desiccants in both categories have economic and environmental concerns. All single-use desiccants result in the generation of solid wastes and therefore disposal issues and recurring costs. Although molecular sieves have the advantage of being reusable, they require significant amounts of energy for regeneration.3 Therefore it would be advantageous, both economically and environmentally, to have a reusable drying agent that is easily regenerated.The criteria for the ideal drying agent can be defined as follows: able to bind water strongly during the capture stage, able to release water easily during regeneration, robust enough to be recycled, inert to the solvent of interest, and possessing a high water binding capacity. To meet these requirements with respect to the variable binding affinity of the drying agent for H2O, the agent would have to be ‘switchable’ in response to an external trigger; its affinity for water would be different depending on whether it is in capture mode or regeneration mode. The choice of external trigger strongly affects the cost, environmental impact, and ease of switching. In comparison with other triggers such as acids/bases, oxidants/reductants, salts and light, CO2 makes an attractive choice as it is easily-removed, does not accumulate in a system, and is functional for non-transparent systems.4
In recent years, we have developed CO2-triggered switchable solvents,5 surfactants,6 and latex particles7 that are switchable in polarity or hydrophilicity. In general, CO2-switchable materials rely on the protonation of amine- or amidine-containing molecules when CO2 is dissolved into an aqueous solution (eqn (1)).3
| R3N + CO2 + H2O ⇌ [R3NH]+[HCO3]− | (1) |
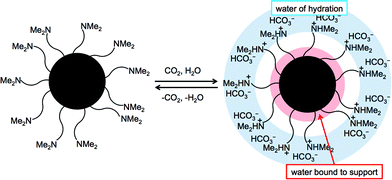
The fact that this reaction both consumes an equivalent of water and simultaneously increases the surface charge and hydrophilicity suggests that there is the possibility of capturing more than one water molecule per nitrogen atom through waters of hydration (Fig. 1). The remaining necessary component of a switchable drying agent is a support that is inert to the solvent of interest, has a high surface area and is physically robust. Silica and alumina are attractive candidates because they satisfy these criteria, in addition to being easily functionalized. Polymer grafting methods have allowed for a wide variety of silica-based stimuli-responsive materials.8 One of the ways polymer grafting can be achieved is through surface initiated atom transfer radical polymerization (SI-ATRP). SI-ATRP has been used to synthesize a CO2-responsive surface previously.9 Porous polymer particles present another option as long as the polymer particle can withstand the conditions outlined in the drying and regeneration steps.
Herein we describe the synthesis and evaluation of switchable drying agents containing tertiary amines in the drying of isobutanol. The conditions for use and regeneration are explored and it is shown that the amine-containing particles are able to dry isobutanol and are easily recycled several times under very mild conditions.
Results and discussion
Synthesis of the particles
For the synthesis of porous polymer particles (P-1), the crosslinkable monomer, 1,4-bis(diethylamino)-2,3-bismethacryoloxybutanoate, 1, was chosen. We hoped that the high nitrogen content (5.4 mmol of nitrogen atoms per gram of material) of 1 would translate into a high accessible nitrogen content in the resulting porous particles. Monomer 1 was synthesized in two steps from the commercially available 2,3-dibromo-1,4-butanediol (Scheme 1), which was converted to 1,4-bis(diethylamino)-2,3-butanediol under basic conditions via the epoxide intermediate,10,11 which reacted in situ with diethylamine. The methacrylate moiety was introduced under basic conditions in dimethyl carbonate (DMC) with methacryloyl chloride. Benign solvents were used in both steps and neither step required chromatographic techniques for product purification. | ||
| Scheme 1 Synthesis of the monomer, 1, for porous CO2-switchable polymer particles. DMC = dimethylcarbonate. | ||
The porous polymer particles were prepared using suspension polymerization of 1 in a water and toluene mixture. The particles were determined to have a mean particle size of 220 μM through diffractive light scattering measurements (see Fig. S1 in the ESI†). Scanning electron microscope (SEM) images of the particles show a surface with many deep pores (Fig. 2), which suggests that the polymer particles are highly porous. The porosity of the polymer particles was confirmed through N2(g) absorption experiments with the BET surface area found to be 40 m2 g−1 (Fig. S2 in the ESI†). Finally the particles were shown to be thermally stable up to 300 °C by thermogravimetric analysis (TGA) (Fig. S9†).
The polymer-grafted silica particles (PGS-1 & PGS-2) were synthesized via a three step method. Firstly, silica particles (SiliaFlash P60, 45–60 μM, 230–400 mesh) were subjected to a three part treatment in order to removed organic contaminants and promote a hydroxyl rich surface (see ESI†). Secondly, the initiator, α-bromoisobutyryl bromide (BIBB) was grafted to the surface via nucleophilic acyl substitution with the surface hydroxyl groups of the silica particle to yield particle 2 (Scheme 2). Lastly, poly-N-[3-(dimethylamino)propyl]methacrylamide (PDMAPMAm) was grafted to particle 2 by SI-ATRP to yield particle 3 (Scheme 2) (see ESI†). The polymer-grafted silica particles (PGS-1 & PGS-2) were washed and dried, then stored under Ar(g) until further use.
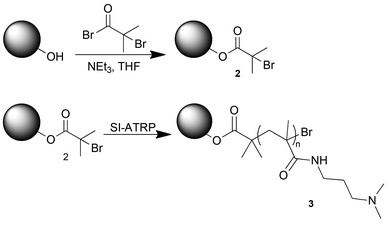 | ||
| Scheme 2 Synthesis and SI-ATRP of the initiator functionalized (2) silica to produce PDMAPMAm functionalized silica (3). | ||
For comparison, commercially available silica, alumina, and silica functionalized with 3-dimethylaminopropyl groups were also tested.
Characterization of surface switchable groups
Commercially available amine-functionalized silica particles are characterized by the loading of amine on the solid support, in units of mmol g−1. Amine loading refers to the amount of amine in a given sample, whereas accessible amine content refers to the number of reactive amine groups and does not include amine functional groups that are physically buried and therefore unreactive.Commonly, amine loading values are determined by elemental analysis (EA), however if one wants to differentiate between the overall loading of amines and the amines that are accessible, a titration with a strong acid such as HCl is recommended. Ojo et al. highlighted the difference between EA values and titration values. In one example, only 85% of the amines reported by EA are reported accessible by titration to low pH (≈2) with HCl.12 The titration method used by Ojo et al. is an effective method for amine-containing particles that have only one protonatable species. In the case of our polymer particles (P-1) every protonatable site is an amine, therefore titration to low pH (≈2) with HCl is again recommended for determining the accessible amine content. In contrast, elemental analysis of P-1 would determine amine loading but would be ineffective for determining accessible amine content because it would account for all amines, including physically buried amines.
Another problem arises when the material in question has two or more protonatable species, as is the case with commercially available amine-functionalized silica particles (DMA-S) and our polymer-grafted silica particles (PGS-1 & PGS-2). High grafting densities are not easily achieved with silica based materials; as a result some surface silanol groups remain active. A full titration of these particles reveals not one, but two equivalence points (ca. pH 7 and at ca. pH 3). This phenomenon can be attributed to both the amine functionalities (pKa ≤ 9.3) and the unfunctionalized surface silanols (pKa = 4.5).13 The pKa of PDMAPMAm in solution is 8.8.14 To accurately report accessible amine content the titration is stopped at pH 4, assuming that at pH 4 all of the amines are protonated and none of the surface silanols have been protonated. Centrifugation followed by back titration of the supernatant allows for the extrapolation of an accessible amine value that can be fully attributed to the reactive amine functional groups. This assumption is supported by the data shown in Table 1. When titrated to pH 4, the unfunctionalized silica sample shows no interaction with HCl. However, when titrated to pH 2, the silica sample shows an interaction with HCl, analogous to a protonatable sites content of 0.8 mmol g−1. Furthermore, when the polymer-grafted silica samples (PGS-1 & PGS-2) are titrated to pH 2 they show an increase in the accessible amine content, an increase which we can confidently contribute to the silica and not the amine functionalities.
| Drying agent | Accessible amine (mmol g−1) | Reported commercially | Protonatable sites (mmol g−1) | |
|---|---|---|---|---|
| pHi to pH 4a | pHi to pH 2a | |||
| a Samples were titrated from the initial pH to the pH indicated. b Loss in protonatable sites is due to cleavage of amine functionalities from the silica particle. | ||||
| DMA-S | 1.1 | 1.51 | 1.1 | 0.7b |
| P-1 | 2.6 | — | 2.6 | 2.6 |
| PGS-1 | 0.3 | — | 0.3 | 0.5 |
| PGS-2 | 0.4 | — | 0.4 | 0.5 |
| Silica | 0 | 0 | 0 | 0.8 |
Interestingly enough, when the silica based DMA-S samples were titrated to pH 2 they showed a decrease in the accessible amine content. Silanes are known to hydrolyze off of a silica support at low pH.15 The reduced accessible amine content when titrated to pH 2 is a result of the Si–O–Si bond being hydrolyzed and the subsequent cleavage of the amino-silanes from the silica support. In contrast, PGS-1 and PGS-2 show no signs of hydrolysis at low pH. The increased hydrolytic stability of PGS-1 and PGS-2 is of great importance and was anticipated. The amide bonds in PDMAPMAm resist hydrolysis at low pH whereas the ester linkages in the more commonly used monomers such as; N,N-dimethylaminoethyl methacrylate (DMAEMA), 2-(diethylamino)ethyl methacrylate (DEAEMA), and 2-(diisopropylamino)ethyl methacrylate (DPAEMA) hydrolyze at ca. pH 3. Regardless of the increased stability of the polymer, the stability of the link between the silica support and the polymer chain was still in question. The stability of the Si–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in comparison to the commonly used Si–O–Si bond was unknown. Since no loss in accessible amine content was detected at low pH for PGS-1 and PGS-2 we can conclude that the Si–O–C(
O) in comparison to the commonly used Si–O–Si bond was unknown. Since no loss in accessible amine content was detected at low pH for PGS-1 and PGS-2 we can conclude that the Si–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) remains stable at low pH (Table 1). We hypothesize that the polymer might also contribute to the overall stability of the Si–O–C(
O) remains stable at low pH (Table 1). We hypothesize that the polymer might also contribute to the overall stability of the Si–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond through steric hindrance, due in part to its large hydrodynamic volume. Extending this body of work to include longer chain polymers will help to elucidate the role of polymer conformation and hydrodynamic volume in the stability and performance of the switchable drying agents.
O) bond through steric hindrance, due in part to its large hydrodynamic volume. Extending this body of work to include longer chain polymers will help to elucidate the role of polymer conformation and hydrodynamic volume in the stability and performance of the switchable drying agents.
Use for the drying of wet solvents
The solvent chosen to compare the various drying agents was isobutanol, which was doped with 5 wt% H2O. The drying agent was added to the ‘wet’ isobutanol solution and CO2(g) was bubbled through the solution for 1 h. The vial was then sealed and stirred for 15 h. Silica and alumina were activated before use by heating the absorbents to 250 °C for 24 h before being added to the wet isobutanol solution. The same procedure was then followed as described above, but in the absence of CO2. The water content of the solvent after filtration was determined by gas chromatography thermal conductivity detector (GC-TCD). Before the various drying agents could be compared in terms of their recyclability, regeneration conditions needed to be established. After the drying process was performed for isobutanol using the polymer particles, TGA was performed on the used polymer particles; they were heated isothermally at 50 °C for 4 h and subsequently heated up to 120 °C (Fig. 3). As shown in Fig. 3, the mass of the ‘wet’ particles continues to drop as CO2 and water are lost at 50 °C, however no further weight loss was observed upon subsequent heating up to 120 °C. Therefore, regeneration by heating to 50 °C for 4 h is sufficient. | ||
| Fig. 3 Scanning TGA for the regeneration evaluation at a rate of 2 °C min−1 to 50 °C, isothermal at 50 °C for 4 h, then increasing to 120 °C. | ||
The drying abilities of multiple drying agents under initial conditions are compared in Table 2. The synthesized porous polymer particles (P-1) and polymer-grafted silica (PGS-1 & PGS-2) showed the greatest ability to capture water (Table 2, rows 2-5), outperforming traditional drying agents over consecutive cycles. The efficacy of most drying agents decreases slightly with continued recycling, with the exception being the silica based drying agents. With an increased CO2 bubbling time of 3 h, the polymer particles remove more water per gram of drying agent and maintain their drying ability through several cycles (Table 2, row 4). Upon increasing the reaction time from 15 to 18 h, the polymer-grafted silica particles remove more water per gram of drying agent than they did previously. Thus, drying performance is a function of CO2 exposure and time.
| Drying agent | Accessible amine (mmol g−1) | Size (μm) | Water removeda,b (mg g−1) | |||
|---|---|---|---|---|---|---|
| Cycle | ||||||
| 1 | 2 | 3 | 4 | |||
| a Reaction conditions: 10 g isobutanol with water at a concentration of 5 wt%, 0.5 g drying agent added, 1 h mixing with CO2 bubbling through solution then continued mixing in a sealed vial for 15 h, water content analyzed by GC-TCD. Drying agent regeneration was performed at 50 °C for 4 h. b Water removed with respect to drying agent used. c An increased bubbling time of 3 h and continued mixing under 1 atm of CO2(g) was employed. d An increased reaction time of 18 h was employed. | ||||||
| DMA-S | 1.1 | 20 | 180 | 140 | 160 | 140 |
| P-1 | 2.6 | 220 | 300 | 220 | 200 | 160 |
| P-1 | 2.6 | 220 | 380c | 520c | 400c | 400c |
| PGS-1 | 0.3 | 60–100 | 490 | 480 | 480 | 470 |
| PGS-2 | 0.4 | 60–100 | 540 | 440 | 430 | 430 |
| PGS-1 | 0.3 | 60–100 | 520d | 580d | — | — |
| PGS-2 | 0.4 | 60–100 | 580d | 580d | — | — |
| Molecular sieves | 0 | 4000 | 380 | 40 | 60 | — |
| Silica | 0 | 74–177 | 150 | 150 | 140 | 140 |
| Alumina | 0 | 37–63 | 39 | 75 | 72 | 83 |
The porous polymer particles (P-1) were compared to molecular sieves, an industrial standard for reusable drying agents. As seen in Table 1, both P-1 and the molecular sieves are effective drying agents for one cycle, but only P-1 is effective after regeneration at 50 °C. A possible explanation for the reduced recyclability of the molecular sieves is that not enough thermal energy was supplied for the release of the water after use. To test this, differential scanning calorimetry was used to compare the energy required to release water from the two drying agents after one cycle (see Fig. S11 in the ESI†). Heating P-1 to the regeneration temperature of 50 °C requires ca. 36 J per g of particles compared to 325 °C and 219 J g−1 for the molecular sieves (see the ESI† for details of the calculations). Once at the regeneration temperature, P-1 requires 16 J g−1 of particles to desorb the water, according to the DSC measurement, compared to 30 J g−1 for the molecular sieves. Thus the lower regeneration temperature and lower desorption enthalpy cause significant energy savings when P1 rather than molecular sieves are used. Although this comparison is an approximation and not for optimized systems, it highlights the advantage of using switchable drying agents in terms of the lower energy required for regeneration.
To evaluate the ability of these agents to dry isobutanol having a lower initial water content, two further experiments were performed using isobutanol doped with less water. P-1 was able to reduce the water content of solutions containing 1 wt% H2O, to a value of 0.8 wt%, but could not remove water from solutions containing only 0.5 wt% H2O. The lower limit for the P-1 to dewater isobutanol is therefore slightly less than 1 wt% H2O but not as low as 0.5 wt% H2O. Presumably, increasing the basicity of the functional group, such as by using amidine groups, would increase the ability of the drying agent to dry solvents to a lower level of residual water.
An important internal measure of performance for a switchable drying agent is the amount of water (mmolw) removed per accessible amine (mmolaa). We introduce the concept of a Molar Hydration Value (mmolw/mmolaa), which allows for a quantitative determination of the amount of water captured per protonatable site. The molar hydration value of the drying agents varied greatly depending on the synthetic strategy employed (Table 3).
| Drying agent | Accessible amines (mmol g−1) | Molar hydration valueb (mmolW/mmolAA) | |
|---|---|---|---|
| Standard procedurea | Modified procedurec | ||
| a Reaction conditions: 10 g isobutanol with water at a concentration of 5 wt%, 0.5 g drying agent added, 1 h mixing with CO2 bubbling through solution then continued mixing in a sealed vial for 15 h, water content analyzed by GC-TCD. Drying agent regeneration was performed at 50 °C for 4 h. b mmol of water removed per mmol of amine. c An increased bubbling time of 3 h and continued mixing under 1 atm of CO2(g) was employed. d An increased reaction time of 18 h was employed. | |||
| DMA-S | 1.1 | 9 | — |
| P-1 | 2.6 | 6 | 8c |
| PGS-1 | 0.3 | 90 | 110d |
| PGS-2 | 0.4 | 75 | 80d |
The commercially available amine functionalized silica particles (DMA-S) as well as the synthesized polymer particles (P-1) behave predictably. Their molar hydration value can be attributed to the previously proposed three ways in which water can bind to a switchable drying agent (Fig. 1). One equivalence of water is captured stoichiometrically in the formation of the bicarbonate salt as shown in eqn (1). The remaining water is bound as waters of hydration around the bicarbonate salt or by adhesion to the support.
The polymer-grafted silica drying agents have molar hydration values that are an order of magnitude greater than P-1 or the commercially available functionalized silica. The hydration model proposed in Fig. 1 does not adequately account for the molar hydration values of the polymer-grafted silica drying agents (PGS-1 & 2), suggesting that the polymeric nature of the amine functionalities plays a crucial role in the hydration of the particle. In one example of polymeric hydration, thermo-responsive poly(N-isopropylacrylamide) (PNIPAAm) and pH-responsive poly(N,N′-diethylaminoethyl methacrylate) (PDEAEMA) polymers were grafted to carboxymethylchitosan (CMC) to form highly water swellable hydrogels with dual responsive properties.16 The hydrogels showed an enhancement in water swellability when grafted with PDEAEMA. The increase in the bulk hydrophilicity of the hydrogel was attributed to the presence of tertiary amino groups in the DEAEMA unit. With agreement to that observation we could hypothesize that the polymeric amines (PDMAPMAm) increase the hydrophilicity of the silica particle by behaving as a hydrogel, therefore capturing more water. We can estimate the water content of the polymeric hydrogel. PGS-1 was determined to be 7.5% polymer by thermogravimetric analysis (Fig. S7 and S8†). If we account for the consistent water uptake of silica (150 mg g−1) and remove it from the equation, 340 mg g−1 of water uptake can be attributed to the polymer. The polymer is therefore able to absorb 82% water by weight. If we look at PGS-1 as a single entity, it can absorb 33% water by weight. In contrast, the CMC grafted PDEAEMA, PNIPAAm co-polymer absorbed 79% water by weight.16 In another study, various free polymers were tested via a comparable method yielding water uptake values ranging from 0.06% (neutral PMMA) to 88% (P(AA) sodium salt).17 With all factors considered, these values indicate hydrogel behavior on the part of the polymer-grafted silica drying agents. This behavior would adequately explain why polymer-grafted silica drying agents have molar hydration values that are an order of magnitude greater than P-1 or the commercially-available amine-functionalized silica. The hydrogel-like nature, and therefore the performance of the polymer-grafted silica drying agents could be improved by increasing the molecular weight of the grafted polymer, thereby increasing the polymer to silica w/w ratio.
The polymer-grafted silica (PGS) particles are also able to dry other solvents (Table 4), although problems are encountered if the solvent is too volatile or is essentially nonvolatile. CO2 was introduced at 5 psi gauge pressure via a needle into a vented vial containing wet solvent, PGS, and a stir bar rotating at 400 rpm. The combination of stirring and CO2 bubbling caused significant solvent loss with ethanol; adding a condenser for the second cycle prevented solvent loss. Retention of ethylene glycol during the particle regeneration step, because that solvent is nonvolatile, caused complete loss of drying performance in subsequent steps. The particles and the drying and regeneration processes would have to be optimized for each solvent individually.
| Solvent | Water removedb (mg g−1) | |
|---|---|---|
| Cycle | ||
| 1 | 2 | |
| a Reaction conditions: 10 g solvent with water at a concentration of 5 wt%, 0.5 g drying agent added, 1 h mixing with CO2 bubbling through solution then continued mixing in a sealed vial for 15 h, water content analyzed by GC-TCD. Drying agent regeneration was performed at 50 °C for 4 h. b Water removed per g of drying agent used. c Without a condenser during the first cycle, with a condenser during the second cycle. | ||
| Isobutanol | 560 | 520 |
| Acetonitrile | 380 | 380 |
| Ethanolc | 220 | 280 |
| Ethylene glycol | 380 | <70 |
Whether these drying agents are greener than current methods is a complex question that can only be resolved by a life cycle assessment of the optimized new process versus the current practice in industry. Until that is possible, we offer instead the following list of advantages and disadvantages of the PGS switchable drying agents relative to the common industrial practice of using non-recyclable salts as drying agents,18 which is the technology we hope to displace. While the synthesis of the particles is likely more environmentally damaging than the synthesis of simple inorganic salts, the impact of the former is amortized over many usages while the latter, because those drying agents are not reusable, is not spread over multiple usages. For example, the use of KOH or K2CO3 in the synthesis of the recyclable drying agent is unlikely to be nearly as damaging as the use of non-recyclable drying agents such as MgSO4, K2CO3, or CaCl2. The other potential concern with the drying agents is the slow release of free amine if the amide linkage in the PGS particles were to slowly hydrolyze. Since polymers are not bioavailable there should be no concern regarding toxicity of the polymer itself but hydrolysis could slowly release low concentrations of N,N-dimethylaminopropanamine; this amine, which is commonly used in cosmetics as a pH-adjuster, has an LD50 of 1857 mg kg−1 (male rat, oral), has no mutagenicity, carcinogenicity, reproductive inhibition or developmental toxicity effects, but can cause dermal sensitization.19 Therefore, apart from possible dermal sensitization, the risk is believed to be low.
Conclusions
In summary, CO2-switchable drying agents have been created for the first time and were evaluated in the drying of wet isobutanol. Through the application of CO2 to suspensions of drying agent, the water content of the isobutanol could be reduced by 490 mg per gram of drying agent. This value exceeds the values obtained for commercial drying agents such as silica or alumina. The closest commercial competitor was molecular sieves at 380 mg of water removed per gram of drying agent; however, the molecular sieves could not be regenerated under the very mild conditions employed for the switchable materials. Nonetheless, molecular sieves are superior in terms of their ability to dry wet solvent to very low concentrations of water. All of the switchable drying agents were superior to normal silica and alumina in terms of the quantity of water removed per g of drying agent. The synthesized switchable drying agents (P1, PGS-1, PGS-2) were superior to the commercially amine-functionalized silica (DMA-S) in terms of drying ability and hydrolytic stability at low pH. The polymer-grafted silica drying agents (PGS-1 & PGS-2) capture 10× more water per amine (mmolW/mmolAA) than the other amine-containing CO2-switchable drying agents.We also reported a new method for the measurement of accessible amine content on surfaces.
Acknowledgements
The authors thank Prof. Daniel W. Armstrong (University of Texas at Arlington) for providing the GCTCD column and Fielding Chemical Technologies Inc. for guidance and funding. We also thank the Ontario Centres of Excellence, Mitacs and the Natural Science and Engineering Research Council for funding.Notes and references
- J. E. Logsdon, in Kirk Othmer Encyclopedia of Chemical Technology: Ethanol, [online]; John Wiley & Sons, Inc., posted online June 18, 2004. http://onlinelibrary.wiley.com/doi/10.1002/0471238961.0520080112150719.a01.pub2/pdf (accessed November 2013) Search PubMed.
- R. French and P. Malone, Fluid Phase Equilib., 2005, 228–229, 27 CrossRef CAS.
- W. L. F. Armarego and C. L. L. Chai, Purification of Laboratory Chemicals, Butterworth-Heinemann, New York, 2003 Search PubMed.
- P. G. Jessop, S. M. Mercer and D. Heldebrant, Energy Environ. Sci., 2012, 5, 7240 CAS.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- Y. Liu, P. G. Jessop, M. F. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958 CrossRef CAS PubMed.
- X. Su, P. G. Jessop and M. F. Cunningham, Macromolecules, 2012, 45, 666 CrossRef CAS.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schower, C. Sugnaux, S. Tugulu and H. A. Klok, Chem. Rev., 2009, 109, 5437 CrossRef CAS PubMed.
- S. Kumar, X. Tong, Y. L. Dory, M. Lepage and Y. Zhao, Chem. Commun., 2013, 49, 90 RSC.
- V. F. Kalasinsky, S. Subramaniam, C.-F. Su and R. L. Cook, J. Mol. Struct., 2000, 550–551, 52 Search PubMed.
- W. F. Beech, J. Chem. Soc., 1951, 2483 RSC.
- K. O. Ojo, L. V. Golovko, Y. P. Gomza and A. N. Vasiliev, Silicon, 2012, 4, 189 CrossRef CAS.
- S. Ong, X. Zhao and K. Eisenthal, Chem. Phys. Lett., 1992, 191, 327 CrossRef CAS.
- P. van de Wetering, E. E. Moret, N. M. E. Schuurmans-Nieuwenbroek, M. J. van Steenbergen and W. E. Hennink, Bioconjugate Chem., 1999, 10, 589 CrossRef CAS PubMed.
- J. J. Kirkland, J. L. Glajch and R. D. Farlee, Anal. Chem., 1989, 61, 2 CrossRef CAS.
- N. Rodkate, B. Rutnakornpituk, U. Wichai, G. Ross and M. Rutnakornpituk, J. Appl. Polym. Sci., 2014, 132, 41505 Search PubMed.
- H. M. L. Thijs, C. R. Becer, C. Guerrero-Sanchez, D. Fournier, R. Hoogenboom and U. S. Schubert, J. Mater. Chem., 2007, 17, 4864 RSC.
- I. M. Smallwood, Solvent Recovery Handbook, Blackwell Science, Oxford, 2nd edn, 2002 Search PubMed.
- “3-Dimethylaminopropylamine”, report prepared for the National Cancer Institute by Technical Resources Int. Inc. under contract no. N02-CB-07007, http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/dimethylaminopropylamine_508.pdf, accessed August 2015.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including NMR spectra, light scattering analysis for the porous polymer particles, N2 isotherm for the porous polymer particles, TGA of the porous polymer particles, and polymer-grafted silicas (PGS1 and PGS2), solid state NMR for the initiator grafted silicas and NMR spectrum for the analysis of isobutanol retained on the porous polymer particles. See DOI: 10.1039/c5gc01201e |
| This journal is © The Royal Society of Chemistry 2016 |