Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dietary fatty acid composition is sensed by the NLRP3 inflammasome: omega-3 fatty acid (DHA) prevents NLRP3 activation in human macrophages
N.
Martínez-Micaelo
,
N.
González-Abuín
,
M.
Pinent
,
A.
Ardévol
and
M.
Blay
*
Mobiofood Research Group, Department of Biochemistry and Biotechnology, Rovira i Virgili University, Tarragona, Spain. E-mail: mteresa.blay@urv.cat; Tel: +34 977 55 84 97
First published on 28th June 2016
Abstract
The Nod-like receptor protein 3 (NLRP3) inflammasome is considered to be a pivotal host platform responsible for sensing of exogenous and endogenous danger signals, including those generated as a result of metabolic dysregulation, and for the subsequent, IL-1β-mediated orchestration of inflammatory and innate immunity responses. In this way, although the molecular link between diet-induced obesity and inflammasome activation is still unclear, free fatty acids (FFA) have been proposed as a triggering event. We report that dietary fatty acid (FA) composition is sensed by the NLRP3 inflammasome in human macrophages. For this purpose, we have analysed three roles of FA supplementation: as a priming signal for ATP-activated macrophages, in determining where the administration of dietary FAs interferes with LPS-mediated inflammasome activation and by inducing inflammasome activation per se. In this study, we confirm that saturated (SFAs) activated the NLRP3 inflammasome and stimulated the secretion of the IL-1β cytokine, while PUFAs were mainly inhibitors. Moreover, in general, DHA (n-3 PUFA) was more effective in preventing inflammasome activation than arachidonic acid (n-6 PUFA).
Introduction
Immunity and metabolism are considered fundamental systems for survival. In fact, cell homeostasis is linked to the integration and continuous crosstalk between the immune system and metabolism.1 In this way, an immune response is influenced by the nutritional status.Tissues and blood FA are mainly derived from the diet and have been used as dietary intake biomarkers.2 FAs present in the diet can be divided into two major classes depending on their degree of saturation: saturated (SFAs) or unsaturated fatty acids (UFAs). Palmitate (16:0) and stearate (18:0) are the most important SFAs in the blood, representing 90% of the total SFAs.2 Moreover, UFAs can be divided into monounsaturated and polyunsaturated FAs (MUFAs and PUFAs, respectively), and PUFAs are further divided into two categories, namely, omega-6 and omega-3, based on the location of the first double bond of the FA molecule. Arachidonic acid (C20:4 n-6) and docosahexaenoic acid (DHA, C22:6 n-3) are both considered to be key FAs because the omega-6/omega-3 ratio is directly related to the pathogenesis of many diseases, including those related to the dysregulation of the inflammatory or immune response.3
Nod-like receptors (NLRs) form central molecular platforms that are mostly expressed in the cytosol and are organised in signalling complexes named inflammasomes.4 Structurally, inflammasomes are large, multiprotein complexes consisting of Nod-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein (ASC) and caspase-1 that recognise a diverse set of unrelated stimuli including PAMPs, DAMPs and even inorganic molecules. Activation of NLRP3, which is the best-characterised NLR family member, promotes inflammasome assembly, which includes the ASC protein adaptor, and the recruitment and autocatalytic activation of caspase-1 and the subsequent induction of the proteolytic activation of the proinflammatory cytokines interleukin 1β (IL-1β) and IL-18.4 IL-1β is a key proinflammatory mediator that underlies the pathogenesis of several metabolic disorders, including the obesity-associated inflammatory response and the development of insulin resistance.5 Therefore, inflammasome activation must be tightly regulated to avoid potentially harmful inflammation.6
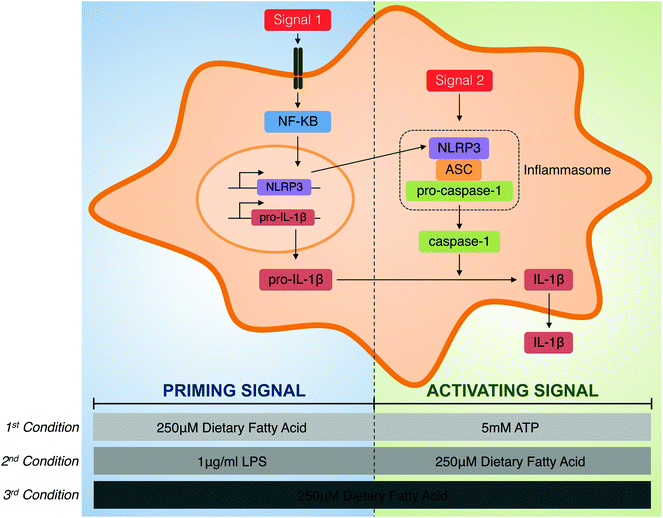
The activation of the inflammasome and IL-1β secretion requires two signals. A first signal is responsible for priming the cell to induce the transcription of the immature form of IL-1β, pro-IL-1β and NLRP3, which are limiting factors required for inflammasome activation, via the toll-like receptor 4-(TLR4) mediated nuclear factor κB (NF-κB) activation pathway. The second signal, the activation signal, promotes conformational changes that induce NLRP3 inflammasome assembly and drives caspase-1 activation and, therefore, IL-1β maturation and secretion.6 In addition, inflammasome activation causes a rapid, proinflammatory form of cell death called pyroptosis.
Otherwise, the NLRP3 inflammasome might be activated by host-derived DAMP signals, which are abundant in metabolic stress. Therefore, inflammasome activation through diet-associated sensing signals can be considered a strong link between inflammation and metabolic disturbances.4,7,8 Indeed, it has been described that obesity-induced inflammasome activation in key metabolic tissues promotes chronic inflammation and contributes to the development of type 1 and type 2 diabetes.9
Obesity is caused by an energy imbalance and not only total fat intake but different dietary FA compositions may cause different effects on the onset of metabolic complications. In the present study, we analysed the inflammasome-mediated inflammatory effects of dietary FAs. For this reason, the two most important SFAs in blood, palmitate and stearate, and two PUFAs, DHA (n-3 PUFA) and arachidonic acid (n-6 PUFA), were tested as physiological inducers of NLRP3 inflammasome activation. Due to the fact that palmitate and stearate acids are both inflammasome activators, the aim of this study was to determine whether the FA type, especially omega-3 and omega-6 PUFAs of the diet, differentially affected NLRP3 inflammasome priming and activation steps in macrophages in a FA-rich environment.
Materials and methods
Preparation of FFA solutions
The FAs sodium palmitate and cis-4,7,10,13,16,19-docosahexaenoic acid were obtained from Sigma-Aldrich (Steinheim, Germany), arachidonic acid from Cayman Chemical (Michigan, USA), and stearic acid from Panreac (Barcelona, Spain). FAs were conjugated to fatty acid-free BSA, which was obtained from Sigma-Aldrich (Steinheim, Germany) at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.
1 molar ratio.
Cell culture and stimulation
Human THP-1 monocytes purchased from the European Collection of Animal Cell Cultures (ECACC no. 88081201) were cultured in RPMI 1640 medium from Gibco (Barcelona, Spain) supplemented with 2 mM L-glutamine, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin, 25 mM HEPES and 10% foetal bovine serum at 37 °C in a humidified incubator with 5% CO2. Cell culture reagents were provided by BioWhittaker (Verviers, Belgium). They were differentiated into macrophages by cultivation in a fresh medium containing 0.5 μg ml−1 phorbol 12-myristate 13-acetate (PMA), from InvivoGen (San Diego, USA), for 24 h. After differentiation, the medium containing PMA was removed, and adherent THP-1 macrophages were incubated in a supplemented medium for 24 h.To explore the role of individual FAs in the modulation of inflammasome priming and activation, three different roles were tested (Fig. 1). First, the priming capacity of each individual fatty was determined and, for this purpose, THP-1 macrophages were primed with 250 μM of BSA-conjugated FAs for the indicated times, and the NLRP3 inflammasome was activated using 5 mM of ATP from Sigma-Aldrich (Steinheim, Germany). Second, the activation capacity of dietary FAs with LPS-induced inflammasome priming was analysed. Therefore, THP-1 cells were primed with 1 μg ml−1 of LPS (E. coli 0111:B4) from Sigma-Aldrich (Steinheim, Germany) for the indicated times before supplementation with 250 μM of each FA. Third, the capacity of each FA to activate the inflammasome per se was determined in terms of IL-1β secretion. In this case, THP-1 macrophages were incubated with 250 μM of FAs alone.
Protein extraction and western blotting
After 2 h of priming and 4 h of activation with the indicated compounds (either fatty acids, LPS or ATP), cytosolic fractions were extracted, and protein expression was determined by western blotting following a previously described procedure.10 Briefly, protein was separated on a 10% SDS-PAGE gel, transferred to a PVDF membrane and blocked prior to detection with anti-COX2 (Bioworld, Barcelona, Spain), anti-NLRP3 or anti-IL-1β (Abcam, Cambridge, UK). Semi-quantification of protein levels was performed using the ImageJ software (National Institutes of Health, Maryland, USA).Caspase-1 activation assay
THP-1 macrophages were primed for 30 min with either 250 μM of the corresponding FAs or LPS (1 μg ml−1), followed by activation for 1 h with the corresponding stimuli according to Fig. 1 (5 mM of ATP or 250 μM of fatty acids) before the addition of FAM-YVAD-fmk (Immunochemistry Technologies), which is a fluorescent, cell-permeable probe that covalently binds only to the active form of caspase-1. Active caspase-1 was determined fluorimetrically. Fluorescence intensity was determined at λex = 490 nm and λem = 520 nm using an FLx800 Multi-Detection Microplate Reader (Biotek, Winooski, USA).IL-1β secretion
Mature IL-1β levels were analysed in culture media using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (BioLegend) and normalised to the protein content measured using the Bradford assay (Bio-Rad).Statistical analysis
All statistical analyses were performed using the SPSS 20.0 software for Windows and were expressed as mean ± S.E.M. The effects of FAs were assessed using the ANOVA test for multiple comparisons followed by Tukey's post-hoc test for pair-wise comparisons. Differences were considered to be statistically significant for P values <0.05.Results
FAs modulate the activation of the NF-κB pathway in human macrophages differentially
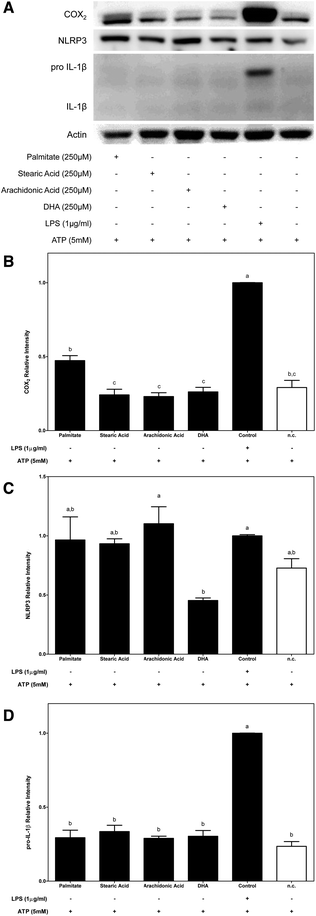
To determine whether the FA composition of a diet might have a crucial role in macrophage inflammasome activation, modulation of the NF-κB signalling pathway was first analysed.Given the potential role of NF-κB in relaying proinflammatory signals, COX2 protein expression was determined to test whether the FA structure (saturated or polyunsaturated) could be involved in activation upstream of the NF-κB signalling pathway. The effects of dietary FAs on NF-κB and subsequent COX2 protein expression were determined under two different conditions (Fig. 2 and 3, respectively). First, we determined the role of dietary FAs as a priming signal and, for this purpose, macrophages were primed for 2 h with each of the studied FAs before their activation with ATP for 4 h. Under the first condition (Fig. 2A and B), only the use of palmitate as a priming signal potentially increased COX2 protein expression in THP-1 macrophages. On the other hand, the use of stearic acid or unsaturated FAs as priming signals for inflammasome activation did not produce any significant effect regarding COX2 protein expression.
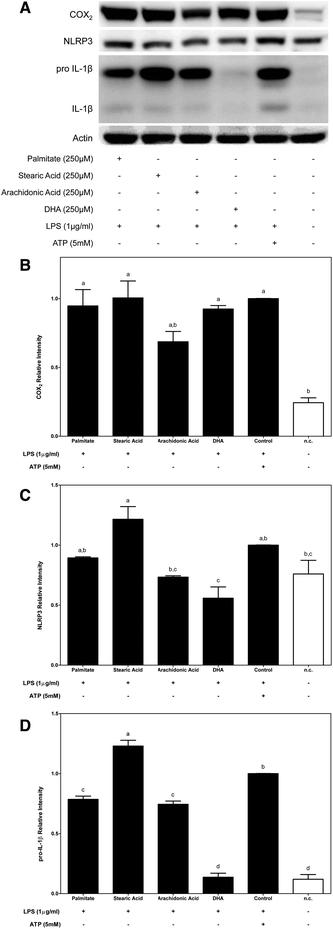
Second, the effects of dietary FAs on COX2 protein expression induced by LPS-primed macrophages were determined. THP-1 macrophages were primed for 2 h with LPS prior to supplementation with the corresponding FA acid for 4 h (Fig. 3A). In this situation, COX2 protein expression that was induced after priming macrophages with LPS was decreased only by supplementation with n-6 arachidonic acid (Fig. 3B).
SFA and UFA omega-3 and omega-6 modulate NLRP3 inflammasome priming differentially
Among the NF-κB up-regulated proinflammatory mediators, the increase in NLRP3 and IL-1β transcription, which is known to be the priming step, is a critical checkpoint that is required for inflammasome activation. In this way, to study the capacities of dietary FAs in modulating the NLRP3 and IL-1β cytosolic pools, their protein expressions were determined.In this way, while supplementation of macrophages with 250 μM of each SFA (palmitate and stearic acid) before ATP activation was slightly effective in increasing the cytosolic pool of NLRP3, conversely the n-3 DHA UFA significantly decreased NLRP3 protein expression in macrophages (Fig. 2A and C). On the other hand, under the conditions where FAs were used as priming signals, no changes in the cytosolic expression of the immature or mature forms IL-1β were observed (Fig. 2A and D).
When macrophages were primed with LPS before fatty acid supplementation, the cytosolic pool of NLRP3 proteins was increased after supplementation with stearic acid (SFA) and was decreased by the n-3 PUFA DHA (Fig. 3A and C).
Moreover, the expression of immature IL-1β that was induced by priming macrophages with LPS was significantly blocked by dietary FAs except stearic acid. In this way, while stearic acid induced an increase in the cytoplasmic expression of pro-IL-1β, palmitate and n-6 and n-3 UFAs significantly decreased its expression. It should be noted that the dramatic inhibition of pro-IL-1β protein expression that was promoted by DHA supplementation lowered the protein amount to levels similar to those of the untreated control (Fig. 3A and D).
All the FAs tested were activators of NF-κB target genes (COX-2, NLRP3 and pro-IL-1β) except for DHA which was an inhibitor.
FAs modulate caspase-1 activation differentially
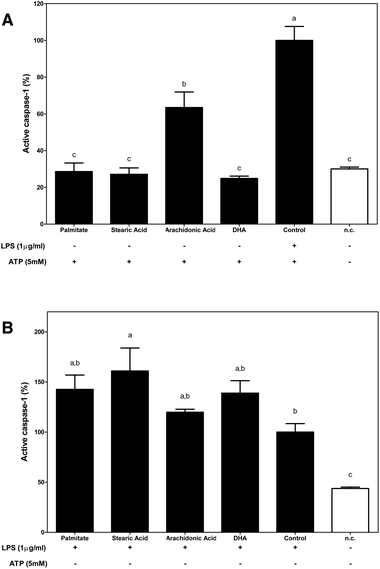
To analyse the role of dietary FA in inflammasome activation, the percentage of activated caspase-1, an essential step in controlling IL-1β maturation after NLRP3 assembly, was determined.Under the first condition that was analysed, FAs were used as a priming stimulus for caspase-1 (Fig. 4A). Fluorometric determinations identified n-6 arachidonic acid as a direct caspase-1 activator.
Although the supplementation of macrophages with palmitate, n-6 and n-3 FAs slightly increased the amount of active caspase-1 when macrophages were primed with LPS, only stearic acid induced a significant increase in caspase-1 activation, which enhanced the activating effects that were induced by LPS (Fig. 4B).
IL-1β secretion is induced by FA administration differentially
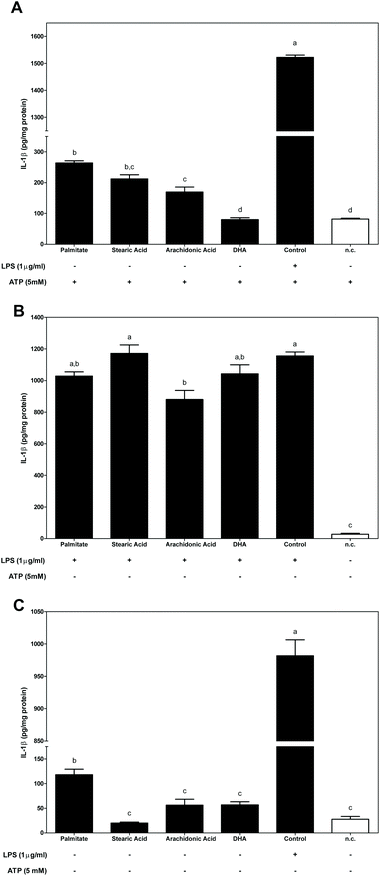
To determine whether dietary FAs modulated inflammasome activation, the secretion of IL-1β was assessed (Fig. 5). The activity of FAs on inflammasome activation and the resulting IL-1β secretion were evaluated using the three different conditions, as detailed in Fig. 1.First, FAs were used as priming signals before inflammasome activation with ATP (Fig. 5A). Priming macrophages with either of the SFAs, palmitate or stearic, or with n-6 arachidonic acid significantly enhanced the secretion of IL-1β. However, DHA was ineffective as a priming stimulus and in the subsequent induction of IL-1β secretion.
Secondly, in LPS stimulated macrophages prior to supplementation with dietary FA (the second condition described in Fig. 1), LPS-induced IL-1β secretion was slightly blocked by treating cells with palmitate and n-3 DHA and was significantly decreased by n-6 arachidonic acid (Fig. 5B).
Finally, the effect of dietary FAs in directly activating IL-1β secretion without any other activating or stimulating signal was measured (Fig. 5C). Under the third condition, only macrophages stimulated by the SFA palmitate were sufficient to induce a significant increase in IL-1β secretion.
Discussion
Obesity is associated with a continuous increase of postprandial FA concentrations, which underlies the development of obesity-related metabolic complications. In this way, under chronic high-fat intake conditions, tissues and plasma-FFA levels are commonly elevated, so we used FAs at physiologically relevant concentrations.2,11Dietary fat has a dual role in human physiology. Besides its function as a source of energy or a cell membrane structural component, dietary FAs can also act as signalling molecules at different levels,12 including nuclear receptor ligands,13,14 or as modulators of the immune system activation. In this sense, the capacity of dietary FAs to interact with TLR4 has been previously described.15 For this reason, in addition to the total dietary fat intake during the onset and progression of metabolic disturbances, the different composition of fat, in terms of the degree of FA saturation, plays a key role.16,17 Under fasting conditions, the levels of total FFAs in human blood can reach up to 700 μM; however, this concentration can be widely modified after the intake of a fatty meal.18 Therefore, blood-circulating cells, such as monocytes or tissue-infiltrated macrophages, would be constantly exposed to relatively high concentrations of FFAs and around 250 μM if we consider each individual form.19
Moreover, dietary FA composition can be sensed by the innate immune response, via macrophages; thus, in a diet-induced obesity context, the hyperlipidaemia-promoted-excess of fat accumulation in metabolic tissues triggers the recruitment and activation of immune cells. In this way, inflammasomes are intracellular, innate immune sensors.6 Subsequently, danger signals resulting from metabolic disturbance can be sensed by NLRP3, a cytosolic pattern recognition receptor that is expressed mostly in monocytes/macrophages, resulting in caspase-1-mediated IL-1β maturation and secretion. This metabolic-triggered inflammasome activation has been linked to the development and progression of many metabolic disorders, including, among others, obesity and type 2 diabetes.2,16,20
In this manner, based on the assumption that dietary FAs might influence the immune system as well the function of various cellular components, the aim of this study was to determine whether the chemical properties that are linked to the degree of FA acid saturation and the site of unsaturation might differentially affect inflammasome activation and IL-1β secretion.
The inflammasome-mediated maturation and secretion of IL-1β secretion is the result of a two-step process. A first signal, known as the priming signal, is responsible for priming the cell, via the toll-like receptor 4-(TLR4) mediated nuclear factor κB (NF-κB) activation pathway. This mediates the transcription of NLRP3 and the immature form of IL-1β. The second signal, the activation signal, induces NLRP3 inflammasome assembly and drives caspase-1 activation and, therefore, IL-1β maturation and secretion.6 IL-1 β exerts proinflammatory actions locally and systemically. We discuss the complex regulatory mechanisms that facilitate a balanced but effective inflammasome-mediated immune response. Although we have gained detailed knowledge about many aspects of inflammasome activation by bacterial lipopolysaccharides, nitric oxide, cholesterol or urate crystals, fatty acids, and ATP, several important questions remain unanswered or poorly understood regarding their mechanisms of regulation.
So, the capacity of four different FAs that are present in diets, two SFAs (palmitate and stearic acid) and two PUFAs (n-6 arachidonic acid and n-3 DHA) as modulators of NLRP3 inflammasome activation were determined by supplementing human macrophages with physiological concentrations of FAs under the three different conditions (Fig. 1). First, the role of FAs as a priming signal before ATP-induced NLRP3 inflammasome activation was studied. Second, the capacity of FAs to activate IL-1β secretion in LPS-primed macrophages was determined. Third, the inflammasome-activating properties of dietary FAs per se, that is, without the administration of any other priming or activating exogenous signal were analysed.
In this way, using the first proposed condition, the effects of dietary FAs acting as priming signals in inflammasome activation were evaluated. We demonstrated that SFAs that were analysed, palmitate and stearic acid, as well as the n-6 PUFA acted as priming signals of inflammasome activation; however, n-3 DHA had no effect. In this way, the supplementation of macrophages with SFAs or n-6 PUFA before ATP-mediated inflammasome activation significantly increased IL-1β secretion, while n-3 DHA supplementation showed no stimulating effect on IL-1β secretion. Regarding, the inflammasome activation checkpoints that are regulated by these FAs, palmitate was the only FA that was able to enhance COX2 protein expression in macrophages, supporting the role of palmitate as a TLR4 agonist and as an activator of NF-κB. Then, the modulation of the cytosolic pool of NLRP3 was only significantly modulated by DHA, which induced a decrease in its protein expression. Otherwise, caspase-1 activation levels were only significantly enhanced when n-6 arachidonic was used as a priming stimulus.
On the other hand, although it has been controversial for years, it has currently been described that acute priming of LPS is solely required to boost the inflammasome and activate caspase-1 and the resulting secretion of mature IL-1β.21–23 Thus, using the second experimental condition, the capacities of dietary FAs to interfere with LPS-induced inflammasome activation were determined. Of the dietary FAs that were tested, supplementation of LPS-primed macrophages with stearic acid potentiated the proinflammatory effects induced by LPS, which enhanced the cytosolic pool of the two limiting components of inflammasome activation, NLRP3 and pro-IL-1β, and caspase-1 activation.
Surprisingly, arachidonic acid interfered inversely with LPS-induced NF-κB activation. We showed that supplementation of LPS-primed macrophages with arachidonic acid decreased COX2, NLRP3 and pro-IL-1β protein abundance in the cytoplasm of LPS-primed macrophages and decreased IL-1β secretion. These results indicate that supplementation of macrophages with n-6 arachidonic acid might modulate the efficiency of LPS to induce NF-κB activation. Although arachidonic acid is a precursor of the cyclooxygenase-mediated synthesis of eicosanoids, it is also stored in the cell membrane. It has been described that the modification of cell membrane fatty acid composition influences membrane fluidity, which, in turn, would modify the membrane receptor function as well as signal transduction mechanisms.24 Thus, an arachidonic acid-rich diet could modify the cell membrane composition, which could affect its fluidity and, then, signal transduction, as was observed for LPS-primed macrophages.
Interestingly, n-3 DHA inhibited LPS-mediated inflammasome activation. This inhibition was primarily mediated by the significant repression of NLRP3 and pro-IL-1β protein expression, which, in turn, translated to a slight decrease in the secretion of mature IL-1β, confirming that DHA modulation of inflammasome activation is mainly through the inhibition of LPS-induced NF-κB activation.
Finally, using the third condition, the abilities of dietary fatty acids to modulate per se the priming and activation of the inflammasome and IL-1β were analysed. We demonstrated that only palmitate was able, per se without any additional stimulus (either priming or activating), to significantly induce the secretion of IL-1β. These results are in accordance with the description of palmitate as a ligand for TLR4, which promotes the activation of NF-κB and the transcription of its proinflammatory target genes.15 Moreover, it has been described previously that the stimulation of macrophages with palmitate can induce the production of reactive oxygen species (ROS).25 Therefore, palmitate may induce the activation of the inflammasome and the secretion of IL-1β in macrophages by directly acting as a priming signal through its TLR4-agonist activity and by sensing NLRP3, which mediates the production of ROS, similar to that described previously for LPS.26
In summary, we can conclude that dietary FA composition can be sensed by NLRP3 inflammasome activation and that the chemical structure of these FAs, regarding the degree of saturation, is critical for its regulating effects. Whereas SFAs, such as palmitate and stearic acid, show mainly a proinflammatory profile by promoting inflammasome activation and IL-1β secretion,27 PUFAs are inhibitors of inflammasome activation by mainly interfering with LPS-TLR4 signal transduction, NF-κB and the inflammasome activation step, n-3 PUFA being stronger inhibitors than n-6 PUFA. With these results, we have provided new insights regarding the molecular-sensing mechanisms involved in signal transduction by high levels of FAs to trigger intracellular inflammatory signalling, which is a mechanism underlying the development of diet-induced chronic inflammation and metabolic disturbances. In this way, the diet and, more accurately, the modification of FA composition, mainly the increase in the proportion of DHA content in the diet, can be a powerful tool for regulating the inflammatory and innate immunity responses and thus for preventing inflammation. However, the ability of a particular trigger to activate inflammasomes and the IL-1β family cytokines in vitro does not necessarily indicate that inflammasomes fully control the inflammatory response to the respective trigger in vivo.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Rovira i Virgili University grant for PhD students and by a grant from the Ministerio de Ciencia e Innovación (MICCIN), AGL2014-55347-R, from the Spanish Government.References
- G. S. Hotamisligil, Nature, 2006, 444, 860–867 CrossRef CAS PubMed.
- L. Hodson, C. M. Skeaff and B. A. Fielding, Prog. Lipid Res., 2008, 47, 348–380 CrossRef CAS PubMed.
- A. P. Simopoulos, Biomed. Pharmacother., 2002, 56, 365–379 CrossRef CAS.
- F. Martinon, A. Mayor and J. Tschopp, Annu. Rev. Immunol., 2009, 27, 229–265 CrossRef CAS PubMed.
- H. Wen, J. P. Y. Ting and L. A. J. O'Neill, Nat. Immunol., 2012, 13, 352–357 CrossRef CAS PubMed.
- T. Strowig, J. Henao-Mejia, E. Elinav and R. Flavell, Nature, 2012, 481, 278–286 CrossRef CAS PubMed.
- K. Schroder and J. Tschopp, Cell, 2010, 140, 821–832 CrossRef CAS PubMed.
- D. De Nardo and E. Latz, Trends Immunol., 2011, 32, 373–379 CrossRef CAS PubMed.
- R. Stienstra, C. J. Tack, T.-D. Kanneganti, L. A. B. Joosten and M. G. Netea, Cell Metab., 2012, 15, 10–18 CrossRef CAS PubMed.
- N. Martinez-Micaelo, N. Gonzalez-Abuin, X. Terra, C. Richart, A. Ardevol, M. Pinent and M. Blay, Biochem. J., 2012, 441, 653–663 CrossRef CAS PubMed.
- B. A. Fielding, J. Callow, R. M. Owen, J. S. Samra, D. R. Matthews and K. N. Frayn, Am. J. Clin. Nutr., 1996, 63, 36–41 CAS.
- E. Duplus, M. Glorian and C. Forest, J. Biol. Chem., 2000, 275, 30749–30752 CrossRef CAS PubMed.
- S. A. Kliewer, S. S. Sundseth, S. A. Jones, P. J. Brown, G. B. Wisely, C. S. Koble, P. Devchand, W. Wahli, T. M. Willson and J. M. Lenhard, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 4318–4323 CrossRef CAS.
- L. Michalik, B. Desvergne and W. Wahli, Nat. Rev. Cancer, 2004, 4, 61–70 CrossRef CAS PubMed.
- H. Shi, M. V. Kokoeva, K. Inouye, I. Tzameli, H. Yin and J. S. Flier, J. Clin. Invest., 2006, 116, 3015–3025 CrossRef CAS PubMed.
- J. E. Schaffer, Curr. Opin. Lipidol., 2003, 14, 281–287 CrossRef CAS PubMed.
- G. A. Bray, J. C. Lovejoy, S. R. Smith, J. P. DeLany, M. Lefevre, D. Hwang, D. H. Ryan and D. A. York, J. Nutr., 2002, 132, 2488–2491 CAS.
- D. B. Jump, Crit. Rev. Clin. Lab. Sci., 2004, 41, 41–78 CrossRef CAS PubMed.
- H. Shapiro, A. Lutaty and A. Ariel, Sci. World J., 2011, 11, 2509 CrossRef PubMed.
- K. Schroder, R. Zhou and J. Tschopp, Science, 2010, 327, 296–300 CrossRef CAS PubMed.
- M. G. Netea, C. A. Nold-Petry, M. F. Nold, L. A. B. Joosten, B. Opitz, J. H. M. van der Meer, F. L. van de Veerdonk, G. Ferwerda, B. Heinhuis and I. Devesa, Blood, 2009, 113, 2324–2335 CrossRef CAS PubMed.
- K. Schroder, V. Sagulenko, A. Zamoshnikova, A. A. Richards, J. A. Cridland, K. M. Irvine, K. J. Stacey and M. J. Sweet, Immunobiology, 2012, 1325–1329 CrossRef CAS PubMed.
- J. C. Kagan, Science, 2013, 341, 1184–1185 CrossRef CAS PubMed.
- P. C. Calder, J. A. Bond, D. J. Harvey, S. Gordon and E. A. Newsholme, Biochem. J., 1990, 269, 807–814 CrossRef CAS PubMed.
- C. Shrestha, T. Ito, K. Kawahara, B. Shrestha, M. Yamakuchi, T. Hashiguchi and I. Maruyama, Biochem. Biophys. Res. Commun., 2013, 573–578 CrossRef CAS PubMed.
- J. Tschopp and K. Schroder, Nat. Rev. Immunol., 2010, 10, 210–215 CrossRef CAS PubMed.
- L. L'homme, N. Esser, L. Riva, A. Scheen, N. Paquot, J. Piette and S. Legrand-Poels, J. Lipid Res., 2013, 54, 2998–3008 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |