Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Food processing and structure impact the metabolizable energy of almonds†‡
Sarah K.
Gebauer
a,
Janet A.
Novotny
a,
Gail M.
Bornhorst
b and
David J.
Baer
*a
aUS Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Building 307B, Room 213, BARC-East, Beltsville, MD 20705, USA. E-mail: David.Baer@ars.usda.gov; Fax: +1-301-504-9098; Tel: +1-301-504-8719
bBiological and Agricultural Engineering, University of California, Davis, 3056 Bainer Hall, 1 Shields Avenue, Davis, CA 95616, USA
First published on 28th September 2016
Abstract
The measured metabolizable energy (ME) of whole almonds has been shown to be less than predicted by Atwater factors. However, data are lacking on the effects of processing (roasting, chopping or grinding) on the ME of almonds. A 5-period randomized, crossover study in healthy individuals (n = 18) was conducted to measure the ME of different forms of almonds (42 g per day), as part of a controlled diet: whole, natural almonds; whole, roasted almonds; chopped almonds; almond butter; and control (0 g per day). After 9 days of adaptation to each diet, participants collected all urine and fecal samples for 9 days. Diets, urine, and feces were analyzed to determine ME. Fracture force and fracture properties of whole and chopped almonds were measured. Measured ME (kcal g−1) of whole natural almonds (4.42), whole roasted almonds (4.86), and chopped almonds (5.04) was significantly lower than predicted with Atwater factors (P < 0.001); ME of almond butter (6.53 kcal g−1) was similar to predicted (P = 0.08). The ME of whole roasted and chopped almonds was lower than almond butter (P < 0.0001). ME of whole natural almonds was lower than whole roasted almonds (P < 0.05). This may be due to lower hardness of whole roasted (298 ± 1.3 N) compared to whole natural almonds (345 ± 1.6 N) (P < 0.05), and to whole natural almonds fracturing into fewer, larger particles, thus inhibiting the release of lipids. Atwater factors overestimate the ME of whole (natural and roasted) and chopped almonds. The amount of calories absorbed from almonds is dependent on the form in which they are consumed.
Introduction
Nut consumption has numerous beneficial effects on risk factors for cardiovascular disease (CVD).1 Recent studies also have shown benefits on other endpoints, including mortality from coronary heart disease, cancer, stroke, diabetes, and metabolic syndrome.2–4 Despite this evidence, nuts have traditionally been excluded from weight loss diets because of the belief that their energy and fat content will promote weight gain; however, epidemiological and clinical data suggest otherwise.5–7 In a recent analysis of NHANES data, tree nut consumption in U.S. adults was associated with lower body mass index (BMI) and waist circumference, as well as lower risk of CVD and better nutrient adequacy and diet quality.8,9Previous studies with nuts have demonstrated that food form impacts macronutrient absorption. Absorption of fat from whole nuts and peanuts is less than that for other forms of nuts, including butter, oil, and flour,10,11 suggesting that the food matrix impacts metabolizable energy (ME) of food. Recent research has focused on several aspects of the physiochemical nature of the almond seed matrix in order to understand the dynamics of food digestion and release of energy. Using in vitro digestion models, Grundy et al. have demonstrated that intact almond cell walls encapsulate lipid which prevents diffusion of lipase into the intracellular space and thus inhibits lipolysis.12 Unless these cell walls are disrupted, lipid digestion is limited. From in vitro duodenal digestion models, Grundy also has shown that particle size has an impact on lipid bioaccessibility.13 Despite the fact that roasting almonds results in smaller particles when masticated,14,15in vitro digestion models (gastric and duodenal) do not show differences in lipid bioaccessibility.15 Nonetheless, the observed in vivo differences in lipemic response of different forms of almonds11,16 suggest that the ME of almonds processed by different methods may be different, which may impact the accurate labeling of food.
The Atwater general factors and Atwater specific factors, developed in the late 1800s and 1950s, respectively, are still the predominantly used methods to calculate the energy content of foods in nutrient databases and on food labels. We have previously shown that Atwater factors overestimate the measured ME of whole nuts (pistachios, almonds), and walnuts pieces, due to incomplete absorption of macronutrients.17–19 However, the measured ME of other forms of nuts is unknown. The objective of the present study was to determine the impact of food structure and processing (roasting, chopping or grinding) on the measured ME of almonds, within the context of a completely controlled diet.
Materials & methods
Study design
The study was a randomized, crossover, controlled-feeding trial consisting of five 3-week diet periods, each separated by a 1-week break. Participants were fed the following controlled diets consisting of a base diet (typical American diet) and varying forms of almonds: whole, natural almonds (42 g d−1); whole, dry roasted almonds (42 g d−1); chopped almonds (dry roasted, 42 g d−1); almond butter (dry roasted, 42 g d−1), and control (0 g d−1). After 9 days of adaptation to each diet, participants collected all urine and fecal samples for 9 days. Study procedures were approved by the Institutional Review Board of the MedStar Health Research Institute, and were in compliance with the principles of the Declaration of Helsinki.Recruitment & participant selection
Participants were recruited from the Washington, DC metropolitan area and required to attend a study information meeting. At that meeting, details and requirements of the study were reviewed and interested individuals completed a study application, provided written informed consent, and were subsequently screened for the study. Screening was conducted following a 12-hour fast and included measurement of height, weight, and blood pressure; collection of blood for lipid profile, comprehensive metabolic panel, complete blood count, thyroid stimulating hormone, and urine for urinalysis; and collection of health history information. Eligibility was determined by a study physician or nurse practitioner. Participants were required to meet the following criteria: age 25–75 years, body mass index (BMI) between 20 and 38 kg m−2, fasting glucose <126 mg dl−1, blood pressure <160/100 mm Hg, fasting total blood cholesterol <280 mg dl−1, and fasting triglycerides <300 mg dl−1. Participants were excluded if they had any of the following characteristics or conditions: presence of type 2 diabetes, kidney disease, liver disease, gout, hyperthyroidism, untreated or unstable hypothyroidism, certain cancers, gastrointestinal disease, pancreatic disease, and other metabolic diseases or malabsorption syndromes; pregnant or lactating women, or women who gave birth during previous 12 months; history of bariatric or certain other surgeries related to weight control; use of tobacco products or smokers (during previous 6 months); history of eating disorders or other dietary patterns not consistent with the dietary intervention, including vegetarians; loss of 10% of body weight during previous 12 months; or allergy to almonds or other nuts.Study participants were randomized to a treatment sequence consisting of each of the 5 diet periods. The randomization scheme was created using a permutation calculator and random number generator. There were 18 sequences that were selected and checked for balance of treatment by period.
Controlled diet
Participants were fed a completely controlled diet for each of the five 3-week diet periods. Participants came in to the Human Studies Facility at the USDA Beltsville Human Nutrition Research Center (BHNRC) in Beltsville, MD for both breakfast and dinner, Monday through Friday, where meals were administered under the supervision of study personnel. Lunch and weekend meals were packed for offsite consumption. Participants were instructed to consume all of the foods provided to them and to not consume any outside food during the 5 diet periods. Outside beverages that were permitted included unlimited water and unlimited calorie free lemonade and diet soda (in the varieties provided). Coffee and tea were limited to no more than 2 cups per day, in the varieties provided. Consumption of alcoholic beverages was not permitted during the diet periods.Diets were designed by a study dietitian using a 7-day menu rotation. The control diet consisted of the base diet, designed as a typical American diet (31% fat, 16% protein, 53% carbohydrate), and 0 g d−1 of almonds. All foods were identical between the control and almond diets, except for the almonds. This was achieved by proportionately reducing the amount of every food on the base diet to allow for the isocaloric inclusion of 42 g d−1 almonds.
All of the almonds (nonpareil variety) fed in the study were provided by the Almond Board of California and originated from the same lot (purchased from Hughson Nut, Inc.). The only difference in the almonds across treatments was whether they remained as whole natural almonds or whether they were dry roasted (154–157° C, 9 min roasting time), and either packaged as whole roasted almonds, chopped (particle sizes of 8.7 & 3.2 mm corresponding to industry standard of medium 22/8), or ground into almond butter (ground to smooth consistency by a commercial processor, Maisie Jane's California Products, Inc., with no additives). The dose of 42 g (1.5 oz) was selected based on the U.S. Food & Drug Administration qualified health claim for nuts. Half of the daily dose of almonds was included at breakfast and half of the dose was included at dinner, so that nuts were consumed while participants were at the facility.
Participants were assigned to an individual calorie level using the Harris Benedict equation, based on an age, height, weight, and activity level. Body weight was measured daily, Monday through Friday, and was closely monitored throughout the intervention. Adjustments in calorie level were made in 200 kcal increments if participants exhibited changes in body weight. Adherence to the diets was assessed by daily interaction with participants, monitoring of body weight, and review of daily questionnaires. Daily questionnaires collected information regarding general health, medications, and exercise; amounts of approved beverages consumed (diet soda, calorie free lemonade, coffee, and tea); documentation of any non-study foods; and verification that all study foods were consumed.
Sample collection & analysis
Diet composites, consisting of a complete 7-day menu cycle, were created during each diet period, as previously described.20 Briefly, foods were prepared as typically consumed (heated, toasted, etc.) and blended in a blender. Diet composites were frozen at −80C and then freeze dried for chemical analysis.Urine was collected in 24 h cycles for 7 days of each diet period during which participants were instructed to collect all urine samples. Participants were provided daily collection supplies, including a 4 L container with boric acid (10 g), an overflow container if needed, and a collection form to record dates/times of collection, any missed samples, and use of medication.
Participants were administered a capsule containing Brilliant Blue to mark the start of the fecal collections. Supplies for fecal collections included a large Styrofoam cooler with dry ice, plastic collection bags, a collection apparatus, and a gym bag to transport samples. Participants were instructed to collect all fecal samples and store them on dry ice until their next visit to the BHNRC. A second capsule of Brilliant Blue was given after 7 days of collection and participants continued collecting until instructed by the study staff to stop. Appearance of the second marker in the feces indicated the end of the collection phase. All samples collected between the appearance of the first and second marker were included for analysis. Daily collection forms were completed with date and time of sample and whether any samples were missed.
Details of chemical analysis have been previously published.18 Briefly, adiabatic bomb calorimetry (Parr Instrument Company; Moline, IL) was used to measure energy of diet, feces, and urine. Samples were analyzed in duplicate. ME was determined by the methods of Novotny et al.,18 using the following equation:
Almond physical property determination
| Carea = 1 − e−(X/X50)b·ln2 |
Particle size distribution of almond butter
Particle size distribution analysis of almond butter was completed using a Mastersizer 2000 (Malvern Instruments Ltd, Malvern, UK), which uses static light scattering to measure the particle size distribution in an emulsion or suspension. The refractive index used was 1.59. To accurately measure the almond particle fragments in the almond butter, isopropanol was used as a dispersant, and samples of almond butter were mixed with isopropanol prior to loading into the measurement chamber (10–15% obscuration) at a pump rate of 2500 rpm. Each sample was measured ten times. Six measurements of four jars of almond butter were completed, resulting in a total of 24 measurements.Statistical analysis
The ME values of different forms of almonds were compared using a mixed model analysis (SAS version 9.3, SAS Institute Inc., Cary, NC) with contrast statements for the following a priori defined comparisons: whole natural almonds versus whole roasted almonds; whole roasted almonds versus chopped almonds (roasted); whole roasted almonds versus almond butter (roasted); and chopped almonds (roasted) versus almond butter (roasted). The model included subject as a random term, and effects of treatment, period, and interaction of treatment × period were tested. A paired t-test was used to compare the measured ME and predicted ME for each of the almond forms. One-way ANOVA using the Tukey Studentized Range (HSD) test was used to analyze differences between natural and roasted almond fracture force, and to analyze differences between whole natural, whole roasted, and roasted chopped fracture properties. Significance was determined based on P < 0.05. Values are expressed as mean ± SEM.Results
Baseline characteristics of the participants (n = 18; 10 M, 8F) were as follows: age = 56.7 ± 2.4 years; height = 170.2 ± 2.1 cm; and body weight = 88.6 ± 5.6 kg. Participants were adherent to the protocol, as indicated by daily questionnaires, collection forms, and daily weekday interactions with participants during breakfast and dinner. The macronutrient composition of the base diet and 4 almond treatments is presented in Table 1. The base diet provided 16%, 25%, and 59% of energy from protein, fat and carbohydrate, respectively, and the diets with almonds provided 15%, 30%, and 55% of energy from protein, fat, and carbohydrate, respectively.| Base diet | Whole, natural almonds | Whole, roasted almonds | Chopped, roasted almonds | Almond butter (from roasted almonds) | |
|---|---|---|---|---|---|
| a Values are shown as mean ± standard deviation (n = 2 to 6 samples). b Protein was determined as N × 5.18 for the almonds and as N × 6.25 for the base diet. c Carbohydrate was calculated by difference. | |||||
| Proteinb | 20.4 ± 0.2 | 20.7 ± 0.1 | 21.8 ± 0.1 | 20.8 ± 0.1 | 19.6 ± 0.1 |
| Lipid | 14.4 ± 0.2 | 57.2 ± 0.7 | 55.6 ± 0.3 | 54.5 ± 0.7 | 56.1 ± 0.5 |
| Ash | 3.68 ± 0.10 | 3.12 ± 0.05 | 3.07 ± 0.09 | 2.91 ± 0.13 | 2.92 ± 0.11 |
| Carbohydratec | 61.5 | 19.0 | 19.5 | 21.8 | 21.4 |
Fracture force of almonds was significantly influenced by almond type (P < 0.0001). The average fracture force was significantly greater for natural almonds (70.0 ± 2.9 N) compared to roasted almonds (52.0 ± 2.2 N) (P < 0.05, Table 2). Hardness of whole natural, whole roasted and roasted chopped almonds was significantly influenced by almond type (P < 0.01). Whole natural almonds had a greater hardness (345 ± 1.6 N) compared to whole roasted almonds (298 ± 1.3 N) (Table 2). Chopped roasted almonds had an intermediate hardness (310 ± 1.4 N), which was not different from either whole natural or whole roasted almonds (Table 2).
| Almond type | Fracture force (N) | Hardness (N) |
|---|---|---|
| a Fracture force was quantified by the three-point bending test on a whole almond kernel, and could not be completed on chopped samples. Hardness was quantified by uniaxial compression of one almond kernel or 1 g of chopped almonds. One-way ANOVA using the Tukey Studentized Range (HSD) test was used to analyze differences between treatment almonds. Values are shown as mean ± standard error (n = 25). Values within each column with different letters are significantly different (P < 0.05). | ||
| Whole, natural almonds | 70.0 ± 2.9a | 345 ± 1.6a |
| Whole, roasted almonds | 52.0 ± 2.2b | 298 ± 1.3b |
| Chopped, roasted almonds | — | 310 ± 1.4ab |
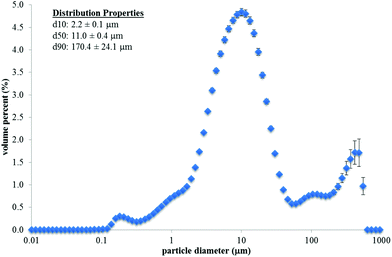
The fracture properties of whole natural, whole roasted, and chopped roasted almonds are shown in Table 3, and example images of one almond (or 1 g chopped almonds) after compression are shown in Fig. 1. The Rosin–Rammler model provided a good fit to the experimental data (R2 = 0.99–1.00). The x50, b, and number of particles per image were all influenced by almond type (P < 0.001). Whole natural almonds had a greater x50 and b value, but a lower number of particles per image compared to whole roasted almonds (P < 0.05). This indicates that as the whole natural almonds were fractured, they broke into fewer, larger pieces compared to whole roasted almonds. The chopped roasted almonds had a larger median particle area (x50) but a fewer number of particles per image compared to the whole roasted almonds (P < 0.05). The particle size distribution of almond butter (as consumed) is shown in Fig. 2. The median particle diameter (d50) was 11.0 ± 0.4 μm. The 90th percentile particle diameter (d90) was 170.4 ± 24.1 μm.
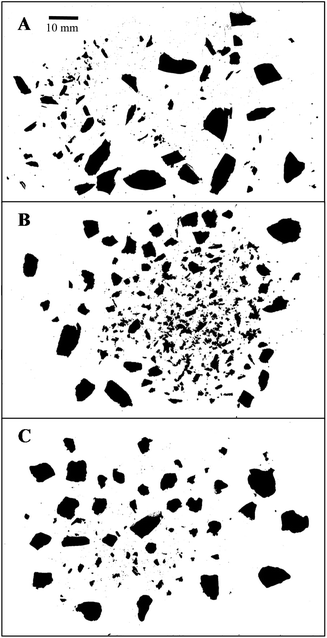 | ||
| Fig. 1 Example binary images from the fracture of (A) one whole natural almond, (B) one whole roasted almond, or (C) 1 g chopped roasted almonds, after compression at 30 mm s−1 to 71% strain. | ||
| Almond type | x 50 (mm2) | b (dimensionless) | Number of particles/image | R 2 |
|---|---|---|---|---|
| a Values represent mean ± standard error (n = 25). The R2 value represents the average goodness of fit of the Rosin–Rammler distribution to the experimental data sets. One-way ANOVA using the Tukey Studentized Range (HSD) test was used to analyze differences between treatment almonds. Values within each column with different letters are statistically different (P < 0.05). | ||||
| Whole, natural almonds | 27.6 ± 1.7a | 1.1 ± 0.10a | 137 ± 11c | 0.99 |
| Whole, roasted almonds | 13.7 ± 0.4c | 0.9 ± 0.05a | 339 ± 10a | 1.00 |
| Chopped, roasted almonds | 17.5 ± 0.5b | 1.1 ± 0.08a | 185 ± 8b | 1.00 |
The measured ME of whole natural almonds was lower than whole roasted almonds (P < 0.05) (Table 4). When comparing the different forms of roasted almonds, the ME of whole and chopped almonds was lower than that of almond butter (P < 0.0001). The ME of whole roasted almonds was not statistically different from chopped roasted almonds (P > 0.05).
| Almond type | Measured MEa | Estimated ME using atwater general factorsb | Estimated ME using atwater specific factorsb |
|---|---|---|---|
| Metabolizable energy, ME.a Values presented are mean ± standard error (n = 18). Values within each column with different letters are statistically different (P < 0.05). Mixed model analysis was used to compare ME of different forms of almonds, using contrast statements for the following a priori defined comparisons: whole natural almonds versus whole roasted almonds; whole roasted almonds versus chopped almonds (roasted); whole roasted almonds versus almond butter (roasted); and chopped almonds (roasted) versus almond butter (roasted).b Differences in measured metabolizable energy and estimated metabolizable energy were determined by paired t-tests. Estimated metabolizable energy using Atwater General Factors represents the calculated metabolizable energy based on Atwater general factors and the measured macronutrient composition of each almond form. Estimated metabolizable energy using Atwater specific factors represents the calculated metabolizable energy based on Atwater specific factors and the measured macronutrient composition of each almond form.c Statistically different compared with measured metabolizable energy (P < 0.05). | |||
| Whole, natural almonds | 4.42 ± 0.24a | 6.34c | 5.91c |
| Whole, roasted almonds | 4.86 ± 0.24b | 6.44c | 6.01c |
| Chopped, roasted almonds | 5.04 ± 0.20b | 6.47c | 6.04c |
| Almond butter | 6.53 ± 0.19c | 6.62 | 6.18 |
Based on the chemical analysis of each almond treatment (Table 1), the estimated ME (kcal g−1), calculated with Atwater general factors, values of the energy content of macronutrients based on general values for heats of combustion and digestibility, of whole natural almonds, whole roasted almonds, chopped almonds, and almond butter was 6.34, 6.44, 6.47, and 6.62, respectively. When using Atwater specific factors, values of the energy content of macronutrients based on heats of combustion and digestibility of different classes of foods, the estimated ME (kcal g−1) was 5.91, 6.01, 6.04, and 6.18, respectively. The measured ME (kcal g−1) for whole natural almonds (4.42), whole roasted almonds (4.86), and chopped almonds (5.04), was significantly lower than that estimated by Atwater general or specific factors (P < 0.001; Table 4). The Atwater general factors overestimated ME by a greater extent than Atwater specific factors. The percent difference between the measured ME and the estimated ME value using the Atwater specific factors was −25%, −19%, and −17%, respectively for the whole natural almonds, whole roasted almonds, and chopped roasted almonds. The measured ME of almond butter (6.53 kcal g−1) was similar to that predicted when using Atwater general or specific factors (P > 0.05 for both).
Discussion
Overestimation of the ME of nuts may explain, in part, results from epidemiological and clinical studies that show that individuals who consume nuts have a lower body weight,27,28 are less likely to gain weight over time,6,29 and lose more weight on a calorie restricted diet,7 compared to those who do not consume nuts. There are numerous potential mechanisms, related to the unique nutrient profile of nuts, which may be contributing to their effects on body weight.30 Nuts are low in saturated fat, and high in unsaturated fat, plant protein, and fiber, as well as numerous vitamins, minerals and other bioactive compounds. In randomized controlled trials, where diets are tightly controlled and not self-selected, incomplete mastication leads to an increase in macronutrient loss in the feces, which results in lower energy available to the body. In addition to incomplete absorption, in free-living studies where diets are self-selected, nut consumption leads to increases in protein and fiber intake, which results in increased satiety/decreased hunger and a reduction in energy intake.As is common with all plant cells, the energy containing macronutrients contained in each almond cell is surrounded by a cell wall. Disruption of the cell wall can occur by mechanical means, either during mastication, processing, chopping or grinding, or by bacterial fermentation in the colon (after the site of absorption). Previous studies with almonds have shown that incomplete rupturing of cell walls during mastication of almond seed tissue results in a large proportion of intact cells.31 The energy containing macronutrients encapsulated in these intact cells remain inaccessible to digestive enzymes, and are excreted in the feces, such that they are not absorbed. Studies have demonstrated that particle size, an indicator of the proportion of unruptured cells, is related to the bioaccessibility of the macronutrients contained in almonds.14,16 Particle size of almonds decreases as the degree of mastication increases (due to greater cell wall disruption), resulting in less fecal fat excretion and higher energy absorption.
Processing of nuts, such as roasting, chopping, and grinding, impacts mastication, particle size, and lipid bioaccessibility;11,14,32 however, the ME of different forms of almonds has not been previously determined. In the present study, the dry roasting of whole almonds resulted in a higher ME compared with natural almonds. Dehydration during the roasting process causes almond tissue to be more brittle, leading to a higher proportion of smaller particles (and lower proportion of large particles) and increases in energy absorption.14
In the present study, whole natural almonds had a greater fracture force and greater hardness compared to whole roasted almonds. Although the fracture force (52 to 70 N) for either type of almonds is much less than the average bite force exerted by the teeth in adult humans (630 N for males and 424 N for females),33 it may play a role in the fracture properties and resulting particle size distribution during chewing.
Fracture properties of whole natural and roasted almonds were determined after compression of a single almond kernel, as this method has been shown to provide similar results to the particle size after in vivo mastication of almonds.21 After fracture, whole roasted almonds had a greater number of particles with a smaller median particle area (Table 3, Fig. 1) compared to whole roasted almonds. This may indicate that during mastication, whole roasted almonds are broken down to a greater degree. As previously reported, smaller particles result in greater lipid release from the almond cell walls.16,34 Since the whole roasted almonds had smaller particles, a greater amount of lipids may have been released, resulting in greater ME compared to whole natural almonds.
The ME of whole roasted and chopped almonds was lower than almond butter, which is likely due to extensive cell wall disruption during the grinding of almonds into butter. The median particle size of the almond butter was 11 μm. Since the almond butter already had a large particle size reduction during grinding, there was little additional breakdown required during mastication and digestion. This extensive cell wall disruption and small particle size may have allowed more energy to be released from the cells and absorbed by the body compared to the chopped and whole almonds, resulting in the higher observed ME value. The ME of almond butter was similar to what is predicted by the Atwater factors.
The ME of whole, roasted almonds was not statistically different than chopped almonds. The median particle area after fracture was in a similar range for whole and chopped roasted almonds, although the values were significantly different (13.7 vs. 17.5 mm2). However, both of these values were significantly lower than the median particle area for whole natural almonds (27.6 mm2). This may indicate that during roasting, regardless of whether the almonds are whole or chopped, particles break down to a similar degree, which results in a similar ME value.
Variability of ME ranged from 2.84 kcal g−1 to 7.66 kcal g−1 across the almond forms. Individual differences in mastication patterns likely contribute to the variability in ME. Instruction was not provided with regards to chewing so that the mastication of the different almond forms was representative of typical consumption. Variability in individual responses differed based on almond form (data not shown), suggesting that differences in mastication patterns have less of an impact on ME when almonds are ground into butter than when they are consumed whole (i.e., cell walls are already ruptured during grinding and energy is more available regardless of differences in mastication). Individual differences in intestinal microbiota also may contribute to variability of ME. Future studies are warranted to determine whether differences in microbiota explain differences in ME across individuals, which could have broader implications with regards to energy intake and body weight of individuals.
Conclusions
The results from the present study demonstrate that processing and structure impact the ME of almonds. The Atwater specific factors overestimate the ME of whole natural, whole roasted, and chopped roasted almonds by 25%, 19%, and 17%, respectively. These differences are likely related to the hardness and fracture properties of the almond matrix and the degree of food structural breakdown during processing and mastication. Additional studies are warranted to further investigate the impact of food matrix and processing on the metabolizable energy of foods.Conflict of interest
There are no conflicts of interest to declare.Acknowledgements
Support was provided by the USDA Agricultural Research Service and the Almond Board of California. The authors would like to acknowledge Patrick Sullivan and the staff of the Human Studies Facility (BHNRC) for preparation and administration of the diets, and Theresa Henderson and the staff of the Food Components & Health Lab (BHNRC) for the processing and analysis of samples.References
- L. C. Del Gobbo, M. C. Falk, R. Feldman, K. Lewis and D. Mozaffarian, Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials, Am. J. Clin. Nutr., 2015, 102, 1347–1356 CrossRef CAS PubMed.
- G. Grosso, J. Yang, S. Marventano, A. Micek, F. Galvano and S. N. Kales, Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies, Am. J. Clin. Nutr., 2015, 101, 783–793 CrossRef CAS PubMed.
- E. Ros, Nuts and CVD, Br. J. Nutr., 2015, 113(Suppl 2), S111–S120 CrossRef CAS PubMed.
- M. Falasca, I. Casari and T. Maffucci, Cancer chemoprevention with nuts, J. Natl. Cancer Inst., 2014, 106 Search PubMed.
- M. Bes-Rastrollo, J. Sabate, E. Gomez-Gracia, A. Alonso, J. A. Martinez and M. A. Martinez-Gonzalez, Nut consumption and weight gain in a Mediterranean cohort: The SUN study, Obesity, 2007, 15, 107–116 CrossRef PubMed.
- D. Mozaffarian, T. Hao, E. B. Rimm, W. C. Willett and F. B. Hu, Changes in diet and lifestyle and long-term weight gain in women and men, N. Engl. J. Med., 2011, 364, 2392–2404 CrossRef CAS PubMed.
- M. A. Wien, J. M. Sabate, D. N. Ikle, S. E. Cole and F. R. Kandeel, Almonds vs complex carbohydrates in a weight reduction program, Int. J. Obes. Relat. Metab. Disord., 2003, 27, 1365–1372 CrossRef CAS PubMed.
- C. E. O'Neil, V. L. Fulgoni 3rd and T. A. Nicklas, Tree Nut consumption is associated with better adiposity measures and cardiovascular and metabolic syndrome health risk factors in U.S. Adults: NHANES 2005-2010, Nutr. J., 2015, 14, 64 CrossRef PubMed.
- C. E. O'Neil, T. A. Nicklas and V. L. Fulgoni 3rd, Tree nut consumption is associated with better nutrient adequacy and diet quality in adults: National Health and Nutrition Examination Survey 2005-2010, Nutrients, 2015, 7, 595–607 CrossRef PubMed.
- A. S. Levine and S. E. Silvis, Absorption of whole peanuts, peanut oil, and peanut butter, N. Engl. J. Med., 1980, 303, 917–918 CrossRef CAS PubMed.
- S. E. Berry, E. A. Tydeman, H. B. Lewis, R. Phalora, J. Rosborough, D. R. Picout and P. R. Ellis, Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects, Am. J. Clin. Nutr., 2008, 88, 922–929 CAS.
- M. M. Grundy, F. Carriere, A. R. Mackie, D. A. Gray, P. J. Butterworth and P. R. Ellis, The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds, Food Funct., 2015, 7, 69–78 Search PubMed.
- M. M. Grundy, P. J. Wilde, P. J. Butterworth, R. Gray and P. R. Ellis, Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis, Food Chem., 2015, 185, 405–412 CrossRef CAS PubMed.
- M. M. Grundy, T. Grassby, G. Mandalari, K. W. Waldron, P. J. Butterworth, S. E. Berry and P. R. Ellis, Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia, Am. J. Clin. Nutr., 2015, 101, 25–33 CrossRef CAS PubMed.
- G. Mandalari, M. M. Grundy, T. Grassby, M. L. Parker, K. L. Cross, S. Chessa, C. Bisignano, D. Barreca, E. Bellocco, G. Lagana, P. J. Butterworth, R. M. Faulks, P. J. Wilde, P. R. Ellis and K. W. Waldron, The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis, Br. J. Nutr., 2014, 112, 1521–1529 CrossRef CAS PubMed.
- B. A. Cassady, J. H. Hollis, A. D. Fulford, R. V. Considine and R. D. Mattes, Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response, Am. J. Clin. Nutr., 2009, 89, 794–800 CrossRef CAS PubMed.
- D. J. Baer, S. K. Gebauer and J. A. Novotny, Measured energy value of pistachios in the human diet, Br. J. Nutr., 2012, 107, 120–125 CrossRef CAS PubMed.
- J. A. Novotny, S. K. Gebauer and D. J. Baer, Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets, Am. J. Clin. Nutr., 2012, 96, 296–301 CrossRef CAS PubMed.
- D. J. Baer, S. K. Gebauer and J. A. Novotny, Walnuts Consumed by Healthy Adults Provide Less Available Energy than Predicted by the Atwater Factors, J. Nutr., 2016, 146, 1–8 CrossRef PubMed.
- S. C. Chen, J. T. Judd, M. Kramer, G. W. Meijer, B. A. Clevidence and D. J. Baer, Phytosterol intake and dietary fat reduction are independent and additive in their ability to reduce plasma LDL cholesterol, Lipids, 2009, 44, 273–281 CrossRef CAS PubMed.
- P. Varela, A. Salvador and S. Fiszman, On the assessment of fracture in brittle foods: The case of roasted almonds, Food Res. Int., 2008, 41, 544–551 CrossRef.
- G. M. Bornhorst, K. Kostlan and R. P. Singh, Particle Size Distribution of Brown and White Rice during Gastric Digestion Measured by Image Analysis, J. Food Sci., 2013, 78, E1383–E1391 CrossRef CAS PubMed.
- P. Rosin and E. Rammler, Gesetzmassigkeiten in der Kornzusammensetzung des Zementes, Zement, 1933, 31, 427–433 Search PubMed.
- S. C. Hutchings, K. D. Foster, J. E. Bronlund, R. G. Lentle, J. R. Jones and M. P. Morgenstern, Mastication of heterogeneous foods: Peanuts inside two different food matrices, Food Qual. Pref., 2011, 22, 332–339 CrossRef.
- A. van der Bilt, L. W. Olthoff, H. W. van der Glas, K. van der Weelen and F. Bosman, A mathematical description of the comminution of food during mastication in man, Arch. Oral Biol., 1987, 32, 579–586 CrossRef CAS PubMed.
- L. W. Olthoff, A. Van Der Bilt, F. Bosman and H. H. Kleizen, Distribution of particle sizes in food comminuted by human mastication, Arch. Oral Biol., 1984, 29, 899–903 CrossRef CAS PubMed.
- J. Sabate, Nut consumption and body weight, Am. J. Clin. Nutr., 2003, 78, 647S–650S CAS.
- R. C. Brown, S. L. Tey, A. R. Gray, A. Chisholm, C. Smith, E. Fleming and W. Parnell, Association of Nut Consumption with Cardiometabolic Risk Factors in the 2008/2009 New Zealand Adult Nutrition Survey, Nutrients, 2015, 7, 7523–7542 CrossRef CAS PubMed.
- M. Bes-Rastrollo, N. M. Wedick, M. A. Martinez-Gonzalez, T. Y. Li, L. Sampson and F. B. Hu, Prospective study of nut consumption, long-term weight change, and obesity risk in women, Am. J. Clin. Nutr., 2009, 89, 1913–1919 CrossRef CAS PubMed.
- C. L. Jackson and F. B. Hu, Long-term associations of nut consumption with body weight and obesity, Am. J. Clin. Nutr., 2014, 100(Suppl 1), 408S–411S CrossRef CAS PubMed.
- P. R. Ellis, C. W. Kendall, Y. Ren, C. Parker, J. F. Pacy, K. W. Waldron and D. J. Jenkins, Role of cell walls in the bioaccessibility of lipids in almond seeds, Am. J. Clin. Nutr., 2004, 80, 604–613 CAS.
- F. McKiernan and R. D. Mattes, Effects of Peanut Processing on Masticatory Performance during Variable Appetitive States, J. Nutr. Metab., 2010, 2010 Search PubMed.
- R. A. M. de Abreu, M. D. Pereira, F. Furtado, G. P. R. Prado, W. Mestriner Jr. and L. M. Ferreira, Masticatory efficiency and bite force in individuals with normal occlusion, Arch. Oral Biol., 2014, 59, 1065–1074 CrossRef PubMed.
- T. Grassby, D. R. Picout, G. Mandalari, R. M. Faulks, C. W. Kendall, G. T. Rich, M. S. Wickham, K. Lapsley and P. R. Ellis, Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls, Food Funct., 2014, 5, 3096–3106 CAS.
Footnotes |
| † Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). |
| ‡ USDA is an equal opportunity provider and employer. |
| This journal is © The Royal Society of Chemistry 2016 |