Open Access Article
Open Access ArticleIntake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats
Yolanda
Aguilera†
a,
Miguel
Rebollo-Hernanz†
 a,
Teresa
Herrera
a,
L. Tábata
Cayuelas
a,
Pilar
Rodríguez-Rodríguez
b,
Ángel L. López
de Pablo
b,
Silvia M.
Arribas
b and
María A.
Martin-Cabrejas
*a
a,
Teresa
Herrera
a,
L. Tábata
Cayuelas
a,
Pilar
Rodríguez-Rodríguez
b,
Ángel L. López
de Pablo
b,
Silvia M.
Arribas
b and
María A.
Martin-Cabrejas
*a
aInstituto de Investigación de Ciencias de la Alimentación (CIAL), Facultad de Ciencias, Universidad Autónoma de Madrid, Spain. E-mail: maria.martin@uam.es; Tel: +34 91 001 7913; Fax: +34 91 497 3826
bDepartamento de Fisiología, Facultad de Medicina, Universidad Autónoma de Madrid, Spain
First published on 20th January 2016
Abstract
Melatonin is an endogenous antioxidant hormone, which reduces with ageing and the low levels are associated with some chronic diseases. Germination of legumes increases the plant levels of melatonin, making sprouts a suitable food source of this hormone. However, information on its bioavailability after consumption is lacking. We aimed to evaluate in rats the effect of kidney bean sprout intake on the plasma levels of melatonin and metabolically related compounds (serotonin, 6-sulfatoxymelatonin), total phenolic compounds and total antioxidant capacity. In addition, we compared the plasma bioavailability derived from kidney bean sprouts versus synthetic melatonin intake. Kidney beans were germinated for 6 days and an extract was prepared in water. Male young Sprague Dawley rats were used; blood and urine samples were obtained before and after 90 min of administration of kidney bean sprout extract via a gavage. The plasmatic melatonin levels increased after sprout ingestion (16%, p < 0.05). This increment correlated with the urinary 6-sulfatoxymelatonin content, the principal biomarker of plasmatic melatonin levels (p < 0.01). Nevertheless, the phenolic compounds and antioxidant capacity levels did not exhibit any significant variation. The comparison of the bioavailability between the melatonin contained in the kidney bean sprouts and in a synthetic solution evidenced slightly higher levels of plasmatic melatonin (17%) in rats fed with the solution of synthetic melatonin. We conclude that kidney bean sprouts could be a good source of dietary melatonin and other bioactive compounds known to have health benefits.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is a molecule with a wide range of cellular and physiological actions.1 Melatonin shows potent antioxidative properties as a direct free radical scavenger2 as well as through its catabolites.3,4 Moreover, it has the ability to stimulate endogenous antioxidant enzymes (e.g. catalase, superoxide dismutase, etc.).5 An increase in plasmatic melatonin levels has been correlated with reduced oxidative stress.6 Exogenous melatonin is widely used for therapeutic purposes. Up to now, the use of melatonin has been restricted to the improvement of sleep quality, the alleviation of subjective feelings of jet lag, and the reduction of sleep onset latency.7 However, numerous studies concluded that melatonin could also be associated with the prevention of different diseases related to ageing and oxidative stress, including type 2 diabetes, cardiovascular diseases, neurodegenerative disorders or cancer.8–11Most generated knowledge about melatonin beneficial effects has been gained with exogenous synthetic melatonin either in vitro or in vivo (experimental animals and humans) and much less is known about the effect of diet on the synthesis and plasmatic levels of melatonin. Recent findings claim the importance of food intake on the plasmatic level of melatonin.12 It has been detected that fasting periods and energy restriction decrease the nocturnal secretion of melatonin;13 high-calorie food also modifies melatonin secretion.14 In addition to the relevant influence of the light-dark cycle, diet and nutrients might also modulate the melatonin plasmatic levels.14 It has been proposed that the consumption of plant foods containing melatonin may improve human health due to its biologic activities and bioavailability.15 For example, it has been reported that the ingestion of products rich in melatonin increases the plasmatic levels of the hormone or the excreted urinary metabolite 6-sulfatoxymelatonin (aMT6s).6,16 Nevertheless, more studies are needed to explore the melatonin bioavailability and plasma fluctuation levels after intake of foods rich in this compound.
The presence of melatonin in different legumes has recently been studied.17–19 These foods are important sources of proteins, vitamins and minerals. They have recently received further attention because of their health benefits on chronic disease prevention, attributed to their relevant soluble and insoluble fibers, slow digestive starch, prebiotic oligosaccharides and phenolic content.20,21 Some of these compounds possess antioxidant properties that are correlated with their potential health benefits in aging and prevention of oxidative stress-associated diseases.21
Legume germination is a simple and commonly used process for improving their nutritional value in many countries. This processing significantly reduces the non-nutritive components, increases the digestibility of proteins and bioavailability of certain minerals and vitamins. Furthermore, we have previously demonstrated that germination enhances the content of antioxidant bioactive compounds, including melatonin.19,22 Hence, the consumption of germinated legumes could be a strategy to prevent, through the diet, the mentioned diseases associated with oxidative stress.
Thus, this study aims to determine in rats whether the intake of germinated kidney beans (Phaseolus vulgaris L.) would alter the melatonin levels and antioxidant capacity in the plasma, as well as potentially related biomarkers such as serotonin and total phenolic compounds in the plasma, and aMT6s in urine. In addition, it also assessed the food matrix effect on melatonin absorption, comparing its levels and antioxidant capacity after synthetic melatonin or kidney bean sprout extract consumption.
Materials and methods
Kidney bean sprout extracts
Kidney beans (Phaseolus vulgaris L. var. Pinta), provided by Institute of Food Science, Technology and Nutrition (CSIC, Madrid), were germinated according to Aguilera et al.23 This process showed good viability, 98% being the percentage of germination. Sprouts were freeze-dried, milled, packed in vacuum bags, and stored at −20 °C. Analyses of sprouts in triplicate were carried out to determine the melatonin, phenolic compounds, and antioxidant capacity as described previously.19The extract from kidney bean sprouts was prepared as follows: kidney bean sprout flour (20 g) was mixed with ethanol (150 mL) and shaken for 16 h at 4 °C in the dark. The mixture was sonicated for 15 min and filtered under vacuum through 11 μm filters (Whatman). The extract was evaporated at 30 °C to dryness and redissolved in 3 mL PBS buffer. The melatonin content in the extract was analyzed, being 10.6 μg. The extract was dissolved in 3 mL Milli-Q water. A synthetic melatonin (≥98%; Sigma-Aldrich Química, Spain) solution of the same concentration as the sprout extract was prepared in Milli-Q water, shaken, and sonicated for 15 min, at the same level as the kidney bean extract.
Animals and experimental design
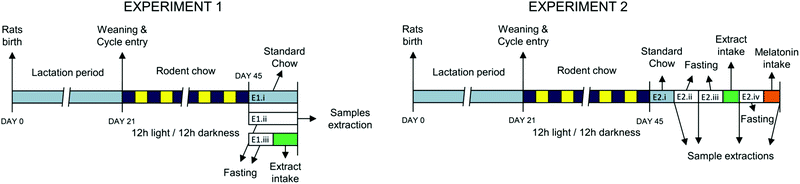
Experiments were performed in Sprague Dawley rats from the colony maintained at the Animal House facility of the Universidad Autónoma de Madrid (Fig. 1). All experimental procedures were approved by the Ethics Review Board of Universidad Autónoma de Madrid and conformed to the Guidelines for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised in 1996), the Spanish legislation (RD 1201/2005) and the Directive 2010/63/EU on the protection of animals used for scientific purposes.| Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|
| Control | Fasting | KB extract | Control | Fasting | KB extract | MEL | |
| Intervention | None | 24 hour-fast | 24 hour-fast | None | 12 hour-fast | 12 hour-fast | 12 hour-fast |
| Bean extract | Bean extract | Melatonin | |||||
| Rat population (N) | 8 ♂ | 8 ♂ | 8 ♂ | 8 ♂ | |||
| Weight (g) | 202 ± 12 | 193 ± 8 | 208 ± 10 | 201 ± 7 | |||
| Entrance into inverse photoperiod (day) | 21 | 29 | 21 | 21 | |||
| Time into inverse photoperiod (days) | 25 | 15 | 31 | 23 | 29 | 35 | 41 |
| Sampling day | 46 | 44 | 52 | 44 | 50 | 56 | 62 |
| Sampling time | 10:30 | 10:30 | 10:30 | 10:30 | 10:30 | 10:30 | 10:30 |
Thereafter, the rats were individually caged and a cling film was placed below the cage to obtain the urine samples. After 90 min of extract administration, all rat groups were anesthetized by CO2. Urine was collected from the cling film with a pipette and transferred to a vial. The blood was collected by cardiac puncture, transferred to vials containing 5% heparin and centrifuged at 4 °C for 15 min at 2100g. The plasma was then divided into aliquots in 1 mL vials and kept frozen at −80 °C to assess several biomarkers related to melatonin metabolism. All experiments were carried out at 10:30 a.m., which was the peak time of melatonin production under the changed light/dark cycle.
Biochemical determinations
Antioxidant capacity
where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence reading at time i. The net AUC corresponding to a sample was calculated as follows:
| net AUC = AUC antioxidant − AUC blank |
The net AUC was plotted against the antioxidant concentration, and the regression equation of the curve was calculated. The ORAC value was obtained by dividing the slope of the latter curve between the slopes of the Trolox curve obtained in the same assay. The final ORAC values were expressed as mM Trolox equivalents (mM TE).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (v/v/v)] were warmed to 37 °C, and then 50 μL of plasma samples were added. The absorbance was recorded at 593 nm against the reagent blank after 10 min. FRAP values were calculated and expressed as μM Trolox equivalents (μM TE).
1) (v/v/v)] were warmed to 37 °C, and then 50 μL of plasma samples were added. The absorbance was recorded at 593 nm against the reagent blank after 10 min. FRAP values were calculated and expressed as μM Trolox equivalents (μM TE).
Statistical analysis
Each sample was analysed in triplicate. Data were expressed as mean ± standard deviation (SD). The data were analysed by one-way analysis of variance (ANOVA) and post hoc Duncan tests. The relationships between the analysed parameters were evaluated by computing the Pearson linear correlation coefficients setting the level of significance at p < 0.05 and p < 0.001. The statistical analysis was performed by using SPSS 21.0. Additionally, for curve-fitting analysis in ELISA assays, the results were processed by using the 4-parameter logistic nonlinear regression model, using OriginPro 8.5.Results and discussion
Characterization of been sprout extract
It has been recently reported that germination has led to improvements in melatonin levels, bringing about significant increases of antioxidant activity in bean sprouts.19,23 However, studies on the bioavailability of melatonin contained in these germinated legume seeds and the possible impact that their intake may have on health have not been performed.Studies have demonstrated that the presence of melatonin in plants is universal and its levels vary widely.27,28 Melatonin in legumes may be related to the protection of highly oxidizable lipids from oxidation, thereby preserving the seed viability for germination.29 The performed germination was already evaluated in previous studies,23 to maximize the load of these compounds and the antioxidant capacity. In the present study, melatonin was identified and quantified in germinated bean extracts with a value of 529.1 ng g−1 (Table 2). The extract also contained phenolic compounds (336.7 mg GAE per 100 g), lower than other bean varieties.30,31
| Melatonin (ng g−1) | TPC (mg GAE per 100 g) | ORAC (μmol TE per g) |
|---|---|---|
| a Results are reported as mean ± SD (n = 3). | ||
| 529.1 ± 27.5 | 336.7 ± 35.8 | 43.1 ± 3.5 |
Both melatonin and phenolic compound levels, as well as other antioxidant phytochemicals, exhibit changes in their contents along the germination process.19,32 The antioxidant capacity of the extract (43.1 μmol TE per g) was mainly due to the relevant content of phenolic compounds. The ORAC data were in agreement with the results reported in the literature for raw common bean varieties,33 and other legumes, as lentils, chickpeas, or lupins.32 Wu et al.34 investigated ORAC in common foods and the results showed that kidney beans exhibited higher levels than other foods, including many fruits commonly believed to be rich in antioxidants. This extract was then used to feed one group of rats (KB extract).
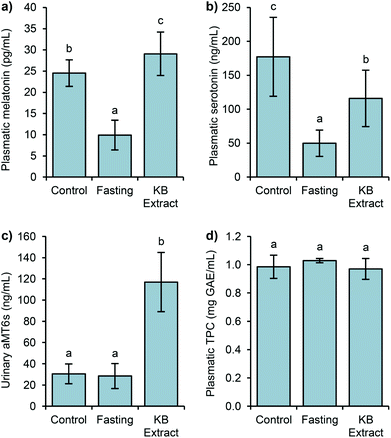
It is worth pointing out that in most studies the addition of exogenous melatonin to laboratory animals is mainly carried out in tap water.37 Our results implied the possibility of using food as the melatonin source. Hence, a relevant content of melatonin contained in the diet may be absorbed by the gastrointestinal tract, increasing its level in the plasma. However, our results were not as high as melatonin levels reported after walnut ingestion.6 Several factors might be involved in the plasmatic levels of melatonin; among them are the age of animals, the amount of ingested melatonin, intake period (from hours to weeks), etc.6,38,39 In addition, the time of blood collection might have an influence.40 It has been demonstrated that consumption of different fruits lead to variations in the plasmatic melatonin levels due to their different bioavailabilities.39,41,42
Furthermore, possible differences in the tryptophan content in plant matrices may also enhance the synthesis of extra-pineal melatonin by the biotransformation via serotonin to melatonin, for example in the gastrointestinal tract.43
Fig. 2b shows the serotonin variations in the above groups of rats. In the control group, the level reached 177 ng mL−1. The 24-hour fasting group produced a drastic decrease of serotonin (72%), being 50 ng mL−1. The group subjected to 24 h of fasting followed by the administration of germinated bean extract reached 116 ng mL−1 of serotonin, representing 1.3 fold higher than the fasting group. After 90 min of intake, an important increase of the serotonin levels was produced. Thus, the influence of diet seems relevant because after 90 min of its administration, the serotonin levels reached 65% of the level from the control group.
The levels of urinary 6-sulfatoxymelatonin (aMT6s), the major metabolite of melatonin in urine is considered to be a good indicator of melatonin in the plasma, showing a correlation with the plasmatic hormone levels.41 The basal aMT6s level was 30 ng mL−1, interestingly these levels remained after fasting (fast, 28 ng mL−1), but a drastic increment of the aMT6s levels (4-fold) was detected after the intake of the bean extract (KB extract), compared to the basal and fasting levels (Fig. 2c). The KB extract results corroborated the previous studies which observed the association of vegetable and fruit intake with significant increases in the urinary aMT6s levels.41,42 The aMT6s levels found after the bean extract intake brought about the high catalytic efficiency for melatonin sulfation in rats.44 Melatonin is rapidly metabolized to 6-hydroxymelatonin which is further conjugated to aMT6s.45 Likewise, the total intrinsic clearance rate of melatonin sulfation presents considerable species differences, being higher in rats than in humans or mice.44 Consequently, the level of aMT6s increases rapidly after the intake of the bean extracts, rich in melatonin.
Regarding plasmatic phenolic compounds (Fig. 2d), all three studied groups exhibited statistically similar TPC levels (1 mg GAE mL−1). In Sprague Dawley rats fed with tea, the achieved levels of TPC were similar to those found in the present work.46 The plasmatic half-life of these compounds usually ranges from 2 to 8 h, but sometimes it can reach up to 12–24 h.47 It has been shown that plasmatic phenolic levels generally exhibit sharp decreases one hour after the ingestion.48,49 However, in our study, the levels of phenolic compounds in the plasma of 24 h-fasted rats did not show any decrease, probably due to their accumulation either in the plasma or in other tissues as some studies have previously demonstrated.50 Their presence in the gut, principally in the large intestine, in higher quantities than in the plasma, seems to be the principal cause for the maintenance of the total phenolic load.49 Because colonic microbiota mediates the formation of phenolic acids from larger phenolic compound polymers through glycoside hydrolysis, ring fission, and oxidation, the resulting metabolites can be absorbed and enter the systemic circulation.49,51,52 Consequently, the TPC measurement after 24 h-fasting would show the content of all those compounds, coming from the diet of the rats, made mainly of plant foods (wheat, corn, wheat bran, barley, soybean, etc.). Likewise, as complex phenolic compounds and glycosides required to be transformed in the colon to be absorbed, in a short term intake of kidney bean extract, phenolic compounds might not reach this digestion stage, and not be bioavailable.
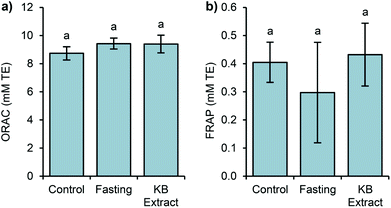
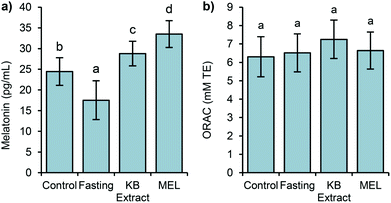
In this study, two assays (ORAC and FRAP) were selected to evaluate the antioxidant capacity because of the different antioxidant mechanisms assessed by these methods (ORAC by hydrogen atom transfer and FRAP by assessing single electron transfer). It is known that most antioxidants act by a combination of both mechanisms, and melatonin is no exception.53 The ORAC data exhibited similar values, with no significant differences (Fig. 3).
In the same way, the FRAP antioxidant levels show no significant difference between the studied groups. Thus, the antioxidant capacity measured by the above assays may not reflect the total influence of melatonin in germinated bean extract on endogenous antioxidant capacity.
FRAP and ORAC assays only measure the free radical scavenging capacity and melatonin exhibits direct and indirect antioxidant actions,54,55 including the stimulation of endogenous antioxidant enzyme expression.5 Since we measured the antioxidant capacity after a single extract administration, we could not detect these indirect antioxidant effects of melatonin. A long term administration with kidney bean sprouts could answer this question. In addition, it is possible that the results imply conservative estimation of antioxidant levels and cannot be just attributed to melatonin. As shown in other studies, the intake of food stuffs rich in melatonin such as cherries, grape juices or beers led to increases in the plasmatic antioxidant capacity. Nonetheless, these results could not be directly related to melatonin levels.16,42,56
Additionally, the Pearson linear correlation between the antioxidant capacity and the studied bioactive compounds was calculated, showing no relationship between them. Even if the statistics showed no correlation, we can assume that the antioxidant capacity measured by ORAC is mainly due to the levels of phenolic compounds, and other compounds not evaluated in this work. As it was mentioned, only the level of aMT6s in urine was correlated with the plasmatic melatonin level (r = 0.713, p < 0.01).
The melatonin levels displayed a similar behaviour as in the Experiment 1, corroborating the observed decrease of melatonin after a fasting period, and its sharp increase when the bean extract was consumed by the rats (Fig. 4).
Regarding the level of plasmatic melatonin in the fourth group (MEL), it showed a significantly higher level, compared to the rest of the groups. Therefore, the melatonin bioavailability in an aqueous solution resulted in 17% higher than that in the matrix of the kidney bean extract. From these results, it was highlighted that the food matrix influenced directly on the absorption of melatonin in the gut. Melatonin in the kidney bean extract is accompanied by other methanolic soluble compounds such as phenolics, which could modify its bioavailability, as described in other studies related to the evaluation of the dietary intake of melatonin from fruits.41
Concerning the antioxidant capacity, as observed in Experiment 1, the data remained similar in all groups, including the MEL group. Thus, the variations in the plasmatic levels of melatonin were not translated into differences in the antioxidant capacity.
Conclusions
Kidney bean sprouts have been demonstrated to contain great amounts of bioactive antioxidant compounds, especially phenolics, which constitute the main antioxidant phytochemicals found in the plasma. In addition, melatonin is available in the plasma after kidney bean sprout ingestion, which indicates that it is readily absorbed. The lack of increased plasma free radical scavenging capacity of kidney bean extract or pure melatonin is likely due to the masking effects of phenolic compounds. However, it is possible that increased plasma melatonin from food sources over prolonged time periods might exert similar indirect antioxidant actions, as previously described for pure compounds. In conclusion, germinated legumes are a suitable natural source of exogenous melatonin. However, additional work is still needed on this issue to determine the long term effects of dietary melatonin consumption on antioxidant defence systems and disease prevention. The health benefits derived from the dietary intake of melatonin are, until now, controversial, as it is not recognized if the chronic consumption of melatonin through the diet has physiological effects.Conflict of interest
The authors declare no conflict of interest.Abbreviations
| aMT6s | 6-Sulfatoxymelatonin |
| FRAP | Ferric reducing ability of plasma |
| GAE | Gallic acid equivalents |
| MEL | Melatonin |
| ORAC | Oxygen radical absorbance capacity |
| TE | Trolox equivalents |
| TPC | Total phenolic compounds |
Acknowledgements
This research was financially supported by the Second Call for Interuniversity Cooperation Projects University Autónoma de Madrid and the Santander Bank with United States (2013–2014). M. R. H. thanks MECD (Ministerio de Educación, Ciencia y Deporte) for his collaboration scholarship.References
- R. J. Reiter, D. X. Tan and L. Fuentes-Broto, Prog. Brain Res., 2010, 181, 127–151 CrossRef CAS PubMed.
- R. J. Reiter, S. D. Paredes, L. C. Manchester and D. X. Tan, Crit. Rev. Biochem. Mol. Biol., 2009, 44, 175–200 CrossRef CAS PubMed.
- A. Galano, D. X. Tan and R. J. Reiter, J. Pineal Res., 2013, 54, 245–257 CrossRef CAS PubMed.
- D. X. Tan, R. Hardeland, L. C. Manchester, A. Galano and R. J. Reiter, Curr. Med. Chem., 2014, 21, 1557–1565 CrossRef CAS PubMed.
- C. Rodriguez, J. C. Mayo, R. M. Sainz, I. Antolín, F. Herrera, V. Martín and R. J. Reiter, J. Pineal Res., 2004, 36, 1–9 CrossRef CAS PubMed.
- R. J. Reiter, L. C. Manchester and D. X. Tan, Nutrition, 2005, 21, 920–924 CrossRef CAS PubMed.
- European Food Safety Authority, EFSA J., 2010, 8, 1461–1467 Search PubMed.
- G. Favero, L. F. Rodella, R. J. Reiter and R. Rezzani, Mol. Cell. Endocrinol., 2014, 382, 926–937 CrossRef CAS PubMed.
- D. Zephy and J. Ahmad, Diabetes Metab. Syndr., 2015, 9, 127–131 CrossRef PubMed.
- G. Polimeni, E. Esposito, V. Bevelacqua, C. Guarneri and S. Cuzzocrea, Front. Biosci., Landmark Ed., 2014, 19, 429–446 CrossRef CAS.
- R. Hardeland, J. Pineal Res., 2013, 55, 325–356 CAS.
- M. Iriti and E. M. Varoni, J. Sci. Food Agric., 2015, 95, 2355–2359 CrossRef CAS PubMed.
- G. S. Roth, V. Lesnikov, M. Lesnikov, D. K. Ingram and M. A. Lane, J. Clin. Endocrinol. Metab., 2001, 86, 3292–3295 CrossRef CAS PubMed.
- A. G. Tavartkiladze, G. V. Simoniia, D. T. Kolbaia, A. G. Shalashvili and T. G. Petriashvili, Georgian Med. News, 2006, 132, 121–123 Search PubMed.
- M. Iriti, E. M. Varoni and S. Vitalini, J. Pineal Res., 2010, 49, 101–105 CAS.
- M. D. Maldonado, H. Moreno and J. R. Calvo, Clin. Nutr., 2009, 28, 188–191 CrossRef CAS PubMed.
- H. Zielinski, B. Lewczuk, B. Przybylska-Gornowicz and H. Kozłowska, in Biologically-active Phytochemicals in Food: Analysis, Metabolism, Bioavailability and Function, ed. W. Pfannhauser, G. R. Fenwick and S. Khokhar, Royal Society of Chemistry, Great Britain, 2001, pp. 110–117 Search PubMed.
- J. Hernández-Ruiz and M. B. Arnao, J. Agric. Food Chem., 2008, 56, 10567–10573 CrossRef PubMed.
- Y. Aguilera, R. Liébana, T. Herrera, M. Rebollo-Hernanz, C. Sanchez-Puelles, V. Benítez and M. A. Martín-Cabrejas, J. Agric. Food Chem., 2014, 62, 10736–10743 CrossRef CAS PubMed.
- C. Bassett, J. Boye, R. Tyler and B. D. Oomah, Food Res. Int., 2010, 43, 397–398 CrossRef.
- R. Campos-Vega, G. Loarca-Piña and B. D. Oomah, Food Res. Int., 2010, 43, 461–482 CrossRef CAS.
- Y. Aguilera, T. Herrera, V. Benítez, S. M. Arribas, A. L. López de Pablo, R. M. Esteban and M. A. Martín-Cabrejas, Food Chem., 2015, 170, 203–211 CrossRef CAS PubMed.
- Y. Aguilera, T. Herrera, R. Liébana, M. Rebollo-Hernanz, C. Sanchez-Puelles and M. A. Martín-Cabrejas, J. Agric. Food Chem., 2015, 63, 7967–7974 CrossRef CAS PubMed.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Methods Enzymol., 1998, 299, 152–178 Search PubMed.
- A. Dávalos, C. Gómez-Cordovés and B. Bartolomé, J. Agric. Food Chem., 2004, 52, 48–54 CrossRef PubMed.
- I. F. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- A. Hattori, H. Migitaka, M. Iigo, M. Itoh, K. Yamamoto, R. Ohtani-Kaneko, M. Hara, T. Suzuki and R. J. Reiter, Biochem. Mol. Biol. Int., 1995, 35, 627–634 CAS.
- R. Dubbels, R. J. Reiter, E. Klenke, A. Goebel, E. Schnakenberg, C. Ehlers, H. W. Schiwara and W. Schloot, J. Pineal Res., 1995, 18, 28–31 CrossRef CAS PubMed.
- L. C. Manchester, D. Tan, R. J. Reiter, W. Park, K. Monis and W. Qi, Life Sci., 2000, 67, 3023–3029 CrossRef CAS PubMed.
- Y. Aguilera, I. Estrella, V. Benitez, R. M. Esteban and M. A. Martín-Cabrejas, Food Res. Int., 2011, 44, 774–780 CrossRef CAS.
- M. Dueñas, C. Martínez-Villaluenga, R. I. Limón, E. Peñas and J. Frias, Food Res. Int., 2015, 70, 55–63 CrossRef.
- M. C. Vaz Patto, R. Amarowicz, A. N. A. Aryee, J. I. Boye, H. J. Chung, M. A. Martín-Cabrejas and C. Domoney, Crit. Rev. Plant Sci., 2015, 34, 105–143 CrossRef CAS.
- B. J. Xu and S. K. C. Chang, J. Food Sci., 2007, 72, S159–S166 CrossRef CAS PubMed.
- X. Wu, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. E. Gebhardt and R. L. Prior, J. Agric. Food Chem., 2004, 52, 4026–4037 CrossRef CAS PubMed.
- S. Röjdmark, S. Rössner and L. Wetterberg, Metab., Clin. Exp., 1992, 41, 1106–1109 CrossRef.
- A. Michalsen, F. Schlegel, A. Rodenbeck, R. Lüdtke, G. Huether, H. Teschler and G. J. Dobos, Ann. Nutr. Metab., 2003, 47, 194–200 CrossRef CAS PubMed.
- S. D. Paredes, M. P. Terrón, A. M. Marchena, C. Barriga, J. A. Pariente, R. J. Reiter and A. B. Rodríguez, Mol. Cell. Biochem., 2007, 304, 305–314 CrossRef CAS PubMed.
- J. Delgado, M. P. Terrón, M. Garrido, J. A. Pariente, C. Barriga, A. B. Rodríguez Moratinos and S. D. Paredes, J. Appl. Biomed., 2012, 10, 109–117 CrossRef CAS.
- M. Sae-Teaw, J. Johns, N. P. Johns and S. Subongkot, J. Pineal Res., 2013, 55, 58–64 CrossRef CAS PubMed.
- J. Delgado, M. del Pilar Terrón, M. Garrido, C. Barriga, J. Espino, S. D. Paredes and A. B. Rodríguez, J. Appl. Biomed., 2012, 10, 41–50 CrossRef CAS.
- N. P. Johns, J. Johns, S. Porasuphatana, P. Plaimee and M. Sae-Teaw, J. Agric. Food Chem., 2013, 61, 913–919 CrossRef CAS PubMed.
- D. González-Flores, E. Gamero, M. Garrido, R. Ramírez, D. Moreno, J. Delgado, E. Valdés, C. Barriga, A. B. Rodríguez and S. D. Paredes, Food Funct., 2012, 3, 34–39 Search PubMed.
- G. A. Bubenik, Dig. Dis. Sci., 2002, 47, 2336–2348 CrossRef CAS PubMed.
- X. Tian, X. Huo, P. Dong, B. Wu, X. Wang, C. Wang, K. Liu and X. Ma, Biochem. Pharmacol., 2015, 94, 282–296 CrossRef CAS PubMed.
- H. Zhao, Y. Wang, B. Yuan, S. Liu, S. Man, H. Xu and X. Lu, J. Pharm. Biomed. Anal., 2016, 117, 390–397 CrossRef CAS PubMed.
- S. Kim, M. Lee, J. Hong, C. Li, T. J. Smith, G. Yang, D. N. Seril and C. S. Yang, Nutr. Cancer, 2000, 37, 41–48 CrossRef CAS PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Remesy and L. Jimenez, Am. J. Clin. Nutr., 2004, 79, 727–747 CAS.
- S. M. Henning, Y. Niu, N. H. Lee, G. D. Thames, R. R. Minutti, H. Wang, V. L. W. Go and D. Heber, Am. J. Clin. Nutr., 2004, 80, 1558–1564 CAS.
- G. Velderrain-Rodríguez, H. Palafox-Carlos, A. Wall-Medrano, J. Ayala-Zavala, C. O. Chen, M. Robles-Sánchez, H. Astiazaran-García, E. Alvarez-Parrilla and G. González-Aguilar, Food Funct., 2014, 5, 189–197 Search PubMed.
- J. H. Moon, R. Nakata, S. Oshima, T. Inakuma and J. Terao, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2000, 279, R461–R467 CAS.
- A. Aura, Phytochem. Rev., 2008, 7, 407–429 CrossRef CAS.
- I. Hasslauer, A. Oehme, S. Locher, A. Valotis, G. van't Slot, H. Humpf and P. Schreier, Mol. Nutr. Food Res., 2010, 54, 1546–1555 CAS.
- A. Galano, Phys. Chem. Chem. Phys., 2011, 13, 7178–7188 RSC.
- A. Korkmaz, R. J. Reiter, T. Topal, L. C. Manchester, S. Oter and D. X. Tan, Mol. Med., 2009, 15, 43–50 CAS.
- D. Bonnefont-Rousselot and F. Collin, Toxicology, 2010, 278, 55–67 CrossRef CAS PubMed.
- M. Garrido, S. D. Paredes, J. Cubero, M. Lozano, A. F. Toribio-Delgado, J. L. Munoz, R. J. Reiter, C. Barriga and A. B. Rodriguez, J. Gerontol., Ser. A, 2010, 65, 909–914 CrossRef PubMed.
Footnote |
| † Both authors contributed equally to this work and should be considered as the first authors. |
| This journal is © The Royal Society of Chemistry 2016 |