Open Access Article
Open Access ArticleThe effect of minimal dietary changes with raisins in NAFLD patients with non-significant fibrosis: a randomized controlled intervention†‡
Andriana C.
Kaliora
*a,
Alexander
Kokkinos
b,
Anastacia
Diolintzi
a,
Maria
Stoupaki
b,
Aristea
Gioxari
a,
Panagiotis T.
Kanellos
a,
George V. Z.
Dedoussis
a,
Jiannis
Vlachogiannakos
c,
Constantinos
Revenas
d,
Spiros D.
Ladas
b and
Vaios T.
Karathanos
ae
aDepartment of Dietetics and Nutritional Science, School of Health Science and Education, Harokopio University, 70 El. Venizelou Ave., 17671, Athens, Greece. E-mail: akaliora@hua.gr; andrianakaliora@gmail.com; Fax: +30 210 95 77 050; Tel: +30 210 95 49 226
bFirst Department of Propaedeutic and Internal Medicine, Laiko General Hospital, Athens University Medical School, 17 Agiou Thoma St., 11527, Athens, Greece
cAcademic Department of Gastroenterology, Athens University Medical School, Laiko General Hospital, Athens, 11527, Greece
dDepartment of Radiology, Laiko Hospital, Athens, Greece
eAgricultural Cooperatives Union Aeghion SA, Korinthou 201, 25 100, Aeghio, Greece
First published on 16th September 2016
Abstract
Aiming at investigating the potential effect of minimal dietary changes in NAFLD patients with non-significant fibrosis, 55 patients with NAFLD were enrolled in a randomized controlled clinical trial. Patients were assigned into two isocaloric dietary treatment groups for 24 weeks: (a) nutritional counseling (Control arm, N = 27), (b) nutritional counseling with currants included (two fruit servings, 36 g per day), substituting snacks of similar caloric content (Currant arm, N = 28). Clinical tests, anthropometrics, inflammatory and oxidative stress markers were conducted pre- and post-intervention. A total of 50 patients completed the trial. Significant differences between the two arms post-intervention were observed in fasting glucose and in IL-6 levels, these being significantly decreased only in Currant patients. Body weight, BMI, HbA1c, CRP and EUS values decreased in both arms, differences being insignificant between the two arms post-intervention. Participants in the Currant arm had significantly reduced total body fat, WC and trunk fat. Ultrasound scanning improved significantly in patients snacking currants daily. Also, volunteers enrolled in the Currant arm showed a reduced intake of saturated fatty acids. Because BW regulation has been officially recognised as a treatment approach in NAFLD an additional analysis was repeated in patients adhering to this. Post-intervention, the decrease in IL-6 and in fasting glucose was significantly higher in Currant patients who lost BW compared to their counterparts in the Control arm. Conclusively, minimal modifications in snacking choices, such as the inclusion of dried grapes in diet, are beneficial in NAFLD patients with non-significant fibrosis.
1 Introduction
Non-alcoholic fatty liver disease and steatohepatitis (NAFLD/NASH) is one of the most common liver disorders and a major public health problem worldwide.1 It includes a spectrum of liver injury beginning from simple steatosis to nonalcoholic steatohepatitis (NASH) that leads to advanced fibrosis and cirrhosis.2 The rising prevalence of NAFLD is closely related to the convergent epidemics of obesity, insulin resistance and type 2 diabetes, while it is considered the hepatic complication of obesity. The estimated worldwide prevalence of NAFLD is approximately 30% and this doubles within the type 2 diabetic population.3 The prevalence of NASH is approximately 7%, and this too is estimated to be at least two-fold higher among people with type 2 diabetes. Since obesity is a growing worldwide epidemic, the prevalence of NAFLD/NASH tends to increase ranging from 2.8% to 46%, depending on the study population and on the diagnostic tool.4,5 Pathogenesis is unclear with the most widely supported theory implicating insulin resistance (IR), which impairs lipid metabolism leading to fat accumulation within the liver, as the key mechanism. Additionally, oxidative injury is required to manifest the necroinflammatory component of steatohepatitis resulting from the combination of oxidative stress, lipid peroxidation, mitochondrial dysfunction, hormonal abnormalities and cellular toxicity from free fatty acids. Mitochondrial dysfunction is crucial in the pathogenesis of NAFLD/NASH, leading to the overproduction of reactive oxygen species that promote hepatocyte injury.6,7 Oxidative stress triggers cell membrane peroxidation, cell degeneration and apoptosis, and the expression of pro-inflammatory and pro-fibrogenic cytokines leading to progressive liver damage. NAFLD is considered a chronic inflammatory disease8 and even mild inflammation has been associated with the risk of disease progression.9 In this context, the changes in adipokine production play a crucial role in the pathogenesis.10 For example, leptin has been shown to exhibit pro-inflammatory effects in the liver.11 To this end, clinical research on the potential management of NAFLD is mounting. Data derived from clinical trials have indicated that managing the body weight may be beneficial in patients with NAFLD or with obesity,12 lowering triglycerides (TG),13 reducing liver fat and inhibiting de novo lipogenesis14 or improving insulin sensitivity.15 The restriction of excessive carbohydrate intake has also been proven to improve several important factors related to cardiovascular diseases16 or to reduce the hepatic triglyceride content.17 Not only should NASH be treated, but so should benign fatty liver.18 The last guidelines on NAFLD clearly report that diet is the first-line treatment for NAFLD.19,20 Adherence to the Mediterranean diet has been reported to reduce liver fat on 1H-MRS, when compared with a low fat/high carbohydrate diet in a cross-over comparison.21 Recent studies in food science have focused on identifying bioactive ingredients that can suppress hepatic lipid accumulation and although weight management is the main intervention, only a few studies on the effect of bioactive microconstituents on NAFLD have been performed. Polyunsaturated n-3 fatty acids from seal oils were efficacious for patients with NAFLD associated with hyperlipidemia and improved their total symptom scores, alanine aminotransferase (ALT), serum lipid levels and normalized ultrasonographic evidence.22 Synbiotic supplementation attenuated inflammatory markers in a randomized, double-blind, placebo-controlled pilot study in NAFLD patients.23 It has been proposed that since NAFLD/NASH pathogenesis involves the increased production of reactive oxygen species and oxidative stress, bioactive phytochemicals may be useful in its management.24 For example, vitamin E has been associated with fibrosis reversal,25 improvement in insulin sensitivity and several of its associated parameters, including ALT levels in overweight otherwise healthy subjects26 or improvement in ALT levels and insulin resistance in children with NAFLD in addition to lifestyle intervention.27 Focusing on improving the clinical features and markers of oxidative stress and inflammation in NAFLD patients, we designed a pilot randomized controlled clinical trial to investigate the effects of two isocaloric diets of different “snacking” choices in between meals in addition to nutritional counseling for minimal body weight decrease. To this end, naturally sun-dried black grapes (currants), previously characterized for their phenolic content (217.4–354.2 mg GAE g−1) and composition,28 were examined vs. other isocaloric snacks.2. Materials and methods
2.1 Patient recruitment and eligibility criteria
Patients were identified and recruited at Laiko University Hospital. The diagnosis of NAFLD was made by ultrasound examination. Included in the trial were patients with no significant fibrosis (evaluated by liver stiffness <7.5 kPa in ShearWave Elastography/Fibroscan). Men and women >18 years of age, without any change in body weight (BW) during the last 6 months prior to the trial, with a body mass index (BMI) >25 kg m−2 and with adherence to the “westernized” diet indicated by MedDietScore values lower than 35 were recruited. Exclusion criteria encompassed the presence of chronic viral hepatitis, as well as the presence of congenital or acquired liver disease, history of prior exposure to hepatotoxic drugs, evidence of hepatic cirrhosis, ultrasonography values less than 1 Hz, bariatric surgery, daily consumption of ethanol more than 20 g for women and more than 30 g for men for over 6 months during the last 5 years, any medication effective on fatty liver disease, the co-presence of reduced life expectancy disease, psychiatric disorders impairing the patient's ability to provide written informed consent, age >65 years, pregnant or lactating women or subjects supplemented with n-3 fatty acids, probiotics/synbiotics, antioxidant vitamins and/or phytochemicals. Additionally, the exclusion criterion was any planned, structured, and repetitive physical activity. Trial investigators excluded from final analyses patients who changed their medication during the trial.2.2 Study design
All eligible patients with NAFLD signed an informed consent form after a full review of the inclusion and exclusion criteria and an explanation of the risks and benefits of the study, which were approved by the Ethics Committees of both Harokopio University and Laiko University Hospital, based on the Helsinki Declaration. In a two-armed, single center, randomized, controlled, 24-week prospective intervention trial, NAFLD patients were randomly assigned either to the Control arm or to the Currant arm. The allocation of patients in the two groups was randomised. In this study simple randomization was chosen and the randomisation sequence was computer generated. An independent statistician used a computer randomisation software. After randomisation, the statistician sent the randomisation list to the trial principal investigator who completed a participant form for each subject, including the treatment and the patient trial number and put it in a sealed envelope. Blinding of the allocated treatment was maintained to data analysts and was exposed only after the assessment of outcomes. Daily energy needs were determined according to the basic metabolic rate equation of Harris–Benedict and sedentary lifestyle. The aim of nutritional counseling was a weight loss of approximately 5% of the initial BW within 6 months. Subjects attended appointments with experienced dieticians to receive guidance regarding calorie restriction. Nutritional counseling was centered on the distribution of nutrients in relation to the total caloric value as follows: 30% of the total energy as fat (<10% as SFAs, ∼10% as MUFAs, and ∼10% as PUFAs), 20% as protein, 50% as carbohydrate, 300 mg d−1 as dietary cholesterol, and 20–30 g fiber per d. Participants in the Control arm received the above dietary counseling. Participants in the Currant arm received dietary counseling and incorporated in their daily diet the consumption of 36 g of Corinthian currants equal to two fruit servings replacing snacks of alike nutritional value (low fat yogurt, mini crackers, or bread with low fat cheese). Currants were provided in packages of 36 g each, kindly donated by the Agricultural Cooperatives Union, Aegion, Greece.2.3 Anthropometrics, blood pressure and clinical analyses
Anthropometric indices such as BW, height, waist and hip circumferences (WC, HC), waist to hip ratio (WHR) and BMI were recorded both at baseline and after the end of the trial. BW was measured early in the morning in the fasting state with subjects in light clothing without shoes using a flat scale (Tanita WB-110MA, Japan) recorded to the nearest 0.1 kg and height was measured on a stadiometer (Seca Model 220, Germany) recorded to the nearest 0.1 cm. Body composition evaluation using bioimpedance analysis was also conducted pre- and post-intervention on the Tanita WB. BMI was calculated as weight (in kg) divided by height2 (in m2). The WC was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest, using a stretch-resistant tape. HC was measured around the widest portion of the buttocks, with the tape parallel to the floor. All anthropometric measurements and body compositions were recorded after a ≥12-hour fast. Diastolic and systolic blood pressure (DBP and SBP in mmHg, respectively) and heart frequency (HF) were evaluated at baseline and after the study completion using an electronic sphygmomanometer (OMRON HEM-907 XL, OMRON, Kyoto, Japan). Participants were asked to lie down and relax for a few minutes, after which, two consecutive blood pressure measurements were recorded at an interval of 1–2 minutes. The recorded value was the mean of the two measurements. Blood samples were drawn at baseline and at the end of the study (week 24) through a catheter in an antecubital vein after a 12 h overnight fast. Freshly drawn blood samples were used for the determination of liver enzymes, lipid profile, glucose, insulin, glycated hemoglobin (HbA1c), albumin, urea, uric acid, ferritin, Fe, transferrin and complete blood count using an automatic analyzer. Low density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula.29 HbA1c was measured using high performance liquid chromatography. For assays to determine inflammation and oxidative stress biomarkers, serum samples were collected, separated by centrifugation at 1800g for 10 min at 4 °C, and stored at −80 °C for subsequent analyses.2.4 Liver imaging
Abdominal ultrasound (US) was applied for the diagnosis of NAFLD. ShearWave elastography/Fibroscan was applied for liver stiffness evaluation and for the identification of the fibrosis stage. The NAFLD Fibrosis Score (NFS) was also calculated.302.5 Dietary history and analysis
Food data were collected. Each participant was asked to keep a 3-day food record (non-consecutive days, including one weekend day). Dietitians trained participants and reviewed unclear descriptions, errors, omissions, or doubtful entries in records and asked the participants to clarify them. The research dietitian supervisor checked all completed records for accuracy. The MedDietScore questionnaire was applied to estimate adherence to the Mediterranean dietary pattern.31 Furthermore, during the trial, dietary counseling was monitored by non-scheduled phone calls receiving 24-hour dietary recalls. To calculate energy intake and macronutrient breakdown (fat, protein, and carbohydrate) nutritional data were analyzed by Nutritionist Pro nutrient analysis software version 5.2.0 (Axxya Systems, Nutritionist Pro, Stafford, TX).2.6 Inflammatory biomarkers
Sera were separated from blood samples after centrifugation at 1800g for 10 min at 4 °C and were stored at −80 °C for further analyses. Sandwich Enzyme Linked-Immunosorbent Assay (ELISA) kits were used to measure CRP (ng ml−1) (Invitrogen Corporation, Camarillo, USA), hsIL-6 (pg ml−1) (R&D Systems Inc., Minneapolis, USA), tumor necrosis factor alpha (hsTNF-α) (pg ml−1) (Invitrogen Corporation, Camarillo, USA), visfatin (ng ml−1) (BioVendor-Laboratorni medicina a.s., Brno, Czech Republic) and leptin (ng ml−1) (Invitrogen Corporation, Camarillo, USA).2.7 Oxidative stress biomarkers
A sandwich ELISA kit (Mercodia AB, Uppsala, Sweden) was used to measure oxidized LDL (oxLDL). The total serum oxidizability was estimated photometrically applying the method described by Esterbauer and Jurgens32 using a Biotek PowerWave XS2 ELISA reader.2.8 Statistical analysis
All analyses were conducted applying the Statistical Package for the Social Sciences (SPSS 21.0 for Windows, Chicago, IL, USA). Descriptive statistics were calculated for all parameters and the Kolmogorov–Smirnov test was applied to investigate if all measures were characterized by normal distribution. Parametric data are expressed as mean values (±SD), while non-parametric data are expressed as medians and interquartile ranges. For variables with a normal distribution, the independent samples’ t-test was applied to compare the differences between the two arms pre- and post-intervention, while for variables without a normal distribution, the Mann–Whitney test was applied. Before the intervention this test served to ensure that the study population was characterized by homogeneity. For investigating possible intra-group differences, a paired sample t-test was applied for parametric variables and the Wilcoxon test for non-parametric ones. After NAFLD patients were classified with the criterion of weight loss (Control arm N = 17, Currant arm N = 18), the above mentioned analyses were repeated. For all statistical analyses significance was set at p < 0.05.3 Results
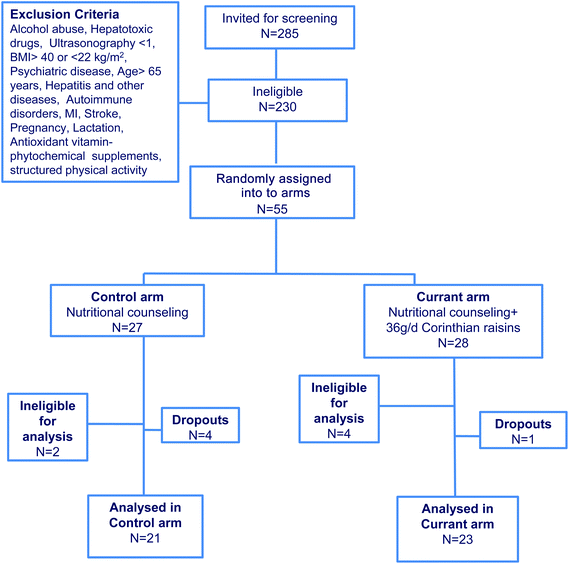
Of the 285 patients invited for screening to this study, 230 were found ineligible to participate. All 55 patients found eligible to participate consented to trial and enrolled in the protocol. No differences were found between the two arms in biochemical and anthropometric indices pre-intervention, except for insulin levels, which were lower in the Control arm compared to the Currant arm (Table 1). Also, energy and nutrient intake did not differ at baseline (Table 1). By the end of the trial, 4 out of 27 patients in the Control and 1 out of 23 in the Currant arm gave personal reasons for dropping out. In addition, 2 patients in the Control and 4 patients in the Currant arm were ineligible, as 5 modified the lipid lowering treatment and one started antimetabolite treatment during the trial. By the end of the study, 21 patients in the Control arm and 23 in the Currant arm were eligible for analysis (Fig. 1).| Parameters | Control arm (N = 27) | Currant arm (N = 28) | P value |
|---|---|---|---|
| BW, body weight; BMR, basic metabolic rate; BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HF, heart frequency; US, ultrasound; EUS, elastography ultrasound stiffness; NFS, NAFLD fibrosis score; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; SGOT/SGPT, SGOT to SGPT ratio; γGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; TPR, total protein; Fe, Iron; Fer, ferritin; HbA1C, glycated hemoglobin; WBC, white blood cells; RBC, red blood cells; Plt, platelets; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; Hb, hemoglobin; Hct, hematocrite; Na, sodium; K, potassium; CRP, C-reactive protein; IL-6, interleukin-6; oxLDL, oxidized LDL; TNF-α, tumor necrosis factor α; SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid. Data are mean values ± deviation (SD). CV, coefficient variation. Nutritional data were derived from food records, while the MedDiet score is given as well. P stands for the difference between the Control and the Currant arm at baseline parameters analyzed by independent sample t test, ≠ indicates the difference between the Control and the Currant arm parameters analyzed by the Mann–Whitney test. Difference was considered significant at P < 0.05. | |||
| Age (y) | 51.6 ± 9.4 | 50.7 ± 10.9 | 0.747 |
| Sex (n) | 0.480 | ||
| Males | 10 | 13 | |
| Females | 17 | 15 | |
| Anthropometrics | |||
| Height (m) | 1.7 ± 0.0 | 1.70 ± 0.0 | 0.390 |
| BW (kg) | 82.0 ± 3.0 | 85.7 ± 14.3 | 0.357 |
| BMR (kcal per 24 h) | 1633.3 ± 349.4 | 1712.2 ± 344.4 | 0.403 |
| BMI (kg m−2) | 29.1 ± 21.8 | 29.7 ± 22.2 | 0.595 |
| Fat (%) | 33.3 ± 9.4 | 33.3 ± 8.5 | 0.993 |
| Trunk fat (%) | 32.0 ± 8.3 | 32.5 ± 6.9 | 0.801 |
| WC (cm) | 99.1 ± 12.5 | 102.2 ± 11.6 | 0.336 |
| HC (cm) | 110.0 ± 10.9 | 111.8 ± 8.9 | 0.522 |
| WHR | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.678 |
| Clinical data | |||
| SBP (mmHg) | 130.2 ± 13.0 | 130.5 ± 13.2 | 0.929 |
| DBP (mmHg) | 81.5 ± 8.3 | 81.5 ± 8.9 | 0.988 |
| HF (bpm) | 70.3 ± 12.0 | 70.2 ± 45.0 | 0.974 |
| US (Hz) | 1.7 ± 5.2 | 2.0 ± 0.5 | 0.108≠ |
| EUS (kPa) | 5.6 ± 1.6 | 5.4 ± 1.0 | 0.655 |
| NFS | −2.4 ± 1.0 | −2.3 ± 1.0 | 0.692 |
| Glucose (mg dl−1) | 90.9 ± 15.6 | 95.7 ± 12.2 | 0.201 |
| TC (mg dl−1) | 203.7 ± 31.2 | 209.1 ± 31.2 | 0.523 |
| HDL-C (mg dl−1) | 57.2 ± 16.6 | 54.4 ± 12.7 | 0.483 |
| LDL-C (mg dl−1) | 122.3 ± 28.6 | 126.8 ± 33.3 | 0.590 |
| TG (mg dl−1) | 112.2 ± 46.8 | 137.2 ± 90.4 | 0.259≠ |
| SGOT (U L−1) | 22.3 ± 6.2 | 22.7 ± 5.3 | 0.809 |
| SGPT (U L−1) | 28.0 ± 12.5 | 29.6 ± 12.7 | 0.624 |
| SGOT/SGPT | 0.9 ± 0.52 | 0.8 ± 0.0 | 0.560 |
| γGT (U L−1) | 101.2 ± 69.7 | 128.5 ± 338.6 | 0.021≠ |
| ALP (U L−1) | 64.6 ± 16.6 | 69.4 ± 23.8 | 0.396 |
| TBIL (mg dl−1) | 0.6 ± 0.0 | 0.7 ± 0.5 | 0.752 |
| DBIL (mg dl−1) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.653 |
| TPR (g dl−1) | 7.3 ± 0.5 | 7.3 ± 0.5 | 0.968 |
| Fe (μg dl−1) | 107.8 ± 44.2 | 94.3 ± 24.3 | 0.164 |
| Fer (ng dl−1) | 94.8 ± 102.4 | 124.4 ± 97.3 | 0.275 |
| HbA1C (%) | 5.5 ± 0.5 | 5.8 ± 0.5 | 0.058 |
| Insulin (μIU ml−1) | 12.5 ± 3.6 | 16.1 ± 6.9 | 0.016 |
| WBC (×103 μl−1) | 6.3 ± 1.6 | 6.8 ± 2.1 | 0.490≠ |
| RBC (×106 μl−1) | 5.0 ± 0.5 | 5.1 ± 0.5 | 0.606 |
| Plt (×103 mm−3) | 256.4 ± 58.2 | 240.7 ± 40.7 | 0.249 |
| MCV (fl) | 83.9 ± 9.4 | 83.0 ± 7.9 | 0.350≠ |
| MCH (pg) | 28.6 ± 3.6 | 28.1 ± 3.2 | 0.448≠ |
| Hb (g dL−1) | 14.2 ± 1.6 | 14.1 ± 1.1 | 0.877 |
| Hct (%) | 41.6 ± 4.2 | 41.7 ± 2.6 | 0.912 |
| Na (mmol L−1) | 141.0 ± 2.1 | 141.5 ± 1.6 | 0.267 |
| K (mmol L−1) | 4.5 ± 0.5 | 4.6 ± 0.5 | 0.656 |
| Urea (mg dl−1) | 27.9 ± 8.8 | 29.0 ± 5.7 | 0.593 |
| Creatinine (mg dL−1) | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.448 |
| Uric acid (mg dL−1) | 5.1 ± 1.0 | 5.3 ± 1.1 | 0.394 |
| CRP (ng ml−1) | 2661.6 ± 2831.4 | 2780.8 ± 2703.7 | 0.528≠ |
| Visfatin (ng ml−1) | 8.4 ± 2.1 | 9.4 ± 5.3 | 0.381 |
| Leptin (ng ml−1) | 75.7 ± 53.6 | 91.8 ± 76.7 | 0.368 |
| IL-6 (pg ml−1) | 1.6 ± 2.6 | 2.4 ± 4.2 | 0.057≠ |
| oxLDL (U L−1) | 125.7 ± 29.6 | 137.5 ± 32.8 | 0.171 |
| Serum oxidizability (s) | 6033.6 ± 1188.2 | 5775.0 ± 1185.0 | 0.424 |
| TNF-α (pg ml−1) | 1.3 ± 1.0 | 0.9 ± 1.1 | 0.120 |
| Nutritional data | |||
| MedDietScore | 32.1 ± 4.7 | 30.0 ± 4.2 | 0.095 |
| Energy (kcal) | 1996.3 ± 617.2 | 1927.2 ± 645.4 | 0.688 |
| Carbohydrates (g) | 197.6 ± 68.1 | 203.0 ± 73.5 | 0.776 |
| Sugars (g) | 59.3 ± 22.9 | 63.1 ± 27.5 | 0.586 |
| Fat total (g) | 103.1 ± 48.4 | 87.2 ± 40.7 | 0.192 |
| SFA (g) | 28.3 ± 17.2 | 30.6 ± 13.2 | 0.572 |
| PUFA (g) | 15.5 ± 9.9 | 12.2 ± 9.0 | 0.209 |
| MUFA (g) | 49.2 ± 25.0 | 35.4 ± 21.2 | 0.051 |
| Protein (g) | 82.5 ± 35.9 | 82.4 ± 32.8 | 0.986 |
| Insoluble fibre (g) | 1.7 ± 2.6 | 0.9 ± 5.8 | 0.241≠ |
| Crude fibre (g) | 2.6 ± 1.6 | 3.2 ± 4.2 | 0.462 |
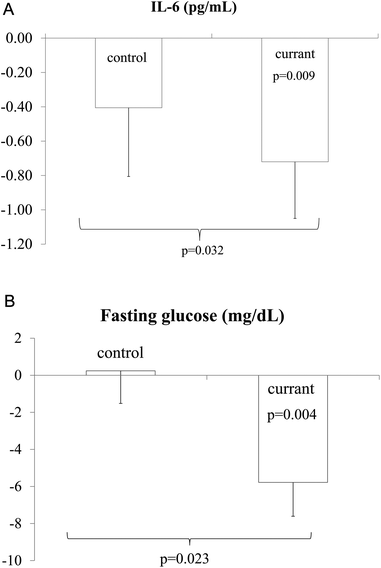
Significant differences between the two arms post-intervention were observed in fasting glucose and IL-6 levels (p = 0.023 and p = 0.032, respectively) (Fig. 2). These were significantly decreased only in Currant patients (variation in baseline glucose 13.6% and in endpoint 16%, p = 0.004; variation in baseline IL-6 87.5% and in endpoint 55.6%, p = 0.009) (Table 3).
At the end of the trial anthropometric measures were improved in both groups compared to the baseline, BW (Control arm: p = 0.001, Currant arm: p = 0.002), BMI (Control arm: p = 0.001, Currant arm: p = 0.001) and HC (Control arm: p = 0.010, Currant arm: p < 0.001), as well as HbA1c (Control arm: p = 0.028, Currant arm: p = 0.001), CRP (Control arm: p = 0.027, Currant arm: p = 0.001) and Elastography Ultrasound Scanning (EUS) values (Control arm: p = 0.043, Currant arm: p = 0.008) (Table 2). However, differences were insignificant between the two arms post-intervention (Table 3). Furthermore, adherence to the Mediterranean dietary pattern, illustrated by MedDietScore, was increased both in the Control arm (p = 0.005), and in the Currant arm (p < 0.001) (Table 4). However, at the end of the trial, the adherence difference was significantly higher in the Currant arm (p = 0.044) (Table 4). Patients in the Control arm had significantly decreased SBP and DBP (p = 0.001 and p = 0.003, respectively), LDL and HDL (p = 0.016 in both), NFS (p = 0.018), as well as the consumption of total fat (p = 0.047) (Tables 2–4). Participants in the Currant arm had significantly reduced total body fat (p = 0.010), WC (p = 0.007) and trunk fat (p = 0.045). Ultrasound scanning improved significantly in patients snacking currants daily (p = 0.029). Volunteers enrolled in the Currant arm showed a reduced intake of saturated fatty acids (SFAs) (p = 0.003). All participants in both arms received a common treatment, meaning nutritional counseling that aimed at a weight loss of approximately 5% of the initial BW within six months. However not all participants adhered to counseling; seventeen patients in the Control arm and eighteen in the arm that used currants as snacks showed a reduced body weight. Since weight loss has been officially recognised as a treatment approach in NAFLD19 a secondary analysis was repeated in patients adhering to this lifestyle change. Overall, NAFLD patients with a decreased BW had a significantly improved total body fat, WC, and HC. Post-intervention, the decrease in fasting glucose and in IL-6 was significantly higher in Currant patients who lost BW compared to their counterparts in the Control arm (p = 0.005 and p = 0.035, respectively) (Table 2S‡). Variation in baseline glucose in the Currant arm was 9.8% and in the endpoint 10.8% while variation in baseline IL-6 in the Currant arm was 69.2% and in the endpoint 50.0%. Also EUS values, CRP, and leptin decreased significantly in these patients after 6 months (Table 2S‡). The assessment of nutritional intake showed a significant increase in MedDietScore values, and a significant decrease in SFA intake in patients who lost weight in both arms (Table 3S‡).
| Variable | Arm | Week 0 | Week 24 | P value | *P value |
|---|---|---|---|---|---|
| Data are mean values ± standard deviation (SD). BW, body weight; BMR, basic metabolic rate; BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HF, heart frequency; US, ultrasound; EUS, elastography ultrasound stiffness; NFS, NAFLD fibrosis score. P values: comparison with the baseline values by the paired samples t test or # the Wilcoxon test, difference was considered significant at P < 0.05. *P values indicate significant differences in the change of risk factors in the raisin group as compared with the change in the Control group applying the independent samples t test # or the Mann–Whitney test difference was considered significant at P < 0.05. | |||||
| BW (kg) | Control | 79.8 ± 61.8 | 76.9 ± 12.4 | 0.001 | 0.343 |
| Currant | 84.9 ± 14.4 | 82.9 ± 14.4 | 0.002 | ||
| BMR (kcal per 24 h) | Control | 1631.6 ± 318.8 | 1592.4 ± 292.6 | 0.003 | 0.151 |
| Currant | 1689.5 ± 348.5 | 1674.7 ± 351.8 | 0.221 | ||
| BMI (kg m−2) | Control | 28.2 ± 3.7 | 27.1 ± 3.7 | 0.001 | 0.241 |
| Currant | 29.5 ± 4.3 | 28.8 ± 4.3 | 0.001 | ||
| Fat (%) | Control | 31.2 ± 8.7 | 30.0 ± 8.2 | 0.133 | 0.965 |
| Currant | 33.6 ± 8.2 | 32.4 ± 8.7 | 0.010 | ||
| Trunk fat (%) | Control | 30.8 ± 7.3 | 29.5 ± 7.8 | 0.205 | 0.866 |
| Currant | 32.6 ± 7.2 | 31.5 ± 7.7 | 0.045 | ||
| WC (cm) | Control | 96.7 ± 11.5 | 94.5 ± 13.0 | 0.094 | 0.969 |
| Currant | 101.0 ± 11.5 | 98.9 ± 11.5 | 0.007 | ||
| HC (cm) | Control | 107.0 ± 10.1 | 105.3 ± 9.2 | 0.010 | 0.207 |
| Currant | 112.5 ± 9.1 | 109.6 ± 9.1 | <0.001 | ||
| WHR | Control | 0.9 ± 0.0 | 0.9 ± 0.0.09 | 0.565 | 0.588 |
| Currant | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.972 | ||
| SBP (mmHg) | Control | 130.3 ± 13.2 | 121.8 ± 11.0 | 0.001 | 0.179 |
| Currant | 129.1 ± 13.4 | 124.8 ± 13.0 | 0.057 | ||
| DBP (mmHg) | Control | 81.4 ± 8.7 | 76.5 ± 8.7 | 0.003 | 0.030 |
| Currant | 80.3 ± 9.1 | 79.8 ± 10.1 | 0.737 | ||
| HF (bpm) | Control | 68.4 ± 18.8 | 68.5 ± 13.3 | 0.967 | 0.255 |
| Currant | 71.4 ± 8.6 | 69.0 ± 8.2 | 0.078 | ||
| US (Hz) | Control | 1.6 ± 0.5 | 1.5 ± 0.5 | 0.058# | 0.833# |
| Currant | 1.9 ± 1.0 | 1.8 ± 0.5 | 0.033# | ||
| EUS (kPa) | Control | 5.5 ± 1.8 | 5.0 ± 0.9 | 0.043 | 0.684 |
| Currant | 5.3 ± 1.0 | 4.9 ± 1.0 | 0.008 | ||
| NFS | Control | −2.3 ± 1.4 | −2.1 ± 1.4 | 0.018 | 0.279 |
| Currant | −2.3 ± 1.0 | −2.2 ± 1.0 | 0.102 | ||
| Variable | Arm | Week 0 | Week 24 | P value | *P value |
|---|---|---|---|---|---|
| Data are mean values ± standard deviation (SD). TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; SGOT/SGPT, SGOT to SGPT ratio; γGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; HbA1C, glycated hemoglobin; CRP, C-reactive protein; IL-6, interleukin-6; oxLDL, oxidized LDL; TNF-α, tumor necrosis factor α. P values: comparison with the baseline values by the paired samples t test # or the Wilcoxon test, difference was considered significant at P < 0.05. *P values indicate significant differences in the change of risk factors in the raisin group as compared with the change in the Control group, applying the independent samples t test # or the Mann–Whitney test, difference was considered significant at P < 0.05. | |||||
| Glucose (mg dL−1) | Control | 92.1 ± 16.9 | 92.3 ± 16.9 | 0.894 | 0.023 |
| Currant | 95.7 ± 13.0 | 89.9 ± 14.4 | 0.004 | ||
| TC (mg dL−1) | Control | 202.8 ± 32.0 | 209.2 ± 35.7 | 0.309 | 0.479 |
| Currant | 209.6 ± 33.6 | 211.4 ± 32.2 | 0.548 | ||
| HDL-C (mg dL−1) | Control | 56.7 ± 15.1 | 52.5 ± 13.7 | 0.016 | 0.853 |
| Currant | 56.1 ± 14.4 | 52.4 ± 11.0 | 0.065 | ||
| LDL-C (mg dL−1) | Control | 120.8 ± 29.8 | 134.9 ± 32.5 | 0.016 | 0.310 |
| Currant | 126.7 ± 37.4 | 133.1 ± 34.1 | 0.225 | ||
| TG (mg dL−1) | Control | 114.1 ± 51.8 | 109.1 ± 47.6 | 0.602# | 1.000# |
| Currant | 137.3 ± 97.9 | 132.3 ± 79.7 | 0.399# | ||
| SGOT (U L−1) | Control | 23.1 ± 6.4 | 22.1 ± 6.4 | 0.511 | 0.827 |
| Currant | 22.8 ± 5.8 | 22.2 ± 6.7 | 0.634 | ||
| SGPT (U L−1) | Control | 29.5 ± 13.7 | 27.2 ± 15.6 | 0.394# | 0.991# |
| Currant | 30.0 ± 14.0 | 27.7 ± 14.0 | 0.402# | ||
| SGOT/SGPT | Control | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.352 | 0.991 |
| Currant | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.071 | ||
| γGT (U L−1) | Control | 32.7 ± 29.3 | 36.6 ± 49.5 | 0.504# | 0.465# |
| Currant | 32.1 ± 30.2 | 35.0 ± 37.4 | 0.475# | ||
| ALP (U L−1) | Control | 59.9 ± 14.2 | 61.2 ± 16.5 | 0.492 | 0.118 |
| Currant | 70.1 ± 26.9 | 67.6 ± 28.3 | 0.108 | ||
| TBIL (mg dL−1) | Control | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.793 | 0.317 |
| Currant | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.219 | ||
| DBIL (mg dL−1) | Control | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.135 | 0.185 |
| Currant | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.905 | ||
| HbA1C (%) | Control | 5.5 ± 0.5 | 5.4 ± 0.5 | 0.028# | 0.090# |
| Currant | 5.8 ± 0.5 | 5.4 ± 1.5 | 0.001# | ||
| Insulin (μIU mL−1) | Control | 11.7 ± 3.7 | 11.5 ± 6.0 | 0.839 | 0.288 |
| Currant | 15.8 ± 7.2 | 17.5 ± 9.6 | 0.210 | ||
| Urea (mg dL−1) | Control | 28.5 ± 8.2 | 32.3 ± 9.6 | 0.052 | 0.191 |
| Currant | 29.4 ± 6.2 | 30.5 ± 7.2 | 0.239 | ||
| Uric acid (mg dL−1) | Control | 5.0 ± 0.9 | 5.1 ± 1.4 | 0.585 | 0.556 |
| Currant | 4.9 ± 1.4 | 4.84 ± 1.6 | 0.791 | ||
| CRP (ng mL−1) | Control | 2399.2 ± 3048.9 | 841.6 ± 1087.8 | 0.023# | 0.748 |
| Currant | 2136.9 ± 1861.0 | 825.0 ± 721.9 | 0.002# | ||
| Visfatin (ng mL−1) | Control | 8.4 ± 1.8 | 4.7 ± 3.2 | 0.066 | 0.233 |
| Currant | 9.6 ± 5.3 | 9.2 ± 3.4 | 0.696 | ||
| Leptin (ng mL−1) | Control | 63.5 ± 48.6 | 55.2 ± 39.4 | 0.090 | 0.794 |
| Currant | 95.9 ± 81.6 | 85.2 ± 76.8 | 0.190 | ||
| IL-6 (pg mL−1) | Control | 1.7 ± 3.2 | 1.3 ± 1.4 | 0.322# | 0.032# |
| Currant | 1.6 ± 1.4 | 0.9 ± 0.5 | 0.009# | ||
| oxLDL (U L−1) | Control | 125.7 ± 26.1 | 115.5 ± 26.1 | 0.072 | 0.828 |
| Currant | 137.5 ± 29.8 | 125.1 ± 33.6 | 0.160 | ||
| TNF-α (pg mL−1) | Control | 1.3 ± 1.0 | 0.8 ± 0.5 | 0.004 | 0.066 |
| Currant | 0.9 ± 1.0 | 1.3 ± 1.4 | 0.063 | ||
| Serum oxidizability (s) | Control | 6033.6 ± 1046.5 | 6145.1 ± 946.2 | 0.569 | 0.851 |
| Currant | 5579.0 ± 658.1 | 5745.9 ± 896.6 | 0.462 | ||
| Variable | Arm | Week 0 | Week 24 | P value | *P value |
|---|---|---|---|---|---|
| Data are mean values ± standard deviation (SD). SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid. P values: comparison with the baseline values by the paired samples t test # or the Wilcoxon test, difference was considered significant at P < 0.05. *P values indicate significant differences in the change of risk factors in the raisin group as compared with the change in the Control group, applying the independent samples t test, difference was considered significant at P < 0.05. | |||||
| MedDietScore | Control | 32.0 ± 4.58 | 35.1 ± 5.0 | 0.005 | 0.044 |
| Currant | 29.8 ± 4.3 | 35.6 ± 5.3 | <0.001 | ||
| Energy (kcal) | Control | 1981.1 ± 521.7 | 1831.8 ± 618.7 | 0.429 | 0.988 |
| Currant | 1923.1 ± 683.5 | 1777.4 ± 590.0 | 0.343 | ||
| Carbohydrates (g) | Control | 205.5 ± 70.1 | 205.3 ± 76.5 | 0.992 | 0.577 |
| Currant | 200.5 ± 75.8 | 185.6 ± 58.6 | 0.363 | ||
| Sugars (g) | Control | 60.8 ± 22.0 | 68.8 ± 35.7 | 0.367 | 0.575 |
| Currant | 62.6 ± 27.8 | 65.2 ± 27.4 | 0.551 | ||
| Protein (g) | Control | 82.6 ± 30.2 | 76.5 ± 35.7 | 0.545 | 0.874 |
| Currant | 78.3 ± 31.7 | 74.2 ± 28.8 | 0.576 | ||
| Fat total (g) | Control | 99.5 ± 38.0 | 80.3 ± 26.1 | 0.047 | 0.313 |
| Currant | 89.8 ± 41.3 | 84.9 ± 41.8 | 0.642 | ||
| SFA (g) | Control | 28.0 ± 15.1 | 22.7 ± 9.2 | 0.130 | 0.353 |
| Currant | 31.9 ± 12.4 | 22.6 ± 11.0 | 0.003 | ||
| PUFA (g) | Control | 14.8 ± 6.9 | 12.9 ± 6.4 | 0.274# | 0.442 |
| Currant | 12.5 ± 9.6 | 13.0 ± 8.2 | 0.503# | ||
| MUFA (g) | Control | 46.0 ± 18.3 | 36.3 ± 16.0 | 0.031 | 0.054 |
| Currant | 35.8 ± 21.6 | 41.7 ± 26.9 | 0.350 | ||
| Insoluble fiber (g) | Control | 2.0 ± 2.8 | 1.2 ± 1.4 | 0.287# | 0.461 |
| Currant | 0.9 ± 1.4 | 0.6 ± 1.4 | 0.099# | ||
| Crude fiber (g) | Control | 2.8 ± 1.4 | 4.3 ± 4.6 | 0.170# | 0.642 |
| Currant | 3.0 ± 3.8 | 3.9 ± 2.9 | 0.144# | ||
4 Discussion
To the best of our knowledge, this is the first randomized clinical trial that evaluated the effect of snacking along with standard nutritional counseling for a minimal body weight decrease in NAFLD patients of non-significant fibrosis. More specifically, in addition to nutritional counseling, the effect of incorporating a dried fruit in diet as a snack was evaluated. The main outcome of this study was that minimal dietary modifications in patients with NAFLD of non-significant fibrosis result in the reduction of serum IL-6 and in the reduction of fasting glucose.Dietary counseling was effective in all NAFLD patients, both in the Control and Currant arms, as it significantly reduced BW, BMI and HC, and significantly improved HbA1c, CRP, EUS and MedDietScore. Overall, the findings of our study are consistent with the findings of other clinical trials that have shown the beneficial effects of nutritional counseling and diet-induced weight loss in NAFLD patients31,33–36. Achieving and maintaining a 5% weight reduction has been suggested to improve several components of the metabolic syndrome and liver function.37 A 9% weight loss in obese older adults resulted in significant decreases in the intrahepatic fat content accompanied by considerable improvements in insulin sensitivity.15 Similarly, 6–8% weight loss reduced hepatic fat and improved glucose metabolism in younger NAFLD patients.38,39 Corinthian currants are the dried products of their fresh homologues, grapes (Vitis vinifera L.). MedDietScore values were significantly different between the two groups at the end of the trial, a rather expected result as the snack was a dried fruit, however, its importance should be stressed. Adherence to the Mediterranean diet has been found to be a significant predictor of changes in the fat content of the liver in overweight patients with NAFLD.40 It has been shown that dried fruit consumption in quantities more than 20 g on a daily basis was associated with improved nutrient intakes, a higher overall diet quality score, and lower body weight/adiposity measures, compared to those who did not consume such a quantity.41 In our study, NAFLD patients in the Currant arm showed an additional decrease in the intake of SFAs and a higher adherence to the Mediterranean dietary pattern. In general, Vitis vinifera derived phenols, have known antioxidant properties and have been described to have beneficial effects also in NAFLD.42,43 The beneficial effect of snacking in NAFLD, according to the results reported, could have also been influenced by the higher adherence to diet and by the intrinsic antioxidant effects of phenols.44 Currants have been indicated as dried fruits of a medium glycemic index that regulate insulin and glucose response in both diabetic and non-diabetic subjects referenced to glucose.45 In general, foods with a low glycemic index have been shown to exert anti-inflammatory effects,46,47 as well as beneficial effects in glycemic control and blood pressure.48 Furthermore, currants contain considerable amounts of phenolic compounds such as vanillic acid, protocatechuic acid, syringic acid, p-coumaric acid, gallic acid, ferulic acid, caffeic acid, quercetin and the flavonoid chrysin.47 Seventeen out of 25 phytochemicals present in Corinthian currants have been found to increase in plasma after consumption, providing evidence for their absorption and bioavailability.49 Phenolic compounds have been attributed with several health benefits, mainly with effects on vascular function, on cognitive performance and neurogenesis, on the inhibition of DNA oxidation and tumor development, and on the inhibition of adhesion molecules and inflammatory mediators.50 The regulation of fasting glucose appearing herein might be attributed to the phenolic content of currants, as phenolic compounds, i.e. gallic acid, have been shown to ameliorate impaired glucose in experimental NAFLD/NASH.51 In NAFLD, pro-inflammatory cytokines such as TNF-α and IL-6 are secreted by adipocytes. TNF-α and IL-6 have been proven pro-inflammatory in the liver.11 Herein, TNF-α did not differ between the two arms post-intervention. Leptin was significantly decreased in patients with reduced BW concurrently with currants but not in those with reduced BW in the Control arm, however the decrease was not significantly different between these groups. NAFLD patients snacking polyphenol-rich currants daily experienced reduced IL-6 concentrations, the reduction being significant in all analyzed patients in the Currant arm irrespective of BW loss. Pro-inflammatory IL-6 is expressed in several tissues and is related to insulin resistance and inflammation.52 Plasma IL-6 has been associated with its expression in the liver, which in turn has been positively associated with the stage of inflammation, as well as with fibrosis severity.53 IL-6 is highly specific in confirming the absence of NASH at normal values and normal values are strongly associated with a fatty liver.54 In our study, after including only patients who had lost weight, EUS values remained significantly decreased only in the Currant arm. Although the patients enrolled in the study did not have significant fibrosis at baseline, the decrease in liver stiffness could be attributed to the decrease in inflammation.55 In obesity related NAFLD, IL-6 is largely produced by macrophages, but not only of adipose tissue derivation. In fact the main role is played by spleen and it has been demonstrated that spleen cells produce high levels of inflammatory cytokines and that NASH patients demonstrate high IL-6 blood levels and spleen longitudinal diameter values.56 However, it has also been demonstrated that spleen also mediates a protective role, as it supports a pool of innate-like B cells in white adipose tissue that protect against obesity-associated insulin resistance.57
Surprisingly, we did not observe any change in the markers of oxidative stress in serum. In a previous study in patients with type 2 diabetes,58 the daily consumption of 36 g of Corinthian currants for a total of 6 months resulted in an increase of the plasma antioxidant capacity, the increase being significantly different to that in the Control group post-intervention. As in the case of leptin, also in markers of oxidative stress, the relatively small study size may have limited our power to detect the differences among the Currant and Control groups for respective improvements in leptin or oxidative stress.
While this study reports interesting findings regarding the effect of lifestyle modifications on the health of patients with NAFLD, however it has some limitations. Apart from the study size, liver biopsies were not performed, precluding our ability to comment on any histologic alterations. Additionally, there is substantial potential for observing effects due to the multiple tests included herein. Also, an important limitation of the study is that the CV% are in some parameters very high suggesting that some of the measures are not of sufficient precision to yield unquestioning results. On the other hand, these limitations were compensated in part by the very tight control of the Currant and Control groups to assess the potential effects of the daily consumption of currants by patients with NAFLD. Nevertheless, both mechanistic and larger-scale clinical trials are essential to determine the effect of polyphenol-rich currants on the health of patients with NAFLD and the potential mechanisms underlying any effect.
5 Conclusions
NAFLD has the potential for major economic impact on healthcare costs because of liver-related morbidity and mortality and it already represents an important economic burden for European countries. For example, patients with a sonographic fatty liver disease and increased serum liver enzymes have been shown to incur 26% higher overall health-care costs at a 5-year follow-up.59 NAFLD patients of non-significant fibrosis can take advantage of the adoption of a balanced diet, while a minimal lifestyle change, such as changes in snacking with the increase of bioactive phytochemicals, can potentially ameliorate fasting glucose, inflammation and fibrosis stage. The quantity of raisins consumed herein by the volunteers corresponds to two fruit servings, a realistic dose to be incorporated in the diet of these patients. This modification in diet related to medium calorie restriction and to snacks rich in antioxidant phytochemicals could be an effective strategy for treating NAFLD patients with no significant fibrosis.Author contributions
Andriana C Kaliora and Alexander Kokkinos had the concept and designed this study; Anastacia Diolintzi, Aristea Gioxari, Maria Stoupaki, Jiannis Vlachogiannakos and Constantinos Revenas were the investigators who recruited and treated patients; George V. Z. Dedoussis, Spyros D. Ladas and Vaios T. Karathanos took part in trial coordination; Andriana C. Kaliora, Anastacia Diolintzi, Panagiotis T. Kanellos and Aristea Gioxari collected, managed, analyzed and interpreted the data; Anastacia Diolintzi, Panagiotis T. Kanellos and Aristea Gioxari contributed to the statistical analyses; Andriana C. Kaliora and Anastacia Diolintzi drafted the manuscript; Andriana C. Kaliora made the final version of the manuscript to be published, approved also by Alexander Kokkinos.Conflict of interest
No potential conflicts of interest relevant to this article were reported.References
- M. Masarone, A. Federico, L. Abenavoli, C. Loguercio and M. Persico, Non alcoholic fatty liver: epidemiology and natural history, Rev. Recent Clin. Trials, 2014, 9, 126–133 CrossRef PubMed.
- N. M. de Alwis and C. P. Day, Non-alcoholic fatty liver disease, the mist gradually clears, J. Hepatol., 2008, 48, S104–S112 CrossRef PubMed.
- M. Raman and J. Allard, Nonalcoholic fatty liver disease: A clinical approach and review, Can. J. Gastroenterol., 2006, 20, 345–349 CrossRef PubMed.
- G. Vernon, A. Baranova and Z. M. Younossi, Systematic review, the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults, Aliment. Pharmacol. Ther., 2011, 34, 274–285 CrossRef CAS PubMed.
- M. Lazo and J. M. Clark, The epidemiology of nonalcoholic fatty liver disease, a global perspective, Semin. Liver Dis., 2008, 28, 339–350 CrossRef PubMed.
- Q. M. Anstee and C. P. Day, The genetics of NAFLD, Nat. Rev. Gastroenterol. Hepatol., 2013, 10, 645–655 CrossRef CAS PubMed.
- A. Wree, L. Broderick, A. Canbay, H. M. Hoffman and A. E. Feldstein, From NAFLD to NASH to cirrhosis-new insights into disease mechanisms, Nat. Rev. Gastroenterol. Hepatol., 2013, 10, 627–636 CrossRef CAS PubMed.
- S. Coulon, F. Heindryckx, A. Geerts, C. Van Steenkiste, I. Colle and H. Van Vlierberghe, Angiogenesis in chronic liver disease and its complications, Liver Int., 2011, 31, 146–162 CrossRef CAS PubMed.
- R. Pais, F. Charlotte, L. Fedchuk, P. Bedossa, P. Lebray and T. Poynard, et al., LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver, J. Hepatol., 2013, 59, 550–556 CrossRef CAS PubMed.
- N. Kobyliak, L. Abenavoli, T. Falalyeyeva, O. Virchenko, B. Natalia, T. Beregova, P. Bodnar and M. Spivak, Prevention of NAFLD development in rats with obesity via the improvement of pro/antioxidant state by cerium dioxide nanoparticles, Clujul. Med., 2016, 89, 229–335 CrossRef PubMed.
- V. G. Giby and T. A. Ajith, Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease, World J. Hepatol., 2014, 570–579 Search PubMed.
- C. Finelli and G. Tarantino, Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J. Gastrointestin, Liver Dis., 2012, 21, 293–302 Search PubMed.
- D. E. Larson-Meyer, B. R. Newcomer, L. K. Heilbronn, J. Volaufova, S. R. Smith and A. J. Alfonso, et al., The Pennington CALERIE Team. Effect of a 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function, Obesity (Silver Spring), 2008, 16, 1355–1362 CrossRef CAS PubMed.
- K. Sevastianova, A. Santos, A. Kotronen, A. Hakkarainen, J. Makkonen and K. Silander, et al., Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans, Am. J. Clin. Nutr., 2012, 96, 727–734 CrossRef CAS PubMed.
- K. Shah, A. Stufflebam, T. N. Hilton, D. R. Sinacore, S. Klein and D. T. Villareal, Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults, Obesity (Silver Spring), 2009, 17, 2162–2168 CrossRef PubMed.
- R. Jin, J. A. Welsh, N. A. Le, J. Holzberg, P. Sharma and D. R. Martin, et al., Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD, Nutrients, 2014, 6, 3187–3201 CrossRef PubMed.
- J. D. Browning, J. A. Baker, T. Rogers, J. Davis, S. Satapati and S. C. Burgess, Short-term weight loss and hepatic triglyceride reduction, evidence of a metabolic advantage with dietary carbohydrate restriction, Am. J. Clin. Nutr., 2011, 93, 1048–1052 CrossRef CAS PubMed.
- C. Fierbinteanu-Braticevici, L. Negreanu and G. Tarantino, Is fatty liver always benign and should not consequently be treated?, J. Physiol. Pharmacol., 2013, 64, 3–9 CAS.
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease, European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO), J. Hepatol., 2016, 64, 1388–1402 Search PubMed.
- C. Thoma, C. P. Day and M. I. Trenell, Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review, J Hepatol., 2012, 56, 255–266 CrossRef PubMed.
- M. C. Ryan, C. Itsiopoulos, T. Thodis, G. Ward, N. Trost, S. Hofferberth, K. O'Dea, P. V. Desmond, N. A. Johnson and A. M. Wilson, The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease, J. Hepatol., 2013, 59, 138–143 CrossRef CAS PubMed.
- F. S. Zhu, S. Liu, X. M. Chen, Z. G. Huang and D. W. Zhang, Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia, World J. Gastroenterol., 2008, 14, 6395–6400 CrossRef CAS PubMed.
- T. Eslamparast, H. Poustchi, F. Zamani, M. Sharafkhah, R. Malekzadeh and A. Hekmatdoost, Synbiotic supplementation in nonalcoholic fatty liver disease, a randomized, double-blind, placebo-controlled pilot study, Am. J. Clin. Nutr., 2014, 99, 535–542 CrossRef CAS PubMed.
- M. H. Pan, C. S. Lai, M. L. Tsai and C. T. Ho, Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds, Mol. Nutr. Food Res., 2014, 58, 147–171 CAS.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive summary of the third report of the National Cholesterol Enducation Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III), J. Am. Med. Assoc., 2001, 285, 2486–2497 CrossRef.
- M. Kugelmans, D. B. Hill, B. Vivian, L. Marsano and C. J. McClain, Cytokines and NASH, a pilot study of the effects of lifestyle modification and vitamin E, Hepatology, 2003, 38, 413–419 CrossRef PubMed.
- C. L. Wang, L. Liang, J. F. Fu, C. C. Zou, F. Hong and J. Z. Xue, et al., Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children, World J. Gastroenterol., 2008, 14, 1598–1602 CrossRef CAS PubMed.
- A. Chiou, V. T. Karathanos, A. Mylona, F. N. Salta, F. Preventi and N. K. Andrikopoulos, Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity, Food Chem., 2007, 102, 516–522 CrossRef CAS.
- W. T. Friedewald, R. I. Levy and D. S. Fredrickson, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem., 1972, 18, 499–502 CAS.
- P. Angulo, J. M. Hui, G. Marchesini, E. Bugianesi, J. George and G. C. Farrell, et al., The NAFLD Fibrosis Score, a noninvasive system that identifies liver fibrosis in patients with NAFLD, Hepatology, 2007, 45, 846–854 CrossRef CAS PubMed.
- D. B. Panagiotakos, C. Pitsavos, F. Arvaniti and C. Stefanadis, Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults, the accuracy of the MedDietScore, Prev. Med., 2007, 44, 335–340 CrossRef PubMed.
- H. Esterbauer and G. Jurgens, Mechanistic and genetic aspects of susceptibility of LDL to oxidation, Curr. Opin. Lipidol., 1993, 4, 114–124 CrossRef CAS.
- Y. Tamura, Y. Tanaka, F. Sato, J. B. Choi, H. Wataba and N. Masataka, et al., Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients, J. Clin. Endocrinol. Metab., 2005, 90, 3191–3196 CrossRef CAS PubMed.
- M. A. Huang, J. K. Greenson, C. Chao, L. Anderson, D. Peterman and J. Jacobson, et al., One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis, a pilot study, Am. J. Gastroenterol., 2005, 100, 1072–1081 CrossRef PubMed.
- D. E. Larson-Meyer, L. K. Heilbronn, L. M. Redman, B. R. Newcomer, M. I. Frisard and S. Anton, et al., Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects, Diabetes Care, 2006, 29, 1337–1344 CrossRef PubMed.
- E. L. Thomas, A. E. Brynes, G. Hamilton, N. Patel, A. Spong and R. D. Goldin, et al., Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease, World J. Gastroenterol., 2006, 12, 5813–5819 CrossRef CAS PubMed.
- R. Aller, D. A. De Luis, O. Izaola, B. De La Fuente and R. Bachiller, Effect of a high monounsaturated vs. high polyunsaturated fat hypocaloric diets in nonalcoholic fatty liver disease, Eur. Rev. Med. Pharmacol. Sci., 2014, 18, 1041–1047 CAS.
- F. Sato, Y. Tamura, H. Watada, N. Kumashiro, Y. Igarashi and H. Uchino, et al., Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects, J. Clin. Endocrinol. Metab., 2007, 92, 3326–3329 CrossRef CAS PubMed.
- K. F. Petersen, S. Dufour, D. Befroy, M. Lehrke, R. E. Hendler and G. I. Shulman, Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes, Diabetes, 2005, 54, 603–608 CrossRef CAS PubMed.
- F. M. Trovato, D. Catalano, G. F. Martines, P. Pace and G. M. Trovato, Mediterranean diet and non-alcoholic fatty liver disease, the need of extended and comprehensive interventions, Clin. Nutr., 2015, 34, 86–88 CrossRef PubMed.
- D. R. Keast, C. E. O'Neil and J. M. Jones, Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults, National Health and Nutrition Examination Survey, 1999–2004, Nutr. Res., 2011, 31(6), 460–467 CrossRef CAS PubMed.
- N. Dai, Y. Zou, L. Zhu, H. F. Wang and M. G. Dai, Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation, J. Med. Food, 2014, 17, 663–669 CrossRef CAS PubMed.
- M. Aoun, F. Michel, G. Fouret, F. Casas, M. Jullien and C. Wrutniak-Cabello, et al., A polyphenol extract modifies quantity but not quality of liver fatty acid content in high-fat-high-sucrose diet-fed rats, possible implication of the sirtuin pathway, Br. J. Nutr., 2010, 104, 1760–1770 CrossRef CAS PubMed.
- M. Akaberi and H. Hosseinzadeh, Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome, Phytother. Res., 2016, 30, 540–556 CrossRef CAS PubMed.
- P. T. Kanellos, A. C. Kaliora, C. Liaskos, N. K. Tentolouris, D. Perrea and V. T. Karathanos, A study of glycemic response to Corinthian raisins in healthy subjects and in type 2 diabetes mellitus patients, Plant Foods Hum. Nutr., 2013, 68, 145–148 CrossRef CAS PubMed.
- E. B. Levitan, N. R. Cook, M. J. Stampfer, P. M. Ridker, K. M. Rexrode and J. E. Buring, et al., Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein, Metabolism, 2008, 57, 437–443 CrossRef CAS PubMed.
- R. Jiang, D. R. Jacobs, E. Mayer-Davis, M. Szklo, D. Herrington and N. S. Jenny, et al., Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis, Am. J. Epidemiol., 2006, 163, 222–231 CrossRef PubMed.
- D. J. A. Jenkins, K. Srichaikul, C. W. C. Kendall, J. L. Sievenpiper, S. Abdulnour and A. Mirrahimi, et al., The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes, Diabetologia, 2011, 54, 271–279 CrossRef CAS PubMed.
- P. T. Kanellos, A. C. Kaliora, A. Gioxari, G. O. Christopoulou, N. Kalogeropoulos and V. T. Karathanos, Absorption and bioavailability of antioxidant phytochemicals and increase of serum oxidation resistance in healthy subjects following supplementation with raisins, Plant Foods Hum. Nutr., 2013, 68, 411–415 CrossRef CAS PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (poly)phenolics in human health, structures, bioavailability, and evidence of protective effects against chronic diseases, Antioxid. Redox. Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- J. Chao, T. I. Huo, H. Y. Cheng, J. C. Tsai, J. W. Liao, M. S. Lee, X. M. Qin, M. T. Hsieh, L. H. Pao and W. H. Peng, Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice, PLoS One, 2014, 9(2), e96969, DOI:10.1371/journal.pone.0096969.eCollection2014.
- C. Gabay and I. Kushner, Acute-phase proteins and other systemic responses to inflammation, N. Engl. J. Med., 1999, 340, 448–454 CrossRef CAS PubMed.
- A. Wieckowska, B. G. Papouchado, Z. Li, R. Lopez, N. N. Zein and A. E. Feldstein, Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis, Am. J. Gastroenterol., 2008, 103, 1372–1379 CrossRef CAS PubMed.
- G. Tarantino, P. Conca, F. Pasanisi, M. Ariello, M. Mastrolia and A. Arena, et al., Could inflammatory markers help diagnose nonalcoholic steatohepatitis?, Eur. J. Gastroenterol. Hepatol., 2009, 21, 504–511 CrossRef CAS PubMed.
- G. Ferraioli, C. Filice, L. Castera, B. I. Choi, I. Sporea and S. R. Wilson, et al., WFUMB guidelines and recommendations for clinical use of ultrasound elastography, Part 3, liver, Ultrasound Med. Biol., 2015, 41, 1161–1179 CrossRef PubMed.
- G. Tarantino, S. Savastano, D. Capone and A. Colao, Spleen, A new role for an old player?, World J. Gastroenterol., 2011, 17, 3776–3784 CrossRef PubMed.
- L. Wu, V. V. Parekh, J. Hsiao, D. Kitamura and L. Van Kaer, Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 4638–4647 CrossRef PubMed.
- P. T. Kanellos, A. C. Kaliora, N. K. Tentolouris, V. Argiana, D. Perrea and N. Kalogeropoulos, et al., A pilot, randomized controlled trial to examine the health outcomes of raisin consumption in patients with diabetes, Nutrition, 2014, 30, 358–364 CrossRef CAS PubMed.
- M. Blachier, H. Leleu, M. Peck-Radosavljevic, D. C. Valla and F. Roudot-Thoraval, The burden of liver disease in Europe, A review of available epidemiological data, J. Hepatol., 2013, 58, 593–608 CrossRef PubMed.
Footnotes |
| † This study is a sub study of “Obesity and metabolic syndrome: dietary intervention with Greek raisins in NAFLD/NASH. Investigation of molecular mechanisms” reviewed and approved by the Greek Secretariat for Research and Technology (Cooperation 890/2009). Additionally, this study was reviewed by Harokopio University and Laiko General Hospital, Athens University Medical School Institutional Review Boards. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6fo01040g |
| This journal is © The Royal Society of Chemistry 2016 |